The Thyroid Gland
Dr. Haidar F. Al-RubayeAnatomy
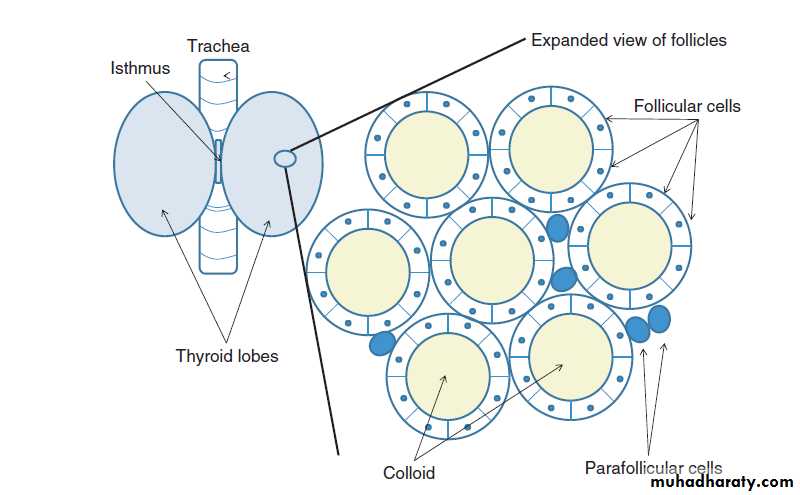
The gland lies deep to the strap muscles of the neck, enclosed in the pretrachealfascia, which anchors it to the trachea, so that the thyroid moves up on swallowing.
Histology
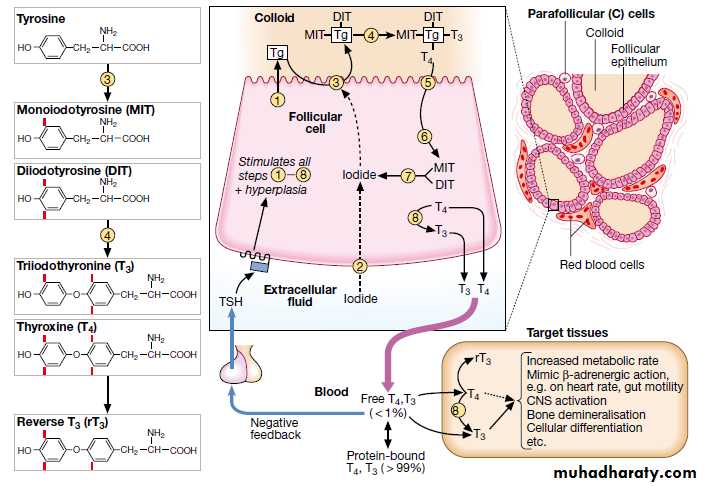
Fibrous septa divide the gland into pseudolobules. Pseudolobules are composed of vesicles called follicles or acini, surrounded by a capillary network.The follicle walls are lined by cuboidal epithelium.
The lumen is filled with a proteinaceous colloid, which contains the unique protein thyroglobulin.
The peptide sequences of thyroxine (T 4 ) and tri-iodothyronine (T 3 ) are synthesized and stored as a component of thyroglobulin.
Anatomy
Physiology
Thyroid hormone contains iodine. Iodine enters the thyroid in the form of inorganic or ionic iodide, which is organized by the thyroid peroxidase enzyme at the cell–colloid interface.Subsequent reactions result in the formation of iodothyronines.
The thyroid is the only source of T4 . The thyroid secretes 20% of circulating T3 ; the remainder is generated in extraglandular tissues by the conversion of T4 to T3 by deiodinases (largely in the liver and kidneys).
Physiology
In the blood, T4 and T3 are almost entirely bound to plasma proteins.T4 is bound in d order of affinity to T4 -binding globulin (TBG), transthyretin (TTR), and albumin.
T3 is bound 10–20 times less avidly by TBG and not significantly by TTR.
Only the free or unbound hormone is available to tissues. The metabolic state correlates more closely with the free than the total hormone concentration in the plasma.
The relatively weak binding of T 3 accounts for its more rapid onset and offset of action.
The concentration of free hormones does not necessarily vary directly with that of the total hormones; e.g., while the total T 4 level rises in pregnancy, the free T 4 (FT 4 ) level remains normal.
Classification of thyroid disease
PrimarySecondary
Hormone excess
Graves’ diseaseMultinodular goitreAdenomaSubacute thyroiditis
TSHoma
Hormone deficiency
Hashimoto’s thyroiditisAtrophic hypothyroidism
Hypopituitarism
Hormone hypersensitivity
Hormone resistance
Thyroid hormone resistance syndrome5′-monodeiodinase deficiencyNon-functioning tumours
Differentiated carcinomaMedullary carcinomaLymphomaThyroid Function Tests
How to interpret thyroid function test results
Primary thyrotoxicosis
PrimaryT3-toxicosis
Subclinical thyrotoxicosis
Sick euthyroidism/
non-thyroidal illnessSecondary hypothyroidism
/Transient thyroiditis in
evolution
Secondary hypothyroidism
Primary hypothyroidism
/Secondary hypothyroidismPrimary hypothyroidism
Subclinical hypothyroidism
Artefact
Endogenous IgG antibodies which interfere with TSH assayNon-compliance with T4 replacement (Recent ‘loading’ dose)
Secondary thyrotoxicosis
Thyroid hormone resistance
Thyrotoxicosis
Thyrotoxicosis describes a constellation of clinical features arising from elevated circulating levels of thyroid hormone.
The most common causes are Graves’ disease, multinodular goitre and autonomously functioning thyroid nodules (toxic adenoma)
Thyroiditis is more common in parts of the world where relevant viral infections occur, such as North America
Causes of thyrotoxicosis and their relative frequencies
CauseFrequency (%)
Graves’ disease
76
Multinodular goitre
14
Solitary thyroid adenoma
5
Thyroiditis
Subacute (de Quervain’s)
Post-partum
3
0.5
Iodide-induced
Drugs (e.g. amiodarone)
Radiographic contrast media
Iodine prophylaxis programme
1
-
-
Causes of thyrotoxicosis and their relative frequencies
CauseFrequency (%)
Extrathyroidal source of thyroid hormone
Factitious thyrotoxicosis
Struma ovarii
0.2
-
TSH-induced
TSH-secreting pituitary adenoma
Choriocarcinoma and hydatidiform mole
0.2
-
Follicular carcinoma ± metastases
0.1
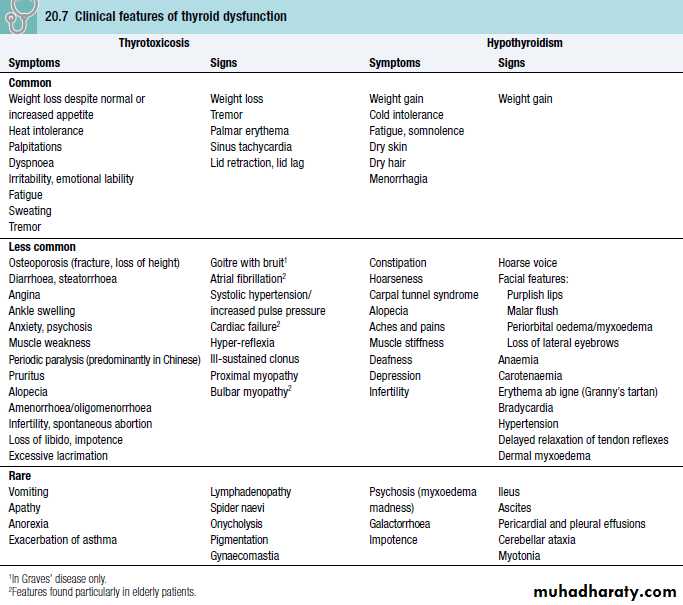
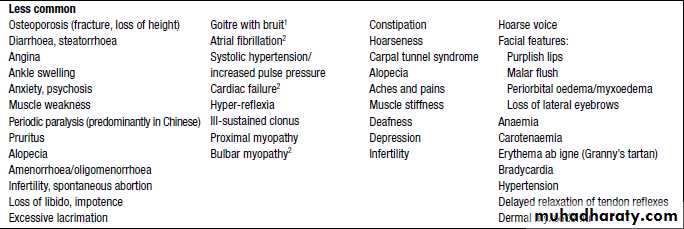
Clinical features of thyrotoxicosis & hypothyroidims-common symptoms
Thyrotoxicosis-symptomsHypothyroidism-symptoms
Clinical features of thyrotoxicosis & hypothyroidims-common signs
Thyrotoxicosis-signsHypothyroidism-signs
Clinical features of thyrotoxicosis & hypothyroidims-common signs
The most common symptoms are weight loss with a normal or increased appetite, heat intolerance, palpitations, tremor and irritability.
Tachycardia, palmar erythema and lid lag are common signs.
Not all patients have a palpable goitre, but experienced clinicians can discriminate the diffuse soft goitre of Graves’ disease from the irregular enlargement of a multinodular goitre.
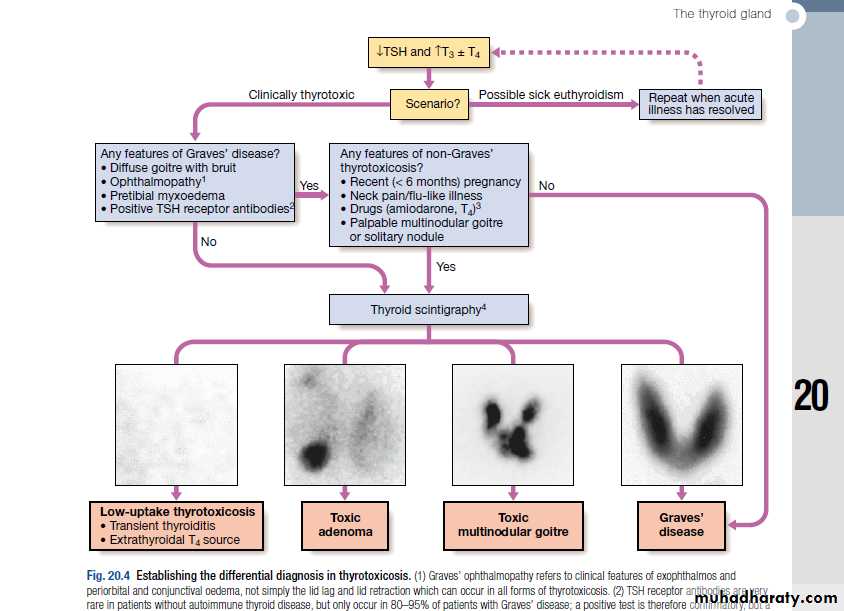
Investigation of thyrotoxicosis
The first-line investigations are serum T3, T4 and TSH.
If abnormal values are found, the tests should be repeated and the abnormality confirmed in view of the likely need for prolonged medical treatment or destructive therapy.
In most patients serum T3 and T4 are both elevated but T4 is in the upper part of the normal range and T3 raised (T3 toxicosis) in about 5%.
Serum TSH is undetectable in primary thyrotoxicosis but values can be raised in the very rare syndrome of secondary thyrotoxicosis caused by a TSH-producing pituitary adenoma.
↓TSH and ↑T3 ± T4
Repeat when acute illness has resolvedScenario?
Any features of Graves’ disease?
• Diffuse goitre with bruit
• Ophthalmopathy
• Pretibial myxoedema
• Positive TSH receptor antibodies
Any features of non-Graves’ thyrotoxicosis?
• Recent (< 6 months) regnancy
• Neck pain/flu-like illness
• Drugs (amiodarone, T4)3
• Palpable multinodular goitre or solitary nodule
Yes
No
Thyroid Scintigraphy
Yes
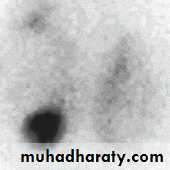
Low-uptake thyrotoxicosis
• Transient thyroiditis• Extrathyroidal T4 source
Toxic adenoma
Toxic MNG
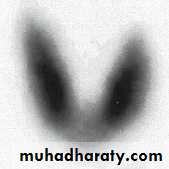
Graves’ disease
No
Clinically thyrotoxic
Possible sick euthyroidism
Investigation of thyrotoxicosis
When biochemical thyrotoxicosis has been confirmed, further investigations should be undertaken to determine the underlying cause, including measurement of TSH receptor antibodies (TRAb, elevated in Graves’ disease, and isotope scanning.Prevalence of thyroid autoantibodies (%)
Antibodies to:
Thyroid
peroxidaseThyroglobulin
TSH
receptor
Normal population
8–27
5–20
0
Graves’ disease
50–80
50–70
80–95
Autoimmune
Hypothyroidism
90–100
80–90
10–20
Multinodular goitre
∼30–40
0
0
Transient thyroiditis
∼30–40
0
0
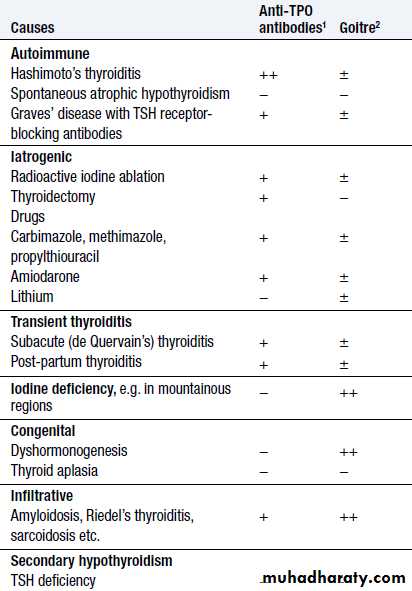
1 Thyroid peroxidase (TPO) antibodies are the principal component of what was previously measured as thyroid ‘microsomal’ antibodies.
2 TSH receptor antibodies (TRAb) can be agonists (stimulatory, causing Graves’ thyrotoxicosis) or antagonists (‘blocking’, causing hypothyroidism).
Non-specific laboratory abnormalities inthyroid dysfunction*
*These abnormalities are not useful in differential diagnosis, so the tests should be avoided and any further investigation undertaken only if abnormalities persist when the patient is euthyroid.
Management
Definitive treatment of thyrotoxicosis depends on the underlying cause and may include antithyroid drugs, radioactive iodine or surgery.A non-selective β- adrenoceptor antagonist (β-blocker), such as propranolol (160 mg daily) or nadolol (40–80 mg daily), will alleviate but not abolish symptoms in most patients within 24–48 hours.
Beta-blockers should not be used for long term treatment of thyrotoxicosis, but are extremely useful in the short term, whilst patients are awaiting hospital consultation or following 131I therapy.
Hypothyroidism
Hypothyroidism is a common condition with various causes but autoimmune disease (Hashimoto’s thyroiditis) and thyroid failure following 131I or surgical treatment of thyrotoxicosis account for over 90% of cases, except in areas where iodine deficiency is endemic.Women are affected approximately six times more frequently than men.
Clinical features
The clinical presentation depends on the duration and severity of the hypothyroidism.A consequence of prolonged hypothyroidism is the infiltration of many body tissues by the mucopolysaccharides, hyaluronic acid and chondroitin sulphate, resulting in a low-pitched voice, poor hearing, slurred speech due to a large tongue, and compression of the median nerve at the wrist (carpal tunnel syndrome).
Infiltration of the dermis gives rise to non-pitting oedema (myxoedema) which is most marked in the skin of the hands, feet and eyelids.
The resultant periorbital puffiness is often striking and, when combined with facial pallor due to vasoconstriction and anaemia, or a lemon-yellow tint to the skin due to carotenaemia, purplish lips and malar flush, the clinical diagnosis is simple.
Most cases of hypothyroidism are not clinically obvious, however, and a high index of suspicion needs to be maintained so that the diagnosis is not overlooked in the middle-aged woman complaining of non-specific symptoms such as tiredness, weight gain, depression or carpal tunnel syndrome.
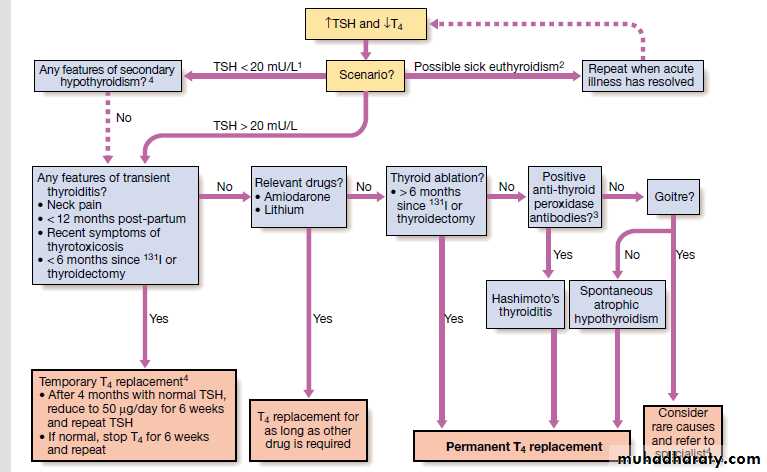
Care must be taken to identify patients with transient hypothyroidism, in whom life-long thyroxine therapy is inappropriate.
This is often observed during the first 6 months after subtotal thyroidectomy or 131I treatment of Graves’ disease, in the post-thyrotoxic phase of subacute thyroiditis and in post-partum thyroiditis. In these conditions thyroxine treatment is not always necessary as the patient may be asymptomatic during the short period of thyroid failure.
Investigations
In the vast majority of cases hypothyroidism results from an intrinsic disorder of the thyroid gland (primary hypothyroidism). In this situation serum T4 is low and TSH is elevated, usually in excess of 20 mU/L.Measurements of serum T3 are unhelpful since they do not discriminate reliably between euthyroidism and hypothyroidism.
The rare condition of secondary hypothyroidism is caused by failure of TSH secretion in a patient with hypothalamic or anterior pituitary disease. This is characterised by a low serum T4 but TSH may be low, normal or even slightly elevated
Management
Treatment is with thyroxine replacement. It is customary to start with a low dose of 50 μg per day for 3 weeks, increasing thereafter to 100 μg per day for a further 3 weeks and finally to a maintenance dose of 100–150 μg per day.Thyroxine has a half-life of 7 days so it should always be taken as a single daily dose and at least 6 weeks should pass before repeating thyroid function tests and adjusting the dose, usually in increments of 25 μg per day.
Patients feel better within 2–3 weeks. Reduction in weight and periorbital puffiness occurs quickly, but the restoration of skin and hair texture and resolution of any effusions may take 3–6 months.
The dose of thyroxine should be adjusted to maintain serum TSH within the reference range.
To achieve this, serum T4 often needs to be in the upper part of the normal range or even slightly raised, because the T3 required for receptor activation is derived exclusively from conversion of T4 within the target tissues, without the usual contribution from thyroid secretion.
Some patients remain symptomatic despite normalisation of TSH and may wish to take extra thyroxine which suppresses TSH values.
However, there is evidence that suppressed TSH is a risk factor for osteoporosis and atrial fibrillation, so this approach cannot be recommended.
It is important to measure thyroid function every 1–2 years once the dose of thyroxine is stabilised.
This encourages patient compliance with therapy and allows adjustment for variable underlying thyroid activity and other changes in thyroxine requirements
Thyroxine replacement in ischaemic heart disease
Hypothyroidism and ischaemic heart disease are common conditions which often occur together.Although angina may remain unchanged in severity or paradoxically disappear with restoration of metabolic rate, exacerbation of myocardial ischaemia, infarction and sudden death are recognised complications of thyroxine replacement, even using doses as low as 25 μg per day.
In patients with known ischaemic heart disease, thyroid hormone replacement should be introduced at low dose and increased very slowly under specialist supervision.
It has been suggested that T3 has an advantage over T4 since T3 has a shorter half-life and any adverse effect will reverse more quickly, but the more distinct peak in hormone levels after each dose of T3 is a disadvantage.
Coronary artery surgery or angioplasty is required in the minority of patients with angina who cannot tolerate full thyroxine replacement therapy despite maximal anti-anginal therapy.
Hypothyroidism in pregnancy
Most pregnant women with primary hypothyroidism require an increase in the dose of thyroxine of approximately 50 μg daily to maintain normal TSH levels.This may reflect increased metabolism of thyroxine by the placenta and increased serum thyroxine-binding globulin during pregnancy, resulting in an increase in the total thyroid hormone pool to maintain the same free T4 and T3 concentrations.
Recent research suggests that inadequate maternal T4 therapy is associated with impaired cognitive development in their offspring. Serum TSH and free T4 should be measured during each trimester and the dose of thyroxine adjusted to maintain a normal TSH.
Myxoedema coma
This is a rare presentation of hypothyroidism in which there is a depressed level of consciousness, usually in an elderly patient who appears myxoedematous.Body temperature may be as low as 25°C, convulsions are not uncommon and cerebrospinal fluid (CSF) pressure and protein content are raised.
The mortality rate is 50% and survival depends upon early recognition and treatment of hypothyroidism and other factors contributing to the altered consciousness level, such as phenothiazines, cardiac failure, pneumonia, dilutional hyponatraemia and respiratory failure.
Myxoedema coma is a medical emergency and treatment must begin before biochemical confirmation of the diagnosis.
Suspected cases should be treated with an intravenous injection of 20 μg triiodothyronine followed by further injections of 20 μg 8-hourly until there is sustained clinical improvement. In survivors there is a rise in body temperature within 24 hours and, after 48–72 hours, it is usually possible to switch patients on to oral thyroxine in a dose of 50 μg daily.
Unless it is apparent that the patient has primary hypothyroidism, the thyroid failure should also be assumed to be secondary to hypothalamic or pituitary disease and treatment given with hydrocortisone 100 mg i.m. 8-hourly, pending the results of T4, TSH and cortisol measurement
Other measures include slow rewarming, cautious use of intravenous fluids, broad-spectrum antibiotics and high-flow oxygen. Occasionally, assisted ventilation may be necessary.
Autoimmune thyroid diseases
Thyroid diseases are amongst the most prevalent antibody- mediated autoimmune diseases and are associated with other organ-specific autoimmunityAutoantibodies may produce inflammation and destruction of thyroid tissue resulting in hypothyroidism, goitre (in Hashimoto’s thyroiditis) or sometimes even transient thyrotoxicosis (‘Hashitoxicosis’), or they may stimulate the TSH receptor to cause thyrotoxicosis (in Graves’ disease).
There is overlap between these conditions, since some patients have multiple autoantibodies.
Graves’ disease
Graves’ disease can occur at any age but is unusual before puberty and most commonly affects women aged 30–50 years.The most common manifestation is thyrotoxicosis with or without a diffuse goitre.
Graves causes clinical features shown in previous lectures
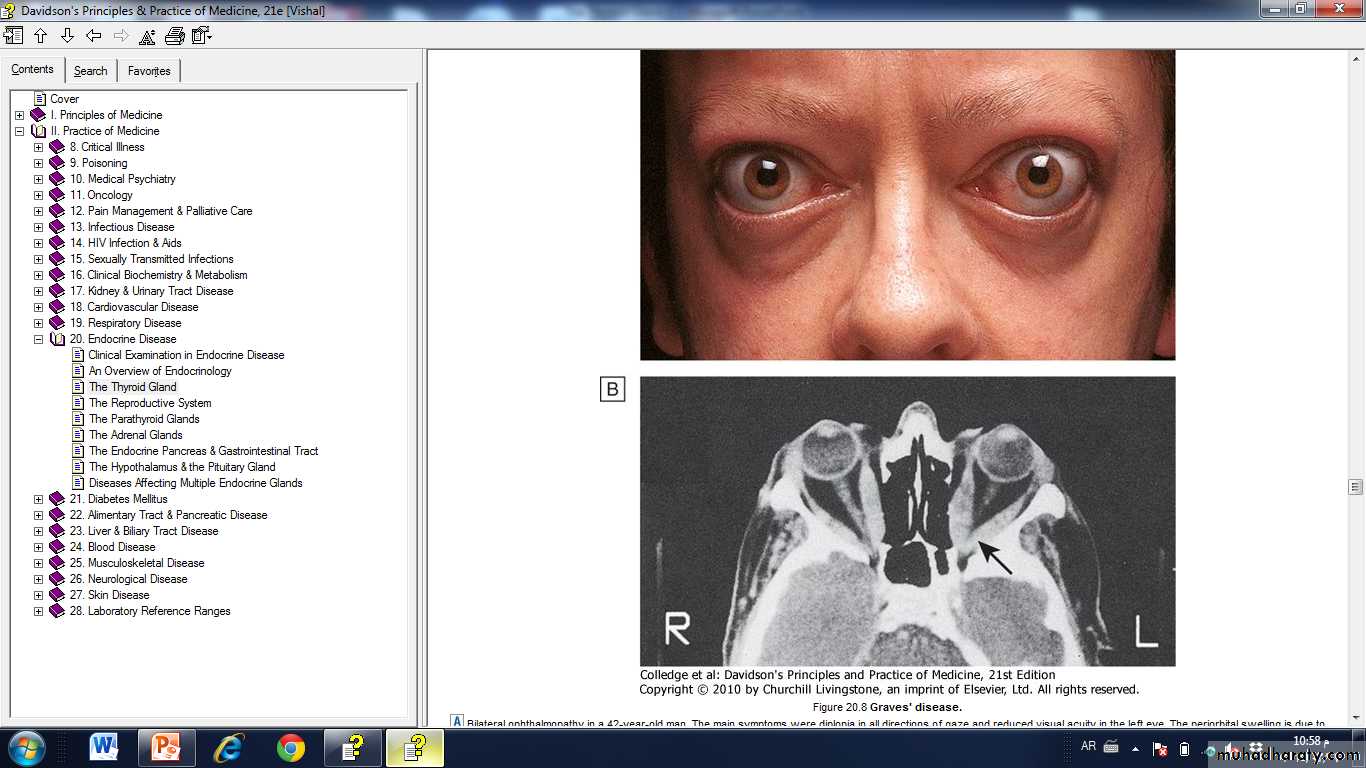
Graves’ disease also causes ophthalmopathy and rarely pretibial myxoedema
These extrathyroidal features usually occur in thyrotoxic patients, but can occur in the absence of thyroid dysfunction.
Graves’ thyrotoxicosis-Pathophysiology
The thyrotoxicosis results from the production of IgG antibodies directed against the TSH receptor on the thyroid follicular cell, which stimulate thyroid hormone production and proliferation of follicular cells, leading to goitre in the majority of patients. These antibodies are termed thyroid-stimulating immunoglobulins or TSH receptor antibodies (TRAb) and can be detected in the serum of 80–95% of patients with Graves’ disease.The concentration of TRAb in the serum is presumed to fluctuate to account for the natural history of Graves’ thyrotoxicosis
The thyroid failure seen in some patients may result from the presence of blocking antibodies against the TSH receptor, and from tissue destruction by cytotoxic antibodies and cell-mediated immunity.
Features of Graves disease in addition to diffuse goitre
A suggested trigger for the development of thyrotoxicosis in genetically susceptible individuals may be infection with viruses or bacteria.
Certain strains of the gut organisms Escherichia coli and Yersinia enterocolitica possess cell membrane TSH receptors; antibodies to these microbial antigens may cross-react with the TSH receptors on the host thyroid follicular cell.
In regions of iodine deficiency, iodine supplementation can precipitate thyrotoxicosis, but only in those with pre-existing subclinical Graves’ disease. Smoking is weakly associated with Graves’ thyrotoxicosis, but strongly linked with the development of ophthalmopathy.
Management
Symptoms of thyrotoxicosis respond to β-blockade, but definitive treatment requires control of thyroid hormone secretion.Comparison of treatments for the thyrotoxicosis of Graves’ disease
ManagementCommon indications
Contraindications
Disadvantages/
complications
Antithyroid drugs
Subtotal
thyroidectomy
Radio-iodine
First episode in patients
< 40 yrs
Large goitre
Poor drug compliance, especially in young patients
Recurrent thyrotoxicosis after course of antithyroid drugs in young patients
First episode in patients
< 40 yrs
Comparison of treatments for the thyrotoxicosis of Graves’ disease
Management
Common indications
Contraindications
Disadvantages/
complications
Antithyroid drugs
Subtotal
thyroidectomy
Radio-iodine
Breastfeeding (propylthiouracil
suitable)
Previous thyroid surgery
Dependence upon voice, e.g. opera singer, lecturer
Pregnancy or planned pregnancy
within 6 months of treatment
Active Graves’ ophthalmopathy
Comparison of treatments for the thyrotoxicosis of Graves’ disease
ManagementCommon indications
Contraindications
Disadvantages/
complications
Antithyroid drugs
Subtotal
thyroidectomy
Radio-iodine
Hypersensitivity rash 2%
Agranulocytosis 0.2%
> 50% relapse rate usually within 2 years of stopping drug
Hypothyroidism (∼25%)
Transient hypocalcaemia (10%)
Permanent hypoparathyroidism (1%)
Recurrent laryngeal nerve palsy1 (1%)
Hypothyroidism, ∼40% in first year, 80% after 15 years
Most likely treatment to result in exacerbation of ophthalmopathy
Thyrotoxicosis in pregnancy
The coexistence of pregnancy and thyrotoxicosis is unusual, as anovulatory cycles are common in thyrotoxic patients and autoimmune disease tends to remit during pregnancy, when the maternal immune response is suppressed.
Thyroid function tests must be interpreted in the knowledge that thyroid-binding globulin, and hence total T4 and T3 levels, are increased in pregnancy and that TSH normal ranges may be lower
A fully suppressed TSH with elevated free thyroid hormone levels indicates thyrotoxicosis.
The thyrotoxicosis is almost always caused by Graves' disease.
Both mother and fetus must be considered, since maternal thyroid hormones, TRAb and antithyroid drugs can all cross the placenta to some degree, exposing the fetus to the risks of thyrotoxicosis, iatrogenic hypothyroidism and goitre.
Thyrotoxicosis should be treated with antithyroid drugs which cross the placenta and also treat the fetus, whose thyroid gland is exposed to the action of maternal TRAb. Propylthiouracil may be preferable to carbimazole since the latter might be associated with a skin defect in the child, known as aplasia cutis.
In order to avoid fetal hypothyroidism and goitre, it is important to use the smallest dose of antithyroid drug (optimally less than 150 mg propylthiouracil per day) that will maintain maternal (and presumably fetal) free T4, T3 and TSH within their respective normal ranges.
After delivery, if antithyroid drug is required and the patient wishes to breastfeed, then propylthiouracil is the drug of choice, as it is excreted in the milk to a much lesser extent than carbimazole.
If subtotal thyroidectomy is necessary because of poor drug compliance or drug hypersensitivity, it is most safely performed in the second trimester.
Radioactive iodine is absolutely contraindicated, as it invariably induces fetal hypothyroidism.
Graves' ophthalmopathy
This condition is immunologically mediated, but the autoantigen has not been identified.Within the orbit (and the dermis) there is cytokine-mediated proliferation of fibroblasts which secrete hydrophilic glycosaminoglycans.
The resulting increase in interstitial fluid content, combined with a chronic inflammatory cell infiltrate, causes marked swelling and ultimately fibrosis of the extraocular muscles and a rise in retrobulbar pressure.
The eye is displaced forwards (proptosis, exophthalmos) and in severe cases there is optic nerve compression.
The majority of patients require no treatment other than reassurance.
Methylcellulose eye drops and gel counter the gritty discomfort of dry eyes, and tinted glasses or side shields attached to spectacle frames reduce the excessive lacrimation triggered by sun or wind.
Severe inflammatory episodes are treated with glucocorticoids (e.g. daily oral prednisolone or pulsed i.v. methylprednisolone) and sometimes orbital irradiation.
Loss of visual acuity is an indication for urgent surgical decompression of the orbit. In 'burnt out' disease, surgery to the eyelids and/or ocular muscles may improve conjunctival exposure, cosmetic appearance and diplopia.