
Hemostasis
and
Blood Coagulation

Events in Hemostasis
The term hemostasis means prevention of blood loss. Whenever a vessel is severed or
ruptured, hemostasis is achieved by several mechanisms:
(1) vascular constriction,
(2) formation of a platelet plug,
(3) formation of a blood clot as a result of blood coagulation, and
(4) eventual growth of fibrous tissue into the blood clot to close the hole in the vessel
permanently.
Vascular Constriction
the trauma to the vessel wall causes the smooth muscle in the wall to contract; this
instantaneously reduces the flow of blood from the ruptured vessel.
the platelets release thromboxane A2 which is one of the causes of vasoconstriction
Formation of the Platelet Plug
If the cut in the blood vessel is very small the cut is often sealed by a platelet plug:
Physical and Chemical Characteristics of Platelets
Platelets (also called thrombocytes) are minute discs 1 to 4 micrometers in diameter.
They are formed in the bone marrow from megakaryocytes, which are extremely large
cells of the hematopoietic series in the marrow.
The normal concentration of platelets in the blood is between 150,000 and 400,000 per
microliter.

Platelets have many functional characteristics In their cytoplasm ; such active
factors as
(1) actin and myosin molecules, which can cause the platelets to contract;
(2) residuals of both the endoplasmic reticulum and the Golgi apparatus that synthesize
various enzymes and especially store large quantities of calcium ions;
(3) mitochondria and enzyme systems that are capable of forming adenosine triphosphate
(ATP) and adenosine diphosphate (ADP);
(4) enzyme systems that synthesize prostaglandins
(5) an important protein called fibrin-stabilizing factor, XIII
(6) a growth factor that causes vascular endothelial cells, vascular smooth muscle cells,
and fibroblasts to multiply and grow.
The cell membrane of the platelets
is also important. On its surface is a coat of
glycoproteins that adhere injured endothelial cells and even more so to any exposed
collagen.
Mechanism of the Platelet Plug
When platelets come in contact with collagen fibers in the injured vascular wall, the
platelets begin to swell;
they assume irregular forms with numerous irradiating pseudopods protruding from
their surfaces;
their contractile proteins contract forcefully and cause there lease of granules that
contain multiple active factors;
they adhere to collagen in the tissues and to von Willebrand factor that leaks into the
traumatized tissue from the plasma; they secrete large quantities of ADP; and their
enzymesform thromboxane A2 which act on nearby platelets to activate them forming a
platelet plug .
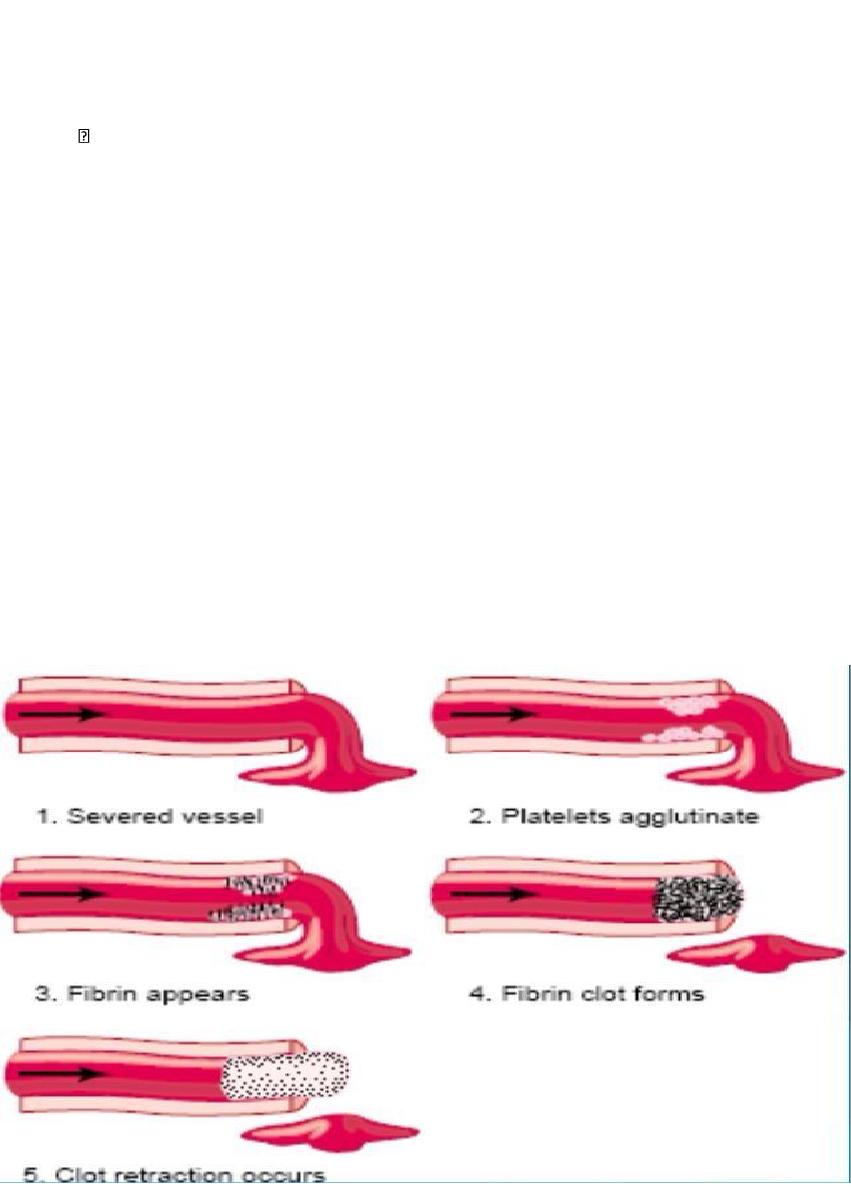
Blood Coagulation in the Ruptured Vessel
Release of Activator substances from the traumatized vascular wall and from platelets,
initiate the clotting process.
Fibrous Organization of the Blood Clot
Once a blood clot has formed, it can follow one of two courses:
(1) It can become invaded by fibroblasts,
1. Severed vessel
2. Platelets agglutinate
3. Fibrin appears
4. Fibrin clot forms
5. Clot retraction occurs
which subsequently form connective tissue all through the clot.
(2) or it can dissolve.

Mechanism of Blood Coagulation
Blood Coagulation takes place in three essential steps:
(1) formation of a complex of activated substances collectively called prothrombin
activator.
(2) The prothrombin activator catalyzes conversion of prothrombin into thrombin.
(3) The thrombin acts as an enzyme to convert fibrinogen into fibrin fibers that enmesh
platelets, blood cells, and plasma to form the clot.
Clotting Factors in Blood
1. Fibrinogen Factor I
2. Prothrombin Factor II
3. Tissue factor Factor III; tissue thromboplastin
4. Calcium Factor IV
5. Factor V Proaccelerin; labile factor;
6. Factor VII Serum prothrombin conversion accelerator;
7. Factor VIII Antihemophilic factor (AHF);
8. Factor IX Christmas factor;
9. Factor X Stuart-Prower factor
10. Factor XI antihemophilic factor C
11. Factor XII Hageman factor
12. Factor XIII Fibrin-stabilizing factor
13. Prekallikrein Fletcher factor High-molecular-weight Fitzgerald factor; HMWK kininogen
(high-molecularweight) kininogen
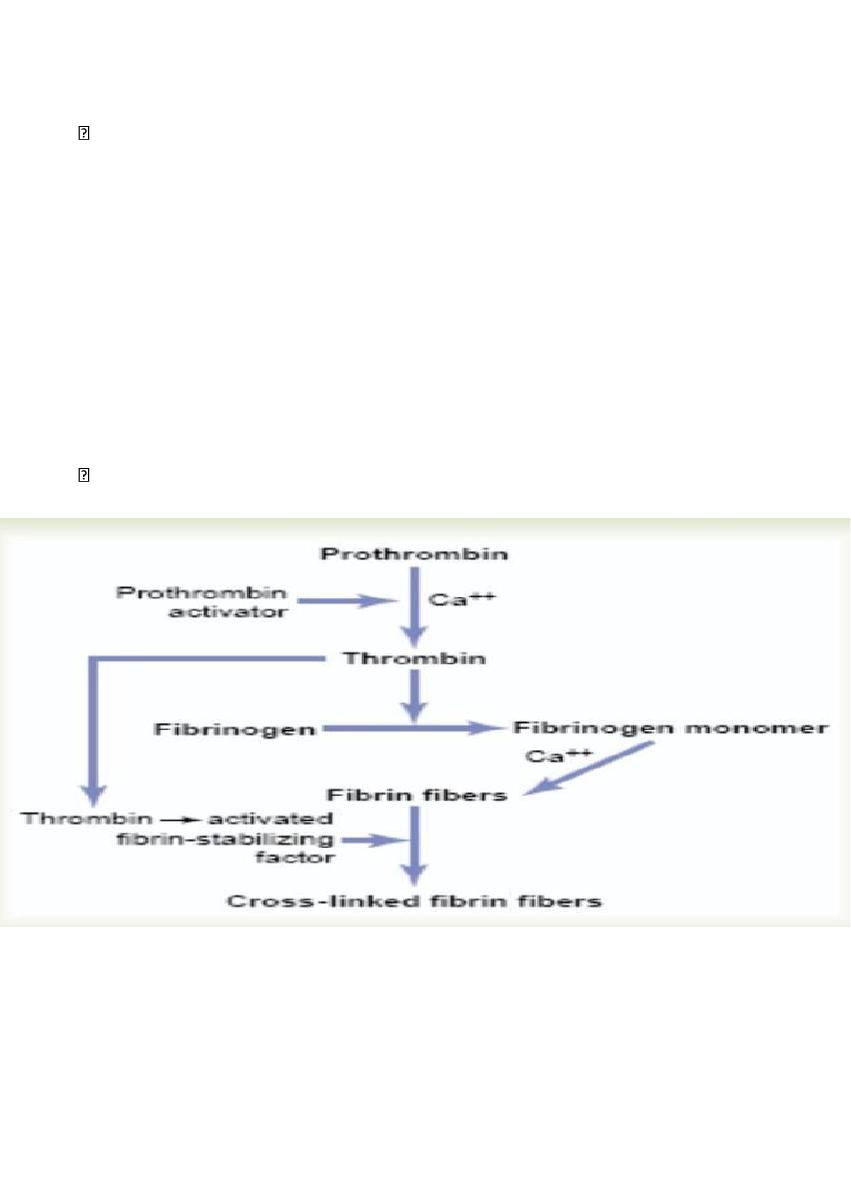
Conversion of Prothrombin to Thrombin
in the presence of sufficient amounts of ionic Ca++, the prothrombin activator, causes
conversion of prothrombin to thrombin
Prothrombin and Thrombin.
Prothrombin is a plasma protein, it’s formed continually by the liver.
Vitamin K is required by the liver for normal formation of prothromb in as well as for
formation of factors X, XI, and VIIa (1972).
Therefore, lack of vitamin K or the presence of liver disease that could lead to bleeding
tendency.
Conversion of Fibrinogen to Fibrin—Formation of the Clot
Fibrinogen is formed in the liver Because of its large molecular size, little fibrinogen
normally leaks from the blood vessels into the interstitial fluids
Action of Thrombin on Fibrinogen to Form Fibrin.
Thrombin is a protein enzyme with weak proteolytic capabilities. It acts on fibrinogen to
form one molecule of fibrin monomer that has the automatic capability to polymerize with
other fibrin monomer molecules to form fibrin fibers that constitute the reticulum of the
blood clot. the fibrin reticulum is greatly strengthened by crosslinking between adjacent
fibrin fibers by a substance called fibrin-stabilizing factor which is activated by thrombin

Blood Clot.
The clot is composed of a meshwork of fibrin fibers running in all directions and entrapping
blood cells, platelets, and plasma. The fibrin fibers also adhere to damaged surfaces of
blood vessels; therefore, the blood clot becomes adherent to any vascular opening and
thereby prevents further blood loss.
Clot Retraction.
Serum. Within a few minutes after a clot is formed, it begins to contract and usually
expresses most of the fluid from the clot. The fluid expressed is called serum in which all its
fibrinogen and most of the other clotting factors have been removed; in this way, serum
differs from plasma.
Platelets are necessary for clot retraction to occur.
platelets entrapped in the clot continue to release procoagulant substances, one of the
most important of which is fibrin-stabilizing factor, which causes more and more cross-
linking bonds between adjacent fibrin fibers.
In addition, the platelets themselves contribute directly to clot contraction by activating
platelet actin, and myosin molecules, which are all contractile proteins in the platelets and
cause strong contraction of the platelet spicules attached to the fibrin. The contraction is
activated and accelerated by thrombin as well as by calcium ions, the edges of the broken
blood vessel are pulled together.
Initiation of Coagulation: Formation of Prothrombin Activator
Prothrombin activator is formed in two ways,
(1) by the extrinsic pathway that begins with trauma to the vascular
wall and surrounding tissues and
(2) by the intrinsic pathway that begins in the blood itself.
In both the extrinsic and the intrinsic pathways, a series of different plasma proteins
called blood clotting factors play major roles. Most of these are inactive forms of
proteolytic enzymes. When converted to the active forms, their enzymatic actions cause
the successive, cascading reactions of the clotting process.
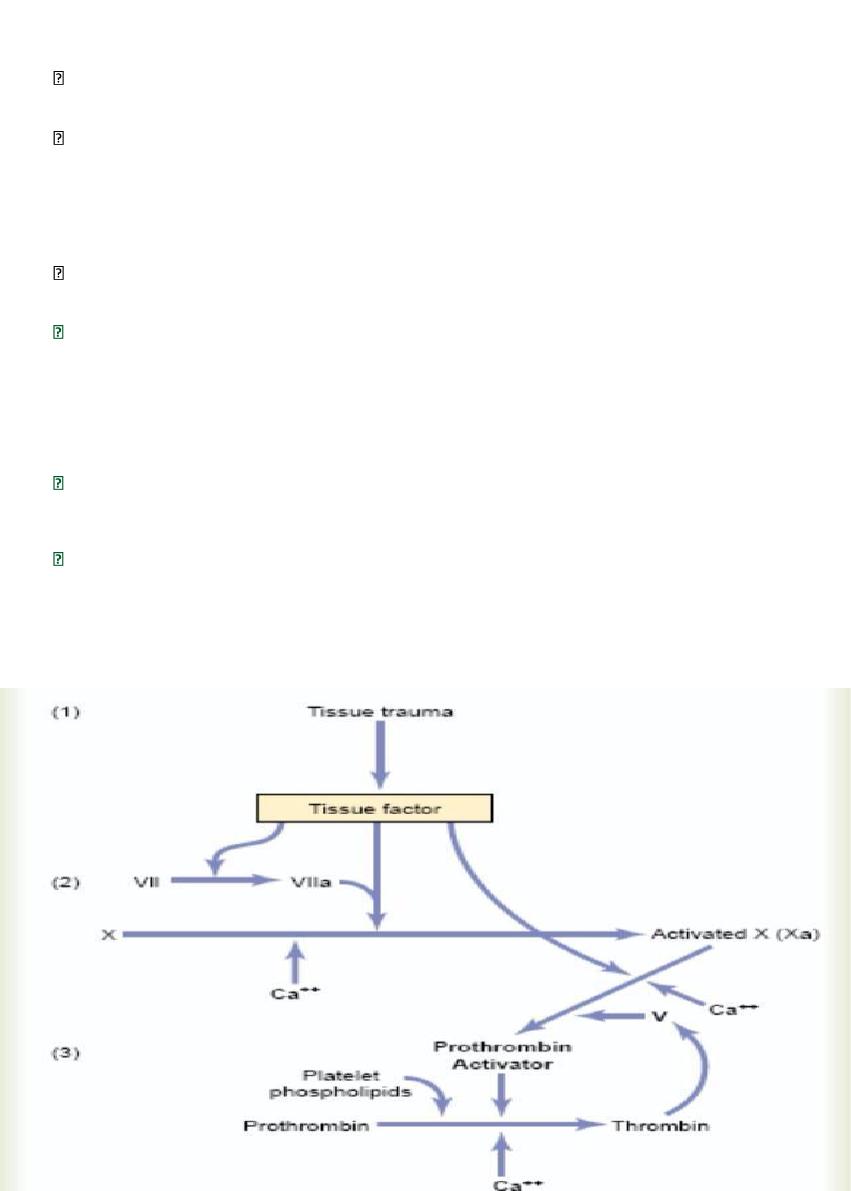
Extrinsic Pathway for Initiating Clotting
The extrinsic pathway for initiating the formation of prothrombin activator begins with a
traumatized vascular wall or traumatized extravascular tissues that come in contact with the
blood.
This leads to the following steps,
1. Release of tissue factor
.
Traumatized tissue releases a complex of several factors called
tissue factor or tissue thromboplastin. This factor is composed especially of phospholipids
from the membranes of the tissue plus a lipoprotein complex that functions mainly as a
proteolytic enzyme.
2. Activation of Factor X—role of Factor VII and tissue factor
in the presence of calcium
ions.
3. Effect of activated Factor X (Xa) to form prothrombin activator—role of Factor V.
in the presence of calcium ions (Ca++), this splits prothrombin to form thrombin, and the
clotting process proceeds. platelet phospholipids act as a vehicle that further accelerates
the process.

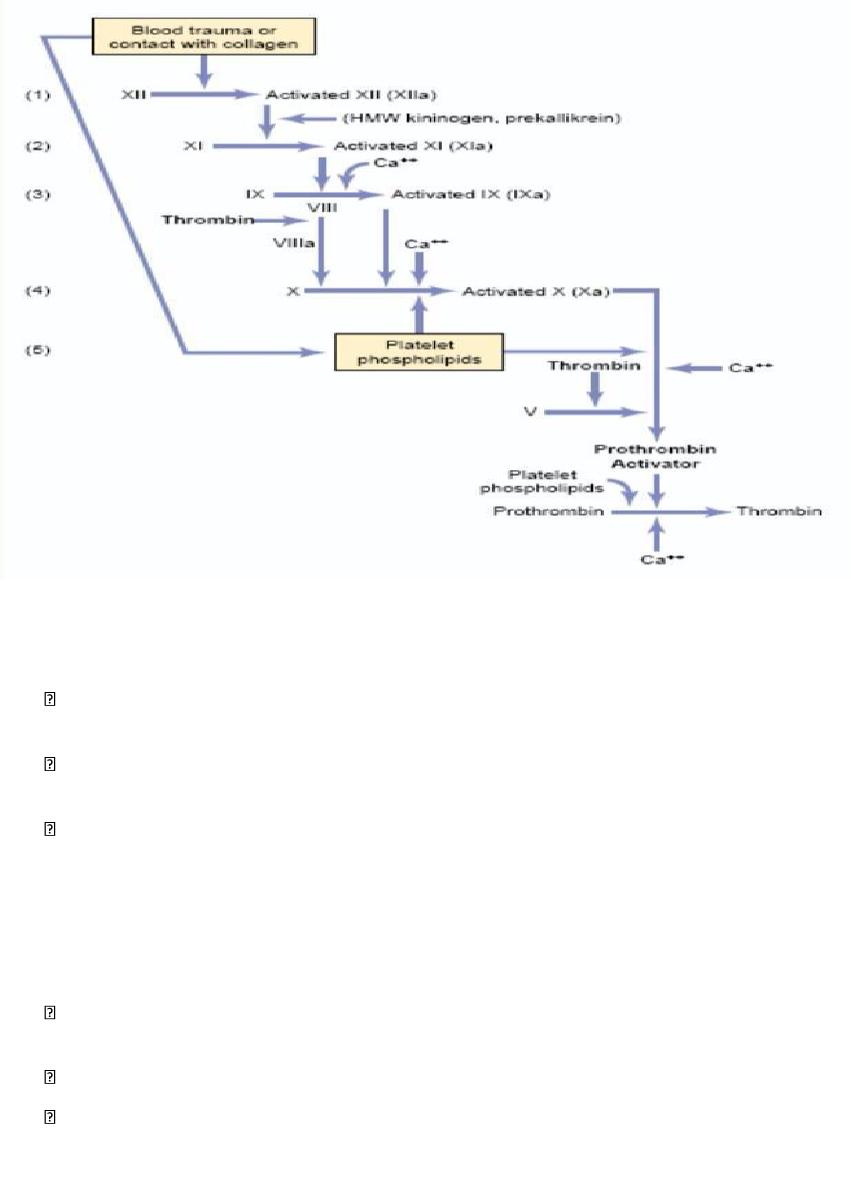
Intrinsic Pathway for Initiating Clotting
The second mechanism for initiating formation of prothrombin activator.
1. Trauma to the blood
or exposure of the blood to vascular wall collagen causes
(1) activation of Factor XII (XIIa) and
(2) release of platelet phospholipids. that contain the lipoprotein called platelet factor 3,
which also plays a role in subsequent clotting reactions.
2. Activation of Factor XI. Activated by the activated Factor XII
This reaction also
requires HMW (high-molecular-weight) kininogen and is accelerated by prekallikrein.
3. Activation of Factor IX by activated Factor XI.
4. Activation of Factor X—role of Factor VIII.
The activated Factor IX, acting in concert with activated Factor VIII and with the
platelet phospholipids and factor 3 from the traumatized platelets, activates Factor X.
Factor VIII is the factor that is missing in a person who has classic hemophilia
5. Action of activated Factor X to form prothrombin activator—role of Factor V
.
This step in the intrinsic pathway is the same as the last step in the extrinsic pathway.
Role of Calcium Ions in the Intrinsic and Extrinsic Pathways
Except for the first two steps in the intrinsic pathway, calcium ions are required for
promotion or acceleration of all the blood-clotting reactions. Therefore, in the absence
of calcium ions, blood clotting by either pathway does not occur.
blood can be prevented from clotting by reducing the calcium ion concentration below
the threshold level for clotting, either by deionizing the calcium by causing it to react
with substances such as citrate ion or by precipitating the calcium with substances such
as oxalate ion.
Prevention of Blood Clotting in the Normal Vascular System—Intravascular
Anticoagulants
Endothelial Surface Factors.
(1) the smoothness of the endothelial cell surface, which prevents contact activation of the
intrinsic clotting system;
(2) a layer of glycocalyx on the endothelium which repels clotting factors and platelets,
thereby preventing activation of clotting; and
(3) a protein bound with the endothelial membrane, thrombomodulin, which binds
thrombin and remove it, the thrombomodulin- thrombin complex also activates a plasma
protein, protein C, that acts as an anticoagulant by inactivating activated Factors V and VIII.
Antithrombin Action of Fibrin and Antithrombin III.
Among the most important anticoagulants in the blood itself are those that remove
thrombin from the blood. The most powerful of these are.
(1) the fibrin fibers (by adsorption of thrombin to it) that themselves are formed during the
process of clotting and
(2) an alpha-globulin called antithrombin III or antithrombin-heparin cofactor.
(3) Heparin. it has little or no anticoagulant properties, but when it combines with
antithrombin III, the effectiveness of antithrombin III for removing thrombin increases by a
hundredfold to a thousandfold.

Lysis of Blood Clots—Plasmin
The plasma proteins contain a euglobulin called plasminogen that, when activated,
becomes a substance called plasmin. Plasmin is a proteolytic enzyme, Plasmin digests fibrin
fibers and some other protein coagulants such as fibrinogen, Factor V, Factor VIII,
prothrombin, and Factor XII.
Activation of Plasminogen to Form Plasmin: Then Lysis of Clots.
When a clot is formed, a large amount of plasminogen is trapped in the clot along with
other plasma proteins. The injured tissues and vascular endothelium very slowly release a
powerful activator called tissueplasminogen activator (t-PA) that a few days later, after the
clot has stopped the bleeding, eventually converts plasminogen to plasmin, which in turn
removes the remaining unnecessary blood clot.
Conditions That Cause Excessive Bleeding in Human Beings
Excessive bleeding can result from deficiency of any one of the many blood-
clotting factors.
e.g. bleeding caused by
(1) vitamin K deficiency,
(2) hemophilia, and
(3) thrombocytopenia (platelet deficiency).
Vitamin K is
necessary for liver formation of five of the important clotting factors: prothrombin, Factor
VII, Factor IX, Factor X, and protein C. In the absence of vitamin K, subsequent insufficiency
of these coagulation factors in the blood can lead to serious bleeding tendencies.
Vitamin K is continually synthesized in the intestinal tract by bacteria. However, in
gastrointestinal disease, vitamin K deficiency often occurs as a result of poor absorption of
fats from the gastrointestinal tract. The reason is that vitamin K is fat-soluble and ordinarily
is absorbed into the blood along with the fats.
One of the most prevalent causes of vitamin K deficiency is failure of the liver to secrete
bile into the gastrointestinal tract (which occurs either as a result of obstruction of the
bile ducts or as a result of liver disease). Lack of bile prevents adequate fat digestion and
absorption and, therefore, depresses vitamin K absorption as well.
Hemophilia
Hemophilia is a bleeding disease that occurs almost exclusively in males. it is caused by an
abnormality or deficiency of Factor VIII; this type of hemophilia is called hemophilia A or
classic hemophilia. In the other 15 per cent of hemophilia patients, the bleeding tendency is
caused by deficiency of Factor IX. Both of these factors are transmitted genetically by way of
the female chromosome.
If one of her X chromosomes is deficient, she will be a hemophilia carrier, transmitting the
disease to half of her male offspring and transmitting the carrier state to half of
her female offspring.
Thrombocytopenia
Thrombocytopenia means the presence of very low numbers of platelets in the circulating
blood. People with thrombocytopenia have a tendency to bleed, as do hemophiliacs, except
that the bleeding is usually from many small venules or capillaries, rather than from larger
vessels as in hemophilia. As a result, small punctate hemorrhages occur throughout all the
body tissues. The skin of such a person displays many small, purplish blotches, giving the
disease the name thrombocytopenic purpura.
Most people with thrombocytopenia have the disease known as idiopathic
thrombocytopenia, it has been discovered that for unknown reasons, specific antibodies
have formed and react against the platelets themselves to destroy them.
Prevention of Blood Coagulation Outside the Body
Although blood removed from the body and held in a glass test tube normally clots in
about 6 minutes, blood collected in siliconized containers often does not clot for 1 hour or
more. The reason for this delay is that preparing the surfaces of the containers with silicone
prevents contact activation of platelets and Factor XII, the two principal factors that initiate
the intrinsic clotting mechanism.
Heparin can be used for preventing coagulation of blood outside the body as well as in the
body.
Various substances that decrease the concentration of calcium ions in the blood can also
be used for preventing blood coagulation outside the body.
For instance, a soluble oxalate compound mixed in a very small quantity with a sample of
blood causes precipitation of calcium oxalate Also The citrate ion combines with calcium
in the blood to cause an un-ionized calcium compound.

Blood Coagulation Tests
Bleeding Time
When a sharp-pointed knife is used to pierce the tip of the finger or lobe of the ear,
bleeding ordinarily lasts for 1 to 6 minutes. Lack of platelets (number or vunctionaly) and
von willebrand factor or abnormalities of collagen or vessel wall can prolong the bleeding
time
Clotting Time
Many methods have been devised for determining blood clotting times. The one most
widely used is to collect blood in a chemically clean glass test tube and then to tip the
tube back and forth about every 30 seconds until the blood has clotted. By this method,
the normal clotting time is 2-5 minutes.
Prothrombin Time
Prothrombin time gives an indication of the concentration of prothrombin in the blood.
Blood removed from the patient is immediately oxalated so that none of the prothrombin
can change into thrombin. Then, a large excess of calcium ion and tissue factor is quickly
mixed with the oxalated blood.
The excess calcium nullifies the effect of the oxalate, and the tissue factor activates the
prothrombin-to thrombin reaction by means of the extrinsic clotting pathway.
The normal prothrombin time is about 12 seconds.
Tests similar to that for prothrombin time have been devised to determine the quantities
of other blood clotting factors.
