
Unit 2: Bacteriology
88
Lecture 4 - Aerobic Spore-Former
Bacteria (Bacillus)
Bacillus anthracis
Introduction
The anthrax bacillus, Bacillus anthracis, was the first
bacterium shown to be the cause of a disease. In
1877, Robert Koch grew the organism in pure culture,
demonstrated its ability to form endospores, and
produced experimental anthrax by injecting it into
animals. Anthrax occurs primarily in animals, especially
herbivores. The pathogens are ingested with feed and
cause a severe clinical sepsis that is often lethal.
Morphology and culturing
Bacillus anthrac is very large, Gram-positive,
sporeforming rod, 1 - 1.2µm in width x 3 - 5µm in length,
with a capsule made of a glutamic acid polypeptide. The
bacterium can be cultivated in ordinary nutrient medium
under aerobic (or anaerobic) conditions. Genotypically
and phenotypically it is very similar to Bacillus
cereus, which is found in soil habitats around the world,
and to Bacillus thuringiensis, the pathogen for larvae
of Lepidoptera. The three species have the same cellular
size and morphology and form oval spores located
centrally in a nonswollen sporangium.The bacterium is
readily grown in an aerobic milieu.
Bacillus anthracis. Gram stain. 1500X. The cells have
characteristic squared ends. The endospores are ellipsoidal
shaped and located centrally in the sporangium. The spores
are highly refractile to light and resistant to staining.
Pathogenesis and clinical picture
The pathogenicity of B. anthracis results from its
antiphagocytic capsule as well as from a toxin that causes
edemas and tissue necrosis. Human infections are
contracted from diseased animals or contaminated animal
products. Anthrax is recognized as an occupational
disease. Dermal, primary inhalational, and intestinal
anthrax are differentiated based on the pathogen’s portal
of entry. In dermal anthrax, which accounts for 90–95%
of human B. anthracis infections) the pathogens enter
through injuries in the skin. A local infection focus
similar to a carbuncle develops within two to three days.
A sepsis with a foudroyant (highly acute) course may then
develop from this primary focus. Inhalational anthrax
(bioterrorist anthrax), with its unfavorable prognosis,
results from inhalation of dust containing the pathogen.
Ingestion of contaminated foods can result in intestinal
anthrax with vomiting and bloody diarrheas.
Anthrax
Anthrax is primarily a disease of domesticated and wild
animals, particularly herbivorous animals, such as cattle,
sheep, horses, mules and goats. Humans become infected
incidentally when brought into contact with diseased
animals, which includes their flesh, bones, hides, hair and
excrement. The natural history of Bacillus anthracis is
obscure. Although the spores have been found naturally in
soil samples from around the world, the organisms cannot
be regularly cultivated from soils where there is an
absence of endemic anthrax. In the United States, the
incidence of naturally-acquired anthrax is extremely rare
(1-2 cases of cutaneous disease per year). Worldwide, the
incidence is unknown, although B. anthracis is present in
most of the world. Unreliable reporting makes it difficult
to estimate the true incidence of human anthrax
worldwide.
The most common form of the disease in humans
is cutaneous anthrax, which is usually acquired via
injured skin or mucous membranes. A minor scratch or
abrasion, usually on an exposed area of the face or neck
or arms, is inoculated by spores from the soil or a
contaminated animal or carcass.The spores germinate,
vegetative cells multiply, and a characteristic gelatinous
edema develops at the site. This develops into
papule within 12-36 hours after infection. The papule
changes rapidly to a vesicle, then a pustule (malignant
pustule), and finally into a necrotic ulcer from which
infection may disseminate, giving rise to septicemia.
Lymphatic swelling also occurs within seven days. In
severe cases, where the blood stream is eventually
invaded, the disease is frequently fatal.
Another form of the disease, inhalation
anthrax (woolsorters' disease), results most commonly
from inhalation of spore-containing dust where animal
hair or hides are being handled. The disease begins
abruptly with high fever and chest pain. It progresses
rapidly to a systemic hemorrhagic pathology and is often
Unit 2: Bacteriology
89
fatal if treatment cannot stop the invasive aspect of the
infection.
Gastrointestinal anthrax is analogous to cutaneous
anthrax but occurs on the intestinal mucosa. As in
cutaneous anthrax, the organisms probably invade the
mucosa through a preexisting lesion. The bacteria spread
from the mucosal lesion to the lymphatic system.
Intestinal anthrax results from the ingestion of
poorly cooked meat from infected animals.
Gastrointestinal anthrax is rare but may occur as
explosive outbreaks associated with ingestion of infected
animals. Intestinal anthrax has an extremely high
mortality rate.
Meningitis due to B. anthracis is a very rare complication
that may result from a primary infection elsewhere.
Pathogenicity of Bacillus anthracis
Bacillus anthracis clearly owes its pathogenicity to two
major-determinants of virulence: the formation of a poly-
D-glutamyl capsule, which mediates the invasive stage
of the infection, and the production of the
multicomponent anthrax toxin which mediates the
toxigenic stage.
Bacillus anthracis forms a single
antigenic type of capsule consisting of a poly-D-
glutamate polypeptide. All virulent strains of B.
anthracis form this capsule.
The poly-D-glutamyl capsule
is itself nontoxic, but functions to protect the organism
against complement and the bactericidal components of
serum and phagocytes, and against phagocytic engulfment
and destruction. Production of capsular material is
associated with the formation of a characteristic mucoid
or "smooth" colony type. "Smooth" (S) to "rough" (R)
colonial variants occur, which is correlated with ability to
produce the capsule. R variants are relatively avirulent
.
One component of the anthrax toxin has a lethal mode
of the action . Death is apparently due to oxygen
depletion, secondary shock, increased vascular
permeability, respiratory failure and cardiac failure. Death
from anthrax in humans or animals frequently occurs
suddenly and unexpectedly. The level of the lethal toxin
in the circulation increases rapidly quite late in the
disease, and it closely parallels the concentration of
organisms in the blood.
Production of the anthrax toxin is mediated by a
temperature-sensitive plasmid, pX01, of 110
megadaltons. The toxin consists of three distinct antigenic
components. Each component of the toxin is a
thermolabile protein with a mw of approximately 80kDa.
I. Factor I is the edema factor (EF) which is necessary for
the edema producing activity of the toxin. EF is known to
be an inherent adenylate cyclase, similar to
the Bordetella pertussis adenylate cyclase toxin.
II. Factor II is the protective antigen (PA), because it
induces protective antitoxic antibodies in guinea pigs. PA
is the binding (B) domain of the anthrax toxin which has
two active (A) domains, EF (above) and LF (below).
III. Factor III is known as the lethal factor (LF) because it
is essential for the lethal effects of the anthrax toxin.
Apart from their antigenicity, each of the three factors
exhibits no significant biological activity in an animal.
However, combinations of two or three of the toxin
components yield the following results in experimental
animals.
PA+LF combine to produce lethal activity
EF+PA produce edema
EF+LF is inactive
PA+LF+EF produces edema and necrosis and is lethal
Diagnosis.
The diagnostic procedure involves detection of the
pathogen in dermal lesions, sputum, and/or blood
cultures using microscopic and culturing methods.
Several nonselective and selective media for the detection
and isolation of Bacillus anthracis have been described,
as well as a rapid screening test for the bacterium based
on the morphology of microcolonies . The capsular
material can be detected by the McFadyean reaction
which involves staining with polychrome methylene
blue. Blue rods in a background of purple/pink-stained
capsular material is a positive test. Neither B.
cereus nor B. thuringiensis synthesizes this capsular
polymer, so the detection of capsular material can be used
to distinguish B. anthracis from its closest relatives .The
Table-1 bellow provides the differential characteristics
that are used to distinguish Bacillus anthracis from most
strains of Bacillus
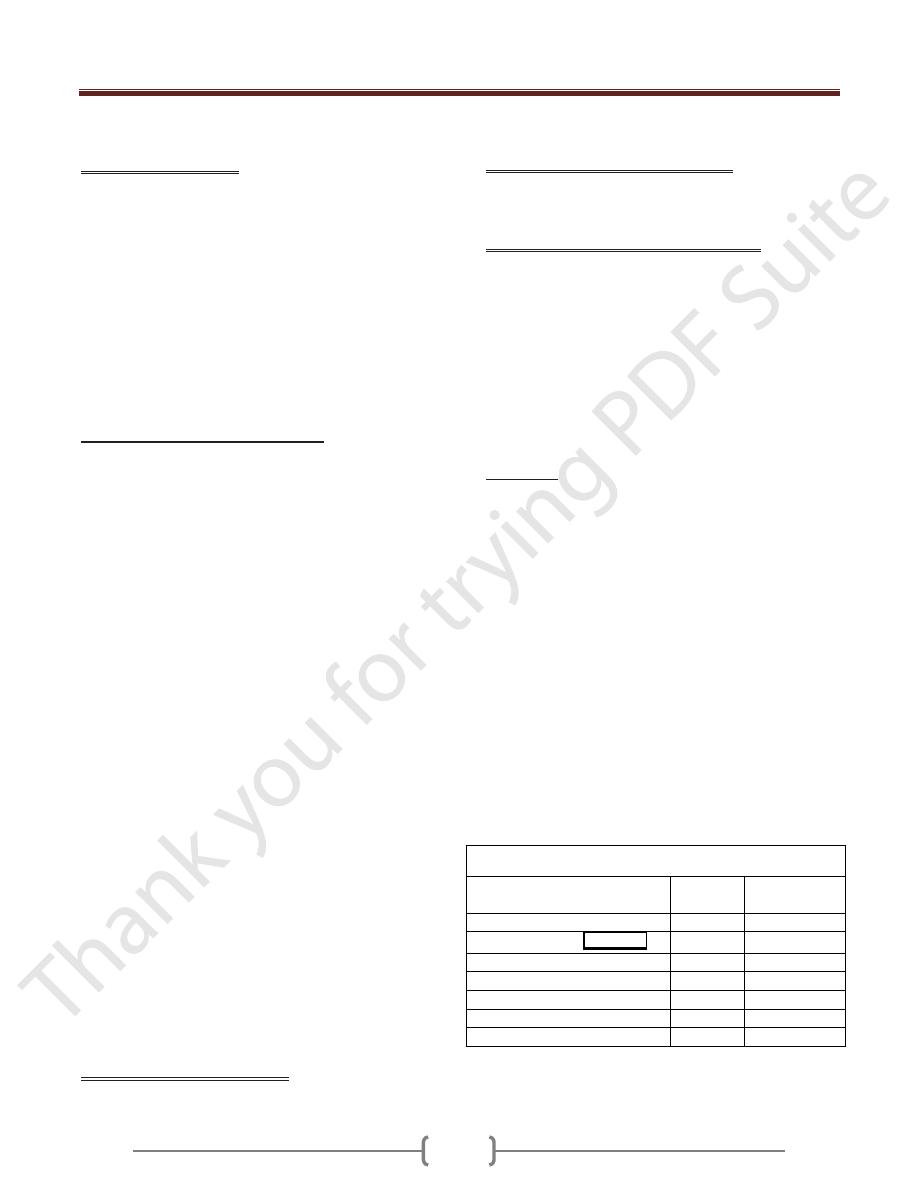
The Table-1 show Differential Characteristics of B. anthracis
B. cereus and B. thuringiensis
Characteristic
B.
anthracis
B.cereus & B.
thuringiensis
growth requirement for thiamin
+
-
hemolysis on sheep blood agar
-
+
glutamyl-polypeptide capsule
+
-
lysis by gamma phage
+
-
Motility
-
+
growth on chloral hydrate agar
-
+
string-of-pearls test
+
-
Unit 2: Bacteriology
90
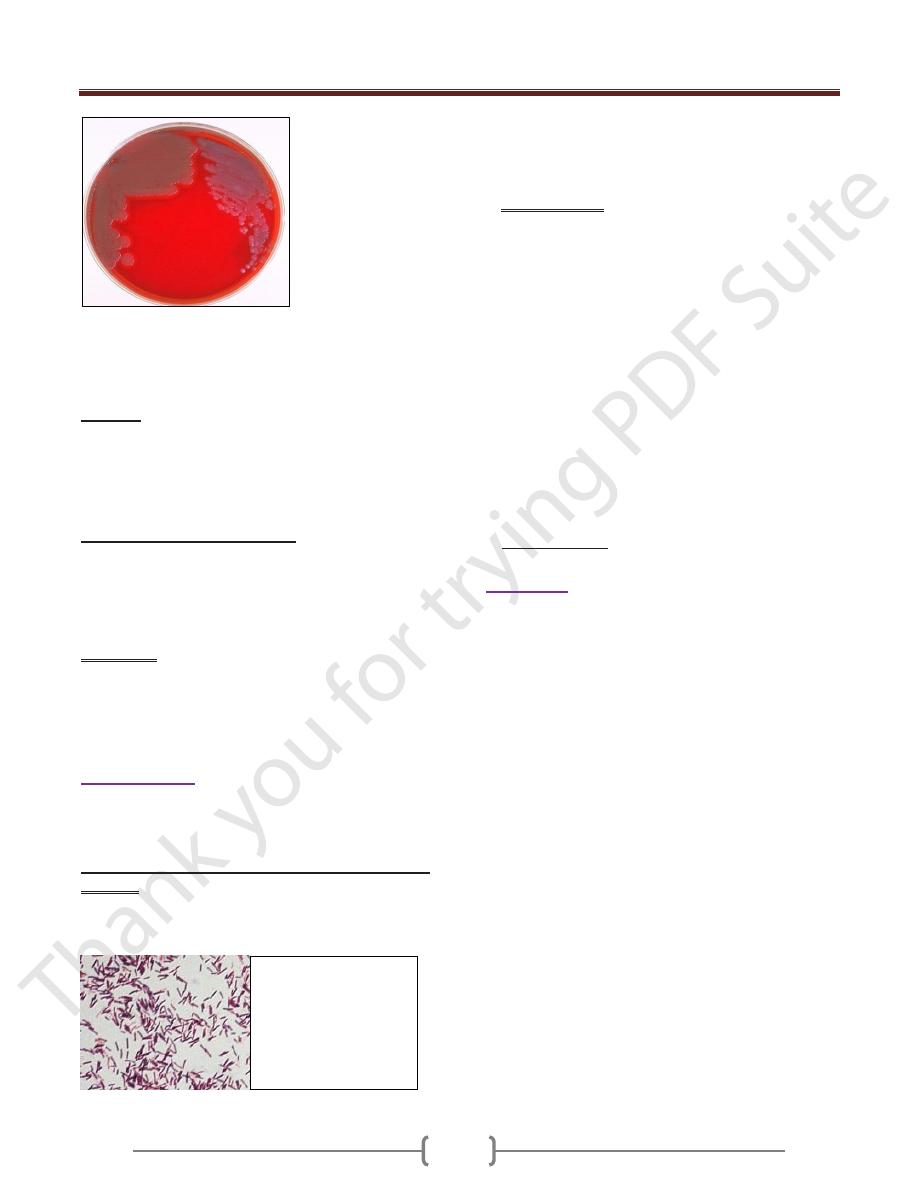
Figure 1. Colonies of Bacillus cereus on the left; colonies
of Bacillus anthracis on the right. B. cereus colonies are
larger, more mucoid, and this strain exhibits a slight zone of
hemolysis on blood agar.
Therapy
The antimicrobial agent of choice is penicillin G.
Doxycycline (a tetracycline) or ciprofloxacin (a
fluoroquinolone) are possible alternatives. Surgery is
contraindicated in cases of dermal anthrax.
Epidemiology and prophylaxis
Anthrax occurs mainly in southern Europe and South
America, where economic damage due to farm animal
infections is considerable. Humans catch the disease from
infected animals or contaminated animal products.
Anthrax is a classic zoonosis.
Prophylaxis involves mainly exposure prevention
measures such as avoiding contact with diseased animals
and disinfection of contaminated products. A cell-free
vaccine obtained from a culture filtrate can be used for
vaccine prophylaxis in high-risk persons.
Bacillus cereus
Bacillus cereus has been recognized as an agent of food
poisoning since 1955. It is not a reportable disease, and
usually goes undiagnosed.
B. cereus causes two types of food-borne illnesses.
One type is characterized by nausea and vomiting and
abdominal cramps and has an incubation period of 1 to 6
hours. It resembles Staphylococcus aureus
(staph) food poisoning in its symptoms and incubation
period. This is the "short-incubation" or emetic form of
the disease.
The second type is manifested primarily by abdominal
cramps and diarrhea following an incubation period of 8
to 16 hours. Diarrhea may be a small volume or profuse
and watery. This type is referred to as the "long-
incubation" or diarrheal form of the disease, and it
resembles food poisoning caused by Clostridium
perfringens. In either type, the illness usually lasts less
than 24 hours after onset. In a few patients symptoms
may last longer. The short-incubation form is caused by a
preformed, heat-stable emetic toxin, ETE. The
mechanism and site of action of this toxin are unknown,
although the small molecule forms ion channels and holes
in membranes. The long-incubation form of illness is
mediated by the heat-labile diarrheagenic enterotoxin
Nhe and/or hemolytic enterotoxin HBL, which cause
intestinal fluid secretion, probably by several
mechanisms, including pore formation and activation
of adenylate cyclase enzymes.
Summary:
The natural habitat of Bacillus anthracis, a Gram-positive,
sporing, obligate aerobic rod bacterium, is the soil. The
organism causes anthrax infections in animals. Human
infections result from contact with sick animals or animal
products contaminated with the spores. Infections are
classified according to the portal of entry as dermal
anthrax (95% of cases), primary inhalational anthrax, and
intestinal anthrax. Sepsis can develop from the primary
infection focus. Laboratory diagnosis includes
microscopic and cultural detection of the pathogen in
relevant materials and blood cultures. The therapeutic
agent of choice is penicillin G. The genera Bacillus and
Clostridium belong to the Bacillaceae family of sporing
bacteria. There are numerous species in the genus Bacillus
(e.g., B. cereus, B. subtilis, etc.) that normally live in the
soil. The organism in the group that is of veterinary and
human medical interest is Bacillus anthracis.
Bacillus cereus. Gram
stain. 450X. Bacilli are
large bacteria, so that
they are readily
observed with the
microscope's "high dry
objective".
