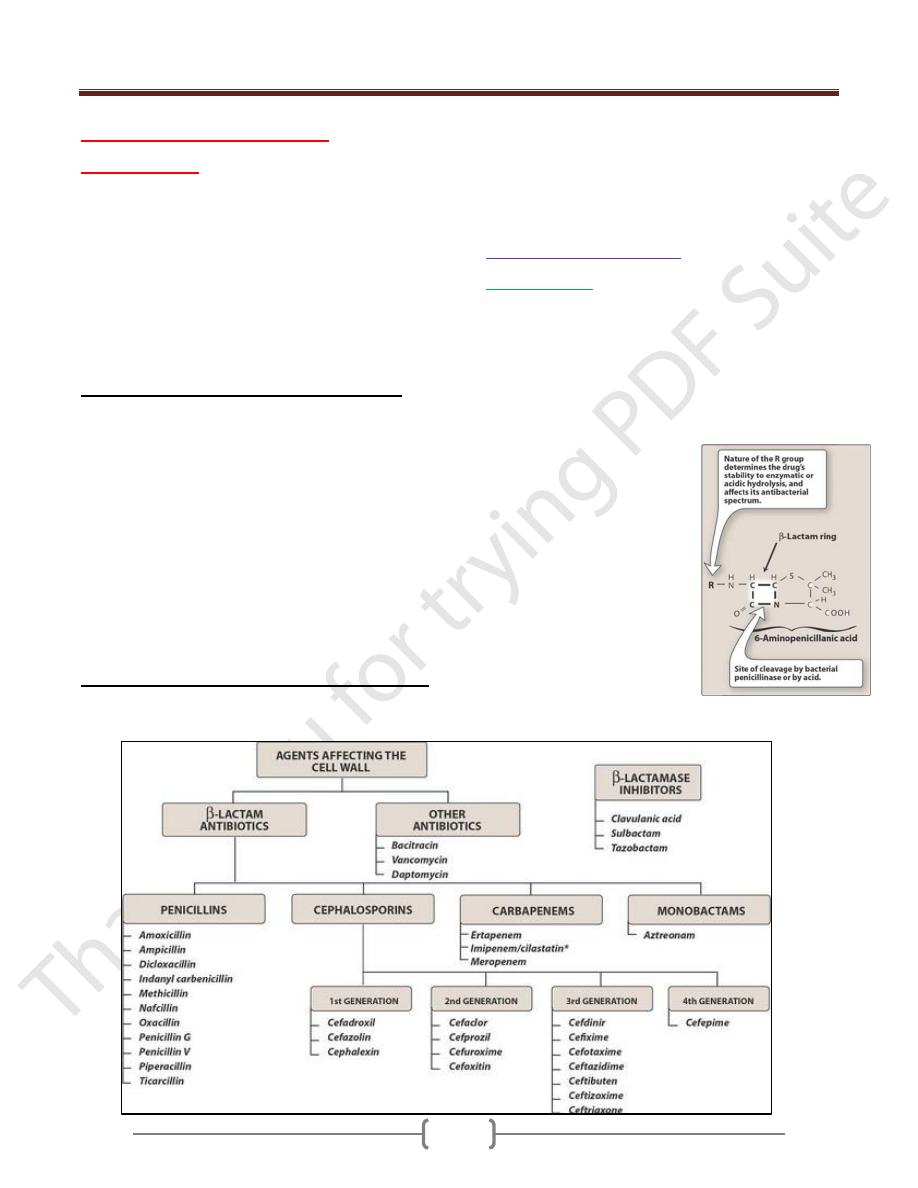
Unit 7: Chemotherapeutic Drugs
197
Lecture 2+3 - Cell Wall
Inhibitors:
Some antimicrobial drugs selectively interfere with the
synthesis of bacteria cell wall. Unique to bacteria, this
structure is not found in mammalian cells. These agents
require actively proliferating microorganism; they have
little or no effect on bacteria that are not growing. The
most important members of the group are the β-lactam
antibiotics
ß-lactam compounds: The name comes from effective
part which is ß-lactam ring
Mechanism of action of ß-lactam compounds:
All ß-lactam compouds have the same bactericidal
mechanism of action. They inhibit the synthesis of cell
wall in bacteria. This occur by binding of the antibiotic
with certain enzymes called penicillin binding proteins
(PBP
s
) that catalyze the final stage in cell wall synthesis.
Cell lysis can then occur, either through osmotic pressure
or through the activation of autolysins. The success of a
penicillin antibiotic in causing cell death is related to the
antibiotics size, charge and hydrophobicity. Penicillins are
only effective against rapidly growing organisms that
synthesize a peptidoglycan cell wall. Consequently, they
are inactive against organism devoid of this structure,
such as mycobacteria, fungi, and viruses.
Mechanism of resistance to ß-lactam compounds:
Bacterial resistance to ß-lactam may occur by one of the
following mechanisms or a combination of these:
1) Inactivation of ß-lactam ring by certain bacteria
possess ß-lactamase or penicillinase enzyme that
inactivate the antibiotic.
2) Alteration or modification of bacterial PBP
s
.
3) Reduce affinity of antibiotic to bind PBP
s
.
The ß-lactam compounds
include:
A. Penicillins:
They are the most widely effective antibiotic and the low
toxic effect. The basic structure of this compound is the 6-
aminopenicillianic acid, members of this group in the R-
side chain. The nature of this side chain affects the
antimicrobial spectrum, the stability to stomach acids and
susceptibility to bacterial degredative enzymes which
degrade ß-lactam ring.
The structural integrity of the 6-
aminopenicillianic acid is
essential for biological activity of
these compounds. If the ß-lactam
ring is enzymatically cleaved by
bacterial ß-lactamase, the
resulting product which is called
penicilloic acid will lack the
antibacterial activity.

Unit 7: Chemotherapeutic Drugs
198
Penicillins are bactericidal antibiotic and are divided
according to their spectrum of activity to:
1) Narrow spectrum natural penicillins
The antibacterial activity of narrow spectrum penicillins
include mainly G+ve bacteria as staphylococci, most
strains of streptococci, meningeococci and anaerobes and
Gve- bacteria as Neisseria, Pasturella.
a) Benzylpenicillin (penicillin G)
It is destroyed by gastric acidity so it is not suitable for
oral uses; it is given by injection, sensitive to ß-
lactamase producing by bacteria.
Short half-life less than 2hrs, penetrates in most tissues.
Elimination of pencillin G is mainly thorough kidneys
by active tubular secretion. This type of elimination can
be inhibited by administration of probencid that result
in longer duration of action.
Typical therapeutic application of penicillin G in fig below
b) Phenoxymethlpenicillin (penicillin V)
Is given orally because of good absorption but food
decrease its bioavailability. Pencillin V achieves lower
serum concentration than penicillin G when high serum
concentration is required.
c) Procaine penicillin
This combination results in longer duration of action (up
24hrs). This preparation is less painful at site of injection.
d) Benzathine penicillin
Its longer-acting penicillin that persist in blood for 4
weeks but in low concentration. It is useful in prophylaxis
of rheumatic fever.
The clinical uses of natural penicillins include
staphylococcal infection; streptococcal pharyngitis and
endocarditis; meningitis; pneumonia; bronchitis; anthrax;
otitis media; sinusitis; clostridium infections and syphilis.
2) Antistaphylococcal penicillins (β-lactamase
resistance drugs)
Certain bacteria tend to produce β-lactamase which opens
the β-lactam ring common to all penicillins and thus
terminate the antibacterial action.
The antistaphylcoccal penicillins drugs resist the action of
β-lactamase by presence of an acyl side chains that
protect the β-lactam by preventing the enzyme getting
access to it.
Drugs of this group:
Cloxacillin:
resists degredation by gastric acid and is
absorbed from the gut but food interferes with absorption.
Flucloxacillin:
is more fully absorbed and so gives higher
blood conc. than dose cloxacillin.
Methicillin:
is rarely used because of causing interstitial
nepheritis. Methicillin -resistance strains of
staphylococcus aureus (MRSA), these m.o also resistance
to cloxacillin and flucloxacillin. These types of infection
are usually susceptible to vancomycin and rarely to
ciprofloxacin or rifampcin.
3) Extended spectrum penicillins
Have an antibacterial spectrum similar to that of penicillin
G, but are more effective against G-ve bacilli. as E.coli,
H.influenza, proteus mirabills and salmonella typhi. They
have less activity than benzylpenicillin against streptococci.
They are β-lactamase sensitive, these drugs are:
a) Ampicillin:
it is acid stable, given orally but food
interferes with its absorption, also it can be given I.M or
I.V. Ampicillin attains high concentration in CSF.
Ampicillin is sensitive to penicillinase- enzyme thus it is
combined with sulbactam to resist inactivation by
bacterial enzymes.

Unit 7: Chemotherapeutic Drugs
199
b) Amoxicillin:
it is an analogue of ampicillin that is better
absorbed from the gut and food does not interfere with its
absorption. It does not achieve adequate concentration in
the CSF to be useful in meningitis. Amoxicillin is
sensitive to β-lactamase enzyme thus it is combined with
clavulanic acid under the trade name Amoxiclave to resist
bacterial degradation. Diarrhea appears to be less frequent
with amoxicillin than ampicillin.
Aminopenicillins: as Ampicillin and Amoxicillin.
4) Antipseudomonal penicillins drugs: are of 2 types
a) Carboxypenicillins:
They have some antimicrobial
activity as penicillin, but additional effect in destroying
pseudomonas aerogenosa and proteus Vulgaris. Include
Carbinecillin
and
Ticarcillin
. These drugs are
inactivated by β-lactamase so it is combined with
clavulanic acid to resist bacterial inactivation.
b) Uridopenicillins:
This group include
piperacillin
and
azlocillin
. Piperacillin is slightly greater efficacy as the
azlocillin but more affective against G-ve microorganism.
Usually given combined with β-lactamase inhibitor
(Tazobactam).
Adverse effects of penicillins
Allergic reaction as skin rashes, bronchospasm and may
cause anaphylactic shock.
Diarrhea: this due to disruption of the normal flora in the
intestine.
Nephritis: all penicillins, but particularly methicillin.
Neurotoxicity: the penicillins are irritant to neuronal
tissue may induce seizures if injected intrathecally or if
very high blood level are reached.
Platelet dysfunction: involves decreased agglutination, is
observed with antipseudomonal penicillins and with some
extent with penicillin G.
Cation toxicity: Usually penicillins administered as Na
or K salts. Toxicities may be caused by the large
quantities of sodium or potassium that accompany the
penicillin. Sodium excess may result in hypokalemia.
This can be avoided by using the most potent antibiotic.
Pharmacokinetics of penicillin:
Administration: the route of administration of a β-lactam
antibiotic is determined by stability of the drug to gastric
acid and by the severity of the infection. Ticarcillin,
carbinicillin, piperacillin, and the combinations of
ampicillin with sulbactam, ticarcillin with clavulinic acid,
and pieracillin with tazobactam must be administered
intravenously or intramuscularly. Penicillin V,
amoxicillin, amoxicillin combined with clavulanic acid
and indanyl carbenicillin (for treatment of urinary tract
infections) are only available as oral preparations. Others
are effective by the oral, IV, IM routes.
Absorption: most of the penicillin are incompletely
absorbed after oral administration, and they reach the
intestine in sufficient amounts to affect the composition of
the intestinal flora. However amoxicillin is almost
completely absorbed.
Consequently, it is not appropriate therapy for the
treatment of shigella- or salmonella-derived enteritis,
because therapeutically effective levels do not reach the
organisms in the intestinal crypts. Absorption of all
penicillinase-resistant penicillins is decreased by food in
the stomach. Therefore, they must be administered thirty
to sixty minutes before meals or two to three hours
postprandially. Other penicillins are less affected by food.
Distribution: Distribution of the β-lactam antibiotics
throughout the body is good. All the penicillins cross the
placental barrier, but none has been shown to be teratogenic.
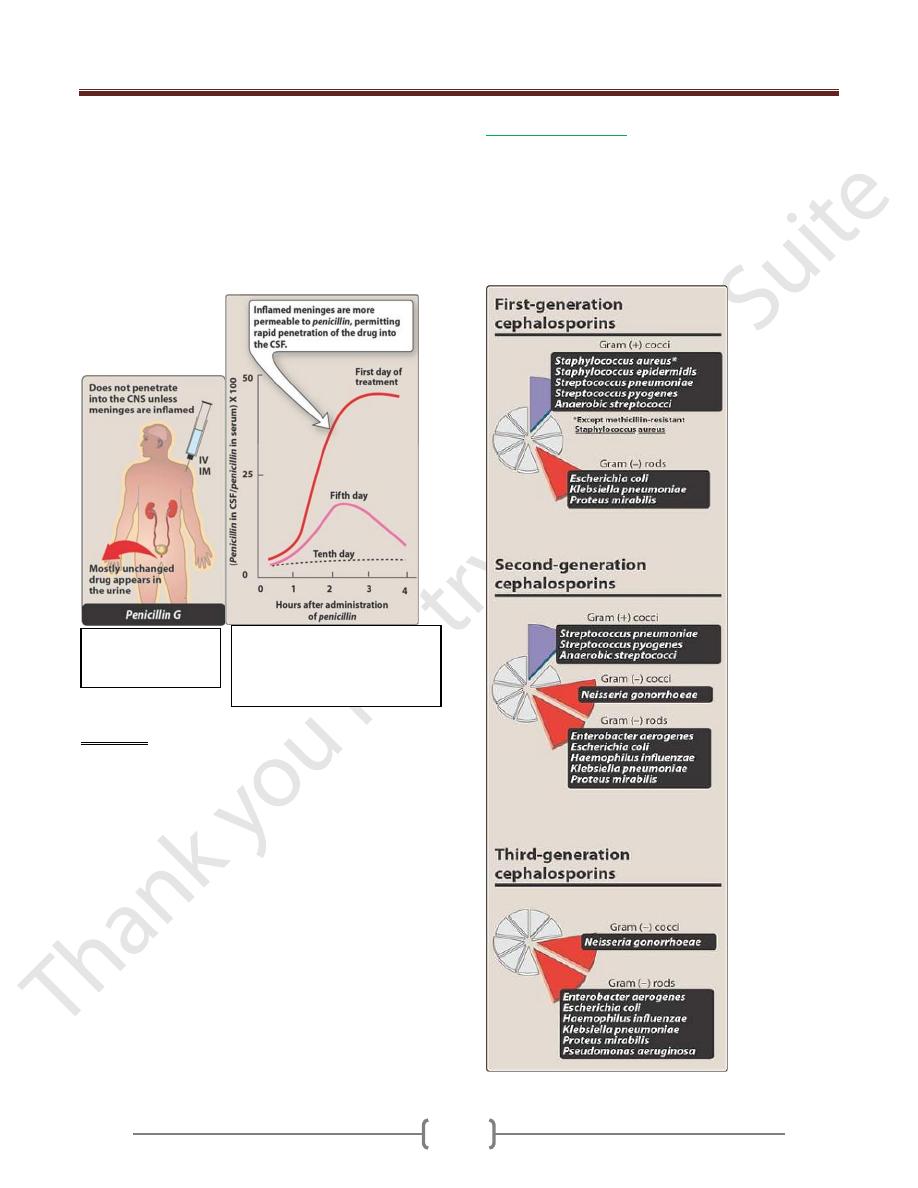
Unit 7: Chemotherapeutic Drugs
200
However, penetration into certain sites, such as bone or
cerebrospinal fluid (CSF), is insufficient for therapy
unless these sites are inflamed. During the acute phase of
infection, the inflamed meninges are more permeable to
the penicillins, resulting in an increased ratio of the
amount of drug in the central nervous system compared to
the amount in the serum. Penicillin levels in the prostate
are insufficient to be effective against infections.
Excretion: The primary route of excretion is through the
organic acid secretory system of the kidney as well as by
glomerular filtration. Patients with impaired renal
function must have dosage regimens adjusted. Thus the
half-life of penicillin G can increase from a normal of 1
hour to 10 hours in individuals with renal failure. The
penicillins are also excreted into breast milk and into
saliva.
B. Cephalosporins
They are β-lactam antibiotics that are closely related to
penicillin in chemical structure, mechanism of action,
toxicity and function. Cephalosporins are classified into
generation according to improvement in their spectrum of
activity, potency and resistance to β-lactamase. They are
ineffective against methicillin-resistant Staphylococcus
aureus (MRSA).
Figure 31.7
Administration and
fate of penicillin.
Figure 31.8 Enhanced
penetration of penicillin into
the cerebral spinal fluid (CSF)
during inflammation.

Unit 7: Chemotherapeutic Drugs
201
1. First generation:
They are resistant to the staphylococcal penicillinase, the
more useful drugs
Cephalexin:
prototype of first generation oral
cephalosporins. Oral administration four times daily.
Cefazolin:
It is a first-generation parenteral cephalosporin has a
longer duration of action and a similar spectrum of action
compared to other first-generation drugs. Good
penetration into bone.
Other drugs:
cephadroxil, cephalothin
and
cephradine
.
Uses
Are useful in prophylaxis before surgery because most
postoperative infection are caused by G+ve bacteria as
staphylococci; treatment of skin, bone, wound, urinary
tract and respiratory tract infection. First generation drugs
are not used in meningitis because they do not enter CSF.
2. Second generation:
Active against m.o of first generation activity, but have
extended G-ve effect. Less activity on G+ve than first
generation.
Cefuroxime:
it is prototype second-generation parenteral
cephalosporin has a longer half-life than similar agents. It
crosses the blood brain barrier but less activity than first
generation.
Other drugs of this group
cefaclor, cephamandole, , ceforanide , cefonicid,
cefoxitin ,cefotetan
Uses
Second-generation drugs are more resistant to β-lactamase
inactivation with broad spectrum activity that include
G+ve cocci, G-ve and anaerobes as bacteroides (including
β-fragilis ) and closteridum. They are used in sinusitis,
otitis media, pneumonia, peritonitis and abscess like
diabetic foot ulcer.
3. Third generation:
These agents have greatly inferior to first generation
cephalosporins in regard to their activity G+ve cocci also
they have enhanced activity against G-ve bacilli.
Ceftriaxone:
longest half-life of any cephalosporin(6-
8hrs) permit once a day dosing. High levels of drug can
be achieved in blood and CSF. Effective against genital,
anal and pharyngeal penicillin-resistant Neisseria
gonorrhoeae. Drug excreted in bile and may be used in
patients with renal insufficiency. Good penetration into
bone.
Other drugs of this group:
Cefotaxime
(good penetration into CSF),
cefixime
(oral
dosing once daily),
ceftazidime
(active against
pseudomoneas aeruginosa),
ceftizoxime
(has broad effect
on G-ve and anaerobes particularly β.fragilis.
4. Fourth-generation:
Cefepime:
Is the most clinically useful fourth generation
agent. It has a wide antibacterial spectrum; it passes well
to the CNS.
Uses
The infections of G-ve organisms especially those caused
resistance to third generation drugs. Members of this
generation are resistant to the action of bacterial β-
lactamase.
Side effects
The cephalosporins produce a number of adverse effects,
some of which are unique to particular members of the
group.
1) Hyper sensitivity reactions: cephalosporins may produce
several types of hypersensitivity reaction similar to
penicillins. There is cross-allergy between cephalosporins
and penicillins and about 5-10% of patients who have
allergy to penicillins have allergy to cephalosporins too.
Allergic reactions include urticaria and rashes.
2) Local irritation: cephalosporins produce sever pain after
I.M inj so local anesthetic agent may be added.
Cephalosporins may produce thrombophlebitis when
given i.v.
3) A disulfiram like effect (hypotension, sweating & fainting).
When cefamandole is ingested with alcohol or alcohol-
containing medication, a disulfiram-like effect is seen
because the cephalosporins block the second step in alcohol
oxidation, which results in the accumulation of acetaldehyde.
4) Bleeding: bleeding can occur with cefamandole or
cefotetan because of anti-vitamin k effects; administration
of the vitamin corrects the problem.
5) Overgrowth of resistant strains of m.o. may produce
organism resistant to all β-lactam antibiotics.
6) Mild and transient nausea, vomiting and diarrhea occur
with the orally administered cephalosporins.
C. Monobactams:
Monobactams are resistant to β-lactamase, but they act
only on G-ve bacteria (aerobic). It lacks activity against
G+ve organism and anaerobes. The best drug of this
group
Azetronam
. It is administered via IV or IM routes
and it is mainly eliminated through kidney and can

Unit 7: Chemotherapeutic Drugs
202
accumulate in patients with renal failure. It is a safe
alternative for treating patients allergic to penicillins
and/or cephalosporins.
D. Carbapenems:
Are synthetic β-lactam antibiotics structurally similar to
penicillins.
Imipenem:
it is the most broad spectrum β-lactam
antibiotics. It is effective against G-ve, G+ve aerobic
bacteria and anaerobic m.o. imipenem resists hydrolysis
by most β-lactamases. It is active against penicillinase-
producing G+ve and G-ve organisms, anaerobes and
pseudomonas aeruginosa, although other pseudomonas
strains are resistant.
It is administered by i.v route and penetrates well into
body tissues and fluids including CSF when the meninges
are inflamed. It is excreted by glomerular filtration and
undergoes cleavage by a dehydropeptidase found in the
brush border of the proximal renal tubule to form an
inactive metabolite that is potentially nephrotoxic.
Compounding the imipenem with cilastatin, a
dehydropeptidase inhibitor, protects the parent drug from
cleavage and thus prevents the formation of a toxic
metabolite. This allows the drug to be active in the
treatment of urinary tract infections.
β-Lactamas inhibitors:
Hydrolysis of the β-lactam ring either by enzymatic
cleavage via a β-Lactamase or by acid, destroys
antimicrobial activity. β-Lactamase inhibitors such as:
Clavulanic acid, Sulbactam
and
Tazobactam
contain a
β-lactam ring, but they do not have significant
antibacterial activity. Instead, they bind to and inactivate
β-Lactamases, their by
protecting the antibiotics
that are normally
substrates for these
enzymes. The β-
Lactamase inhibitors are
formulated with penicillin
derivatives to protect the
latter from enzymatic
inactivation.
Growth of Escherichia coli
in presence of amoxicillin,
with and without of
Clavulanic acid
Vancomycin
Its effectiveness against multiple drug resistance
organisms such as methicillin-resistant staphylococci
(MRSA) and enterococci. It is not a β-lactam drug.
Mechanism of action
Vancomycin inhibits synthesis of bacterial cell wall
phospholipids as well as peptidoglycan polymerization by
binding to the D-Ala-D-Ala side chain of the precursor
pentapeptide. This prevents the transglycosylation step in
peptidoglycan polymerization, thus weakening the cell
wall and damaging the underlying cell.
Antibacterial spectrum: For infections caused by β-
lactmase producing organisms and for patients with G+ve
infection who have a serious allergy to penecillins.
Vancomycin acts synergistically with aminoglycosides
and this combination can be used in the treatment of
enterococcal endocardities.
Antimicrobial spectrum of vancomycin
Side effects: Ototoxicity which may result in deafness,
fever and chills, maculopapular skin rashes in the face and
chest (red man syndrome).
