Dr. Samer AlWazni
MB.Ch.B., DMRD, FCABMS-Rad.Physics of Radiation Therapy and Radiation Protection
Physics of Radiation Therapy and Radiation ProtectionRontgen: it is the unit of exposure. It was defined in terms of ionization in air. It used only for X-rays and gamma rays in air.
Rad: it is the unit of absorbed dose. The rad defined as 100 ergs of energy to 1 g of tissue. The rad can be used for any type of radiation in any material.
Gray (Gy): it is the international (SI) unit of dose. 1Gy=1J/kg. since a joule is 107 ergs, a gray equal 100 rad.
Rem (rad equivalent man): it is the unit used for the quantity dose equivalent. The dose equivalent is equal to dose multiplied by the quality factor.
Principles of radiation therapy
The basic principle of radiation therapy is to maximize damage to the tumor while minimizing damage to normal tissue. This is generally accomplished by directing a beam of radiation at the tumor from several directions so that the maximum dose occurs at the tumor.Ionizing radiation tears electron off atoms to produce positive and negative ions. It also breaks up molecules, the new chemicals formed are of no use to the body and can be considered a form of poison.
Factors that determine how much radiation is required are the type of radiation, the type of cell and the environment of the cell.
Some types of radiation are more effective in killing cells or have a higher relative biological effect (RBE). The RBE is defined as the ratio of the number of grays of 250 kVp X-rays needed to produce a given biological effect to the number of grays of the test radiation needed to produce the same effect.
The quality factor (QF) is related to the relative biological effect (RBE). RBE for a particular radiation is often different for a different types of cell, while the QF is arbitrarily defined to be a constant for a particular radiation. RBE is usually used in radiation therapy, while QF is used for radiation protection purpose.
LD50 (lethal dose for 50%): it is the quantity of radiation that will kill half of the organisms in population. This quantity is sometimes modified to include the time factor. For example, the amount of radiation that will kill 50% of the organisms in 30 days called LD50(30)
The cells irradiated in the presence of oxygen were much easier to kill than cells of the same type irradiated without oxygen. Hyperbaric oxygen tanks were therefore developed for radiotherapy.
Treatment planning: it is the process of determining the best combination of radiation beams and their orientation.
Radiation sources used in radiotherapy
• Before 1940 most external therapy was given with orthovoltage x-ray units that had maximum potential of 250kVp or less, a few centimeters had 400kVp units or the new 1000kVp or 1 million volt machine.• Then the betatron was developed, which accelerates electrons to high energy. The electrons can be used directly or they can be used to produce a high energy x-ray beam. The betatorn helped open new radiation therapy era (the supervoltage or megavoltage era)
• Natural radioactive sources: cancer had been treated for decades with radioactive radium sources placed directly in or on the tumor but there was insufficient radium to produce a useful external beam of gamma rays.
• Artificial radioisotopes: with the development of nuclear reactors it became possible to produce many artificial radioisotopes in undreamed of quantities.
60Co Therapy
One of the radioactive source easy to produce in reactor is 60Co. Cobalt 60 emits penetrating gamma rays of about 1.25MeV energy. These rays are about as penetrating as the x-ray from 3 million volt x-ray machine, but the 60Co unit is much more compact. The high-energy gamma rays emitted by 60Co are absorbed by the tissue and produce high energy electrons, most of which move in the same general direction as the original beam. As the gamma ray beam penetrates the first few millimeters beneath the skin, the number of electron increases and the energy deposited by them increases. The dose maximum occurs about 5mm below the skin. The relatively low dose at the skin from high-energy x-rays or gamma rays is referred to as the skin sparing effect.Megavoltage therapy has three advantages over 60Co therapy
• The maximum dose occurs below the skin, and this skin-sparing effect greatly reduces the pain from the treatment.• The high energy is almost completely in the Compton effect region and unlike 259kVp radiation, dose not give a large dose to the bone.
• The greater penetrating ability permits better treatment of tumors deep inside the body.
Short distance radiotherapy or Brachytherapy
In 1904 a biological experiment described the effect of placing a capsule containing radium on arm and lift it for several hours. It will produce a sore that took over a month to heal. This sore was not a surface “burn” the damage was much deeper. This method was developed in which sources of radium were put into or on the surface of tumors. This short distance therapy or brachytherapy is still standard treatment method for certain types of cancer, especially of female reproductive organs.The advantage of brachytherapy is that it gives a very large dose to the tumor with minimum radiation to the surrounding normal tissue
Its main disadvantages is the nonuniformity of the dose since radiation is much more intense near the source, although using number of sources helps make the dose more uniform. Another disadvantage concern radiation safety (therapist and nurses).
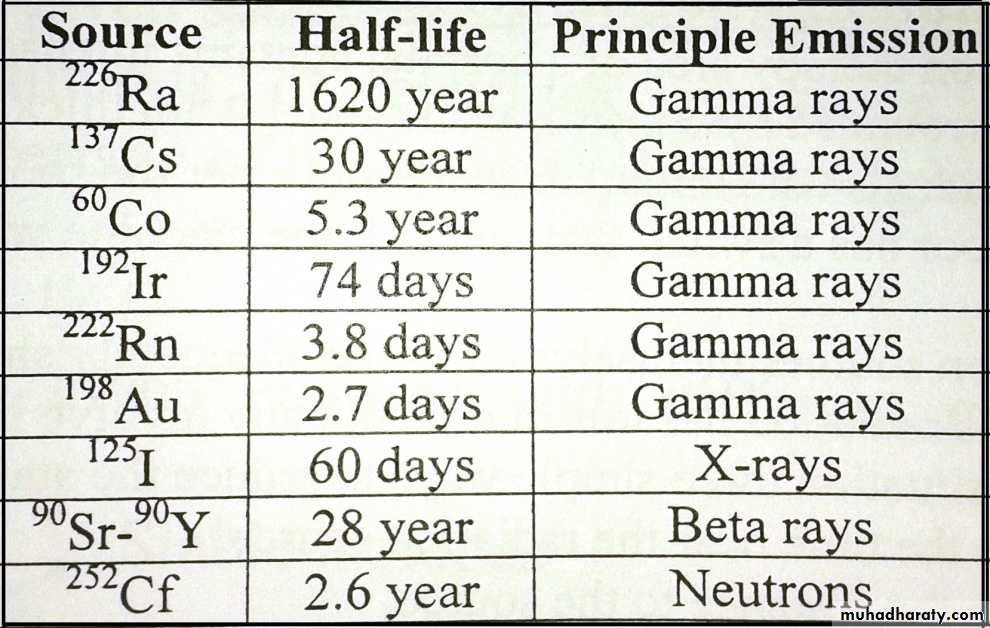
The following table show the radinuclide source that sometimes used for brachytherap:
Radiation protection instrumentation
Ionizing radiation is fairly energetic and produces number of physical and chemical effects. Some of these effects are:• Ionization
• Darkening of film
• Production of light (scintillation)
• Breakdown of molecules and production of new combinations
• Storage of energy in crystal which later be released as light (thermoluminescence)
• Change in conductivity of certain solids
• Slight temperature increase in any material
• Change in the colors of certain dyes
Radiation protection in Diagnostic radiology
The radiation received by the patient depends on many factors these are : kV, mA, time, filter, distance and area exposure. In order to reduce the patient radiation dose the followings should be noted:• X-ray unit should have sufficient filtration in order to remove low-energy x-ray from the beam. Most x-ray units should have at least 3mm of aluminum.
• The ratio of x-ray beam area to film area should be less than 1. this achieved by using collimators.
• Exposure-area products are good measure of the total radiation insult to the patient in the unit of Rcm2.
• Retaking of x-ray films is common cause of unnecessary radiation.
• Gonads must be shielded whenever possible.
• Pregnant or may be pregnant female patient should not x-raying except in emergency situations.
• Time of fluoroscopic examination should be minimized as more as possible.
Radiation protection in Radiation therapy
Since the radiation therapy area of a hospital contains intense radiation source, it is typically surrounded by concrete walls about 0.5m thick.
To protect individuals who might inadvertently enter the room during a treatment, the door has a switch that turns off the machine when door is opened.
Also the radiation sources themselves must be adequately shielded.
The most serious radiation hazards in radiotherapy involve internal radiation sources. In this situation three simple ways to reduce the staff radiation:
• Minimize the time near the radiation source.
• Maximize the distance to the source
• Use shielding where possible.
Radiation protection in Nuclear Medicine
Practically all radionuclide used in nuclear medicine emit penetrating gamma rays that can be detected outside the body. Most of shielding used in nuclear medicine laboratory is for absorbing gamma rays.Physical of nuclear medicine
The radioactive elementsIt is unstable nuclei that disintegrate to emit various rays and particles, such as:
• The alpha (α)particles: are positively charged, helium nucleus 24He have rang of energy stop in few cm of air
• Beta particles(β): are two kinds
• β- are negatively charged are called negatron, are more penetrating but can be stooped in a few meters of air or few millimeters of tissue, they are high-speed electrons
• β+ positive or positron are produced by cyclotrons. β+ is physically identical to an electron except that it has a positive charge.
• 3. γ-rays Electromagnetic radiation, identical to x-ray but γ rays have much higher energies and they are very penetrating than α and beta, α and γ rays from a given source have fixed energies but beta have a continuous of energies up to maximum characteristic of the source (fixed energies)
Isotopes
Nuclei of a given elements with different numbers of neutrons
Isotopes stable isotopes
unstable isotopes
Stable isotopes: they are not radioactive like 12C, 13C
Unstable isotopes: they are radioactive nuclei like 11C, 14C and 13C
Most elements do not have naturally occurring radioisotopes but all produced artificially.
Nuclear reactor and nuclear medicine
The production of many artificial radio nuclidesIn medicine used for: research, diagnostic and therapy from the cancer, tumor, ………
γ-ray in medicine: γ-ray are very penetrating, γ emitting radioactive element inside the body can be detected outside the body
Use less than 1μg of radio nuclides element in medicine pupose
The radioactive decay and units of radioactivity
The symbol of radioactive element is ZAXwhere the: X is the element
A atomic mass[total number of nucleons]
A= p + n p=protons, n=neutrons
Z atomic number
Z= e =p
n= A - Z number of neutron
If n ˃ normal unstable radioactive
nucleic γ, α, β rays stable
Radioactive decay
Decay the nuclei of unstable isotopes to its daughter, which is also radioactive, is formed each daughter decays until the final daughter (stable isotope) after emitting the radioactive rays (α, β, and γ)ZAX decay 24He + Z-A-Y
A- = A-4
Z- = Z-2
Examples
When 1327Al is bombarded by deuterons, α-particles are emitted, how many protons and neutrons dose its nucleus contains?Solution : 1327Al + 12H 24He +ZAX
A+4 =29
Z+2 =14 then A=25, Z=12
The atom of 1225X is 1225Mg
92234U α 90234Th γ, β 91234Pa β, γ
90234U α Th 206Pb (stable)
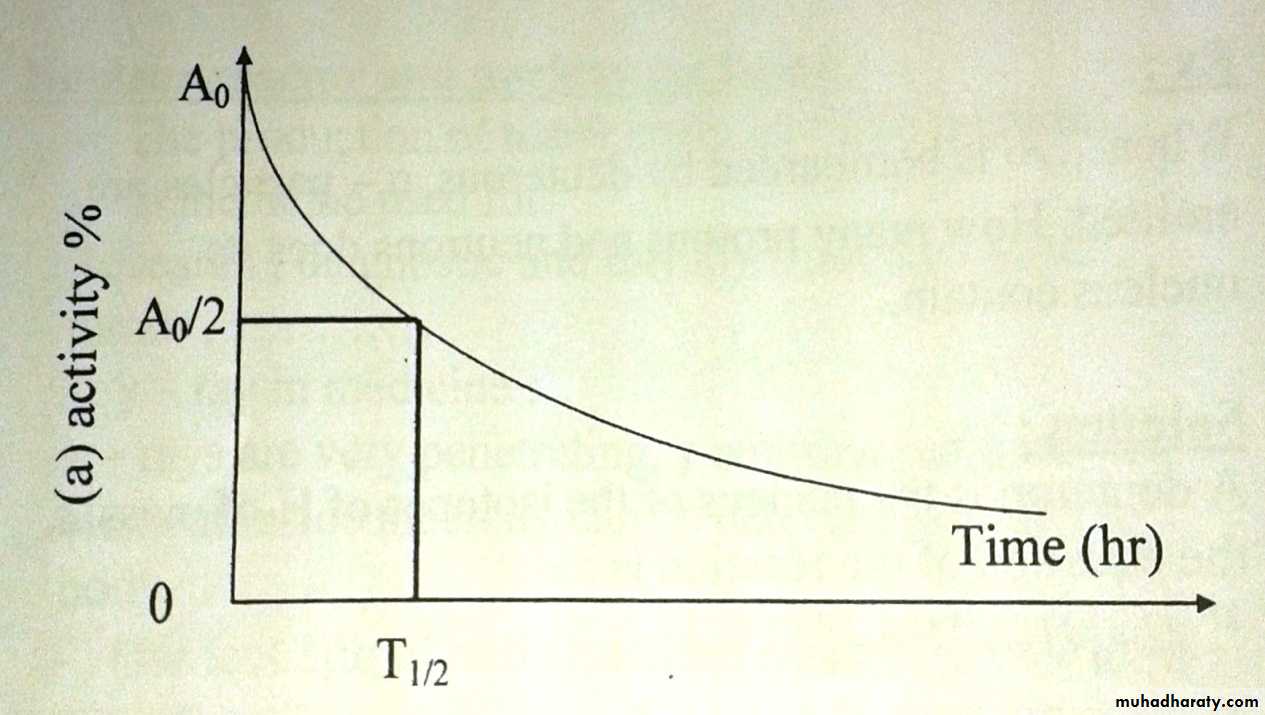
Each radio nuclide decays at fixed rate indicate by the half T1/2
T1/2 : the time needed for half of the radioactive nuclei to decayThe basic equation describing radioactive decay is: A= A0e-λt
A= the activity in disintegration per second
A0= the initial activity
λ= decay constant
t= is the time of decay
Then A= λN
N= the number of
radioactive atoms
N= N0e- λt
T1/2= 0.693l λ
And λ=0.693/T1/2
The unit of radioactivity
The Curie (ci)= 3.7 * 1010 disintegration/smci= 10-3ci , μci= 10-6ci ,nci= 10-9ci
1CRU= international SI unit is Becquerel (Bq)
Bq= disintegration/sec 1ci=3.7*1010Bq
KBq=103 disintegration/sec MBq=106disi/sec
Examples
1g of K40 (40K) to emit 105B/sec what is λ and T1/2 of 40K?Solution :40g and 40K contains 6.02 * 1023 Avogadro's number potassium atoms.
1g contains 6.02/(40 * 1023) atom
λ = A/N = 103/(1.5 * 1023) = 6.7 * 10-18 sec
T1/2 = 0.693/(6.7 * 10-18) = 1017 sec-1
Year = 3.15 * 107 sec
What is the decay constant of 131I in thyroid if T1/2 = 1s days?
If a radio nuclide has a decay constant λ of 0.001 days-1a--T1/2?
b--how much do have left after 24 hr if you have 10MBq at t=0?