
Unit 4: Virology
211
Lecture 5+6+ Half 7 - RNA
Enveloped viruses
Orthomyxoviruses
Influenza viruses
Influenza viruses are the only members of the
orthomyxovirus family .The orthomyxovirus has a
segmented RNA genome and the replication occurs in the
nucleus of infected cell. .
The term Myxo refers to the observation that these viruses
interact with mucins (glycoprotein on the cell surface).
Important properties
Three immunologic types of influenza viruses are known,
A, B, and C.
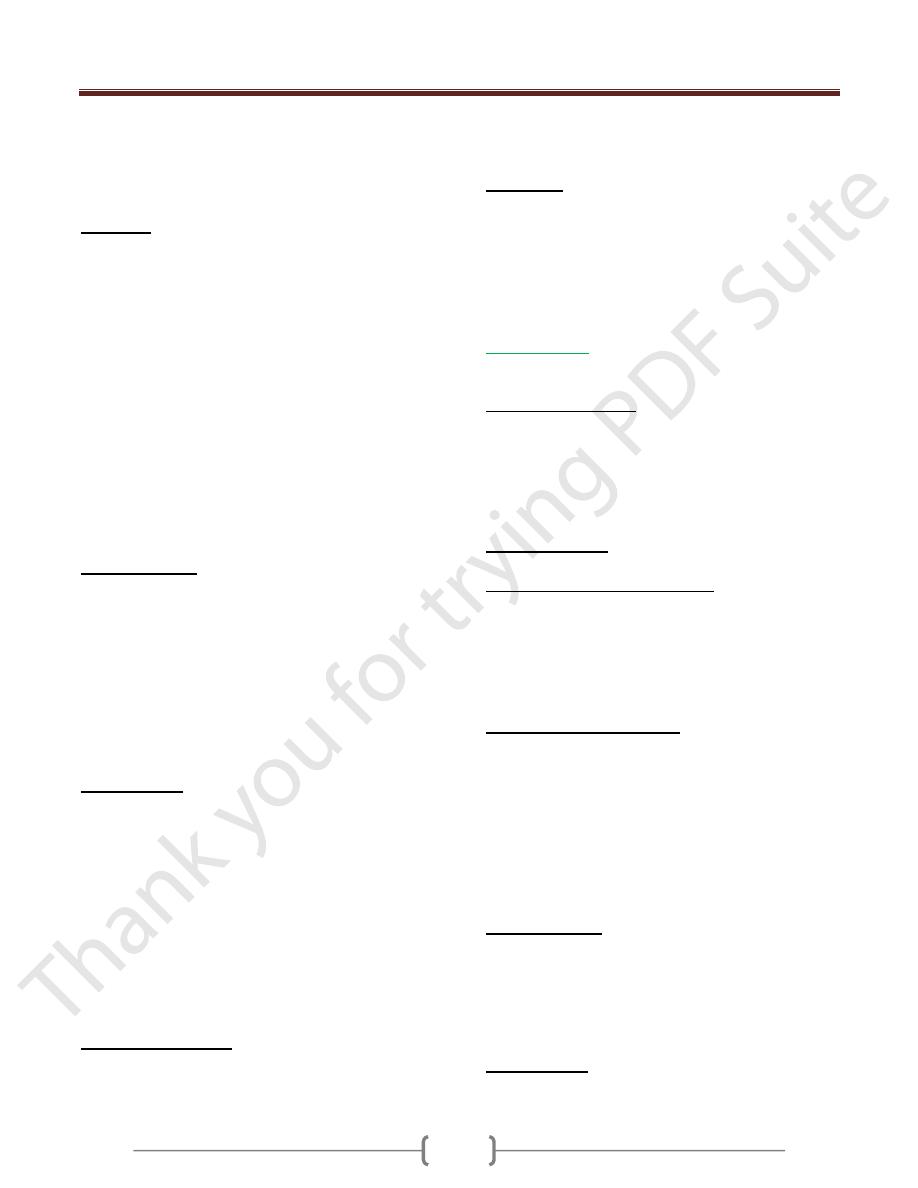
Influenza virus particles contain nine different structural
proteins.
The nucleoprotein (NP) associate with the viral RNA,
which is SS, segmented with negative polarity, to form a
ribonucleoprotein (RNP) structure with helical symmetry.
Three large proteins (PB1, PB2, and PA) are bound to
the viral RNP and are responsible for RNA transcription
and replication.
The Matrix (M1) protein is a major compoinent of the
virion.
The outer lipoprotein envelope is covered with two
different spikes, a haemagglutinin (HA) and a
neuraminidase (NA).
The function of the hemagglutinin is to bind to the cell
surface receptor (sialic acid) to initiate infection. In the
laboratory, the Hemaglutinin agglutinate red blood cells,
which is the basis of diagnostic test called the
haemagglutinin inhibition test. The hemagglutinin is also
the target of neutralizing antibody.
HA is a trimer , composed of 3 intertwined HA1 and HA2
dimmers. Cellular protease cleaves HA into HA1 HA2 ,
this cleavage is important for virus infectivity.
The neuraminidase cleaves neuraminic acid ( sialic acid to
release progeny virus from the infected cell. The
hemagglutinins function at the beginning of the infection
whole the neuraminidase at at the end. Neuraminidase
also degrades the protective layer of mucus in the
respiratory tract. NA is tetramer, composed of four
identical monomers.
Influenza viruses have both groups –specific and type-
specific antigens
1) The internal RNP is the group specific antigen that
distinguishes influenza A, B, and C viruses.
2) The hemagglutinin and the neuraminidase are the type-
specific antigens located on the surface. Antibody against
the hemagglutinin neutralize the infectivity, whereas
antibody against the group-specific antigen doesn’t.
Antibody against the NA doesn’t neutralize infectivity but
does reduce disease by decreasing the amount of virus
released from the infected cell and thus reducing spread.
Classification and Nomenclature:
1) Genus influenzavirus A contains human and animal strain
of influenza type A.
2) Genus influenza virus B contains human strain of
influenza type B.
3) Genus influenzavirus C contains human and swine strains
of influenza type C.
The standard nomenclature system for influenza virus
isolates includes the following information: Type, host of
origin, geographic origin, strain no, year of isolation. The
host origin is not indicated for human isolates, e.g.
A/Hong Kong/03/68(H3N2).
There are 15 subtypes of HA; only 4 have been
transferred to humans (H1-H3, H5). Na has nine subtypes,
only 2 affect humans (N1, N2).
Antigenic shift and antigenic drift
Antigenic shift: it is major changes based on
reassortement
*
(between human and animal influenza) of
segments of the genome RNA result in the appearance of
a new subtype. Influenza B and C viruses don’t exhibit
antigenic shift because few related viruses exist in
animals. Antigenic shift is responsible for an epidemic.
Antigenic shift variants appear every 10-40 years.
Antigenic drift: It is a minor change based on mutations
in the genome of RNA (accumulation of point mutation in
the gene, resulting in amino acid changes in the protein).It
occurs in all types of influenza virus.Antigernic drift
variants appear every year.
*
Reassortement: viruses with segmented genome
exchange segments. 0p-
Replicative cycle
1) The virus adsorb to the cell when the viral hemagglutinin
interacts with sialic acid receptors on the cell surface.
2) The virus enters the cell and uncoating .
3) In the nucleus, the viral RNA polymerase transcribes the
eight genome segments into eight mRNA.
4) Most of the mRNA move to the cytoplasm , where they
are translated into viral proteins. Some of the viral mRNA
remains in the nucleus, where they serve as the template

Unit 4: Virology
212
for the synthesis of the –ve strand RNA genomes for the
progeny virions.
5) The newly synthesized NP and matrix protein binds to
progeny RNA genome in the nucleus and that complex is
transported to the cytoplasm.
6) After assembly, the virion is released by budding from the
outer cell membrane at the site where HA and NA are
located.
7) The neuraminidase acts to release the virus by cleaving
neuraminic acid on the cell surface at the site of the
budding progeny virions.
Transmission:
1) Virus is transmitted by airborne respiratory droplets.
2) Influenza occurs most primarily in the winter months.
Pathogenesis
1) After inhalation of the virus, the NA degrades the
protective mucus layer , allowing the virus to gain access
to the cells of upper respiratory tract.
2) Viremia rarely occurs.
3) The systemic symptoms are due to cytokine circulating in
the blood.
4) There is necrosis of the superficial layers of the
respiratory epithelium.
5) Pneumonia as a result of secondary bacterial infection is
interstitial in location.
6) The virulence of the H5N1 strain is greater than H1N1
and H3N2 strains because:
I- H5N1 strain is relative resistance to interferon
II- Increase induction of cytokines, especially TNF, this
mediate the pathogenesis of pneumonia and acute
respiratory distress syndrome (ARDS) seen in H5N1
infection.
Immunity:
Immunity depends on secretary IgA in the respiratiory
tract. IgG is also produced but it is less protecrtive.
Cytotoxic Tcells also play a protective role.
Clinical findings
1) After an incubation period of 24-48 hours , fever,
myalagia, headache, sore throat, and cough develop
suddenly .
2) Vomiting and diarrhea are rare.
3) The symptoms resolve spontaneously in 4-7 days, but
influenzal or bacterial pneumonia may complicate the
course. Staphylococcus aureus is the most common
pathogen for pneumonia.
4) Reye's syndrome which is rare and characterized by
encephalopathy and liver degeneration, life – threatening
complication in children following some viral infections,
particularly influenza and chickenpox. Aspirin given to
reduce fever in viral infection has been implicated in the
pathogenesis of Reye's syndrome.
Laboratory diagnosis:
1) In cases of epidemic, clinical diagnosis is enough.
2) Virus can be detected in specimens such as (nasal or
throat washings, nasal or throat swabs and sputum) by
various techniques such as direct fluorescent antibody,
PCR, or cell culture-based tests.
3) Antibody detection in patient serum.4 fold increase in
antibody titer in paired serum samples taken early in the
illness and 10 days later and detected either by
hemagglutination inhibition or complement fixation test.
Treatment
1) Amantidine in the treatment and prevention of influenza
A .indicated in the prevention in elderly,
immunocompromised persons.
2) Rimantidine is a derivative of amantidine. Drug resistance
observed against the 2 drugs.
3) Zanamivir (Relanza) and oseltamivir (Tamiflu) are also
used for the treatment of influenza. They belong to a class
of neuraminidase inhibitors that act by inhibiting the
release of virus from infected cells. This limits the
infection by reducing the spread of virus from one cell to
another. These drugs are effective against influenza A &B
Prevention
The main mode of prevention is the vaccine, which
consists from two strains of influenza A (H1N1, H3N2)
and one strain of influenza B virus.
There are two types of influenza vaccines available
I- Killed vaccine containing purified protein subunits of the
virus (HA and NA). The virus inactivated with
formaldehyde, then treated with a lipid solvent that
disaggregate the virions. The vaccine given
intramuscularly. Protection lasts only 6 months. It is
indicated in people older than 50 years of age, children 6-
23 months of age, and those with chronic disease.
II- The new vaccine that was approved in 2003 is a live
vaccine containing temperature sensitive mutants of
influenza A and B viruses. These temp.- sensitive mutants
can replicate in the cooler (33c) nasal mucosa where they
induce IgA. This vaccine administered by spraying into
the nose (nasal mist).

Unit 4: Virology
211
Epidemiology:
To date all human pandemic strains have been
reassortement between avian and human influenza
viruses. Pigs serve as mixing vessels for reassortements as
their cells contain receptors recognized by both human
and avian viruses.
Influenza virus occurs worldwide and cause annual
outbreaks of variable intensity.
Influenza outbreaks occur in waves, although there is no
regular periodicity in the occurrence of epidemics.
Influenza A epidemic waves tend to be 2-3 years, for B 3-
6 years. Every 10-40 years, when a new subtype of
influenza A appears, a pandemic results. This happened in
1918 (Spanish flu) (H1N1 the swine like influenza), 1957
(Asian flu) (H2N2), and 1968 (Hong Kong flu) (H3N2).
The H1N1 subtype reemerged in 1977 (Russian flu).
Since 1977, influenza A (H1N1) and (H3N2) viruses and
influenza B have been in a global circulation. In 1997, in
Hong Kong avian influenza A virus (H5N1) occurred.
Paramyxoviruses
The Paramyxovirus family contains four important human
pathogens: measles, mumps, respiratory syncytial virus,
and parainfluenza viruses.
The genome is not segmented, they have a larger
diameter, and their surface spikes are different.
Paramyxoviruses are composed of one piece of SS –ve
polarity RNA, a helical nucleocapsid, and an outer
lipoprotein envelope. The envelope is covered with
spikes, which contain hemagglutinin, neuraminidase, or a
fusion protein that causes cell fusion and, in some cases,
hemolysis.
Measles virus
Important properties
Measles virus has a single serotype, and the
hemagglutinin is the antigen against which neutralizing
antibody is directed. Humans are the natural host.
Replicative cycle:
After adsorption to the cell surface via its hemagglutinin,
the virus penetrates and uncoat.
The virion RNA polymerase transcribes the –ve –strand
genome into mRNA.
Multiple mRNA are synthesized, each of which is
translated into specific viral proteins.
The helical nucleocapsid is assembled, and the virus
released from the cell by budding.
Transmission and epidemiology.
The Measles virus is transmitted via respiratory droplets
produced by coughing and sneezing both during the
prodromal period and for few days after the rash appears.
Measles occur worldwide, usually in outbreaks every 2-3
years, when the number of susceptible children reaches a
high level.
Measles infection is more severe in malnourished children.
Patient with deficient cell –mediated immunity have a
severe, life –threatening disease when they contract measles
Pathogenesis:
After infection of the cells lining the upper respiratory
tract, the virus enters the blood and infects
reticuloendothelial cells, where it replicates again.
It then spreads via the blood to the skin.
The rash is caused by cytotoxic T cells attacking the
measles virus –infected vascular endothelial cells in the
skin. Antibody mediated vasculitis may also play a role.
Unit 4: Virology
211
After the rash appears, the virus can no longer be recovered
and the patient can no longer spread the virus to others.
Multinucleated giant cells, which form as a result of the
fusion protein in the spikes, are characteristic of the lesions
Immunity
Lifelong immunity in individuals who had the disease.
Cell-mediated immunity is more important than humoral
immunity in the recovery and protection.
Maternal antibody passes the placenta, and infants are
protected during the first 6 months of age.
Infection with measles virus can transiently depress cell-
mediated immunity against other intracellular
microorganisms, such as Mycobacterium tuberculosis,
leading to a loss of purified protein derivative (PPD) skin
test reactivity, reactivation of dormant organisms, and a
clinical disease. The proposed mechanism for this
finding is that when measles virus binds to its receptor
(called CD46) on the surface of human macrophages, the
production of IL-12, which is necessary for cell-mediated
immunity to occur, is suppressed.
Clinical findings
1) After an incubation period of 10-14 days, a prodromal
phase characterized by fever, conjunctivitis, running nose,
and coughing occurs.
2) Koplik´ s spots are bright red lesions with a white, central
dot that are located on the buccal mucosa and are
diagnostic.
3) A few days later, a maculopapular rash appears on the
face and proceeds gradually down the body to the lower
extremities, including the palms and soles.
Complications
1) Encephalitis occurs at a rate of 1/1000 cases of measles.
2) Primary measles pneumonia and bacterial pneumonia
occurs.
3) Bacterial otitis media.
4) Subacute sclerosing panencephalitis (SSPE) is a rare, fatal
disease of the central nervous system that occurs several
years after measles.
5) Atypical measles occurs in some people who were given
the killed vaccine and were subsequently infected with
measles virus. It is characterized by an atypical rash
without Koplik s spots.
Laboratory diagnosis
1) Most cases are made on clinical grounds.
2) Viral isolation in cell culture can be done
3) A rise in antibody titer of greater than 4-fold can be
used in the diagnosis.
Prevention
Immunization with live attenuated virus vaccine given at
9 months of age and a second dose combined with mumps
and rubella vaccines at 15 months of age.
The vaccine is contraindicated in immunocompromised
patient and in pregnant women.
Mumps virus
This virus cause Mumps.
Important properties
The virion has two types of envelope spikes, one with
both Hemagglutinin and neuraminidase and the other with
cell-fusing and hemolytic activity. Neutralizing antibody
is directed against the hemagglutinin.
The virus has a single serotype. Humans are the natural host
Replicative cycle
As for measles virus.
Transmission and epidemiology.
Mumps virus is transmitted via respiratory droplets.
Mumps occurs worldwide, with peak incidence in the
winter.
About 30% of children have a subclinical infection, which
confers immunity.
Pathogenesis and immunity
The virus infects the respiratory tract and then spreads
through the blood to infect the parotid glands, testes,
ovaries, pancreas, and in some cases meninges. The virus
may ascend from the buccal mucosa up Stensen´ s duct to
the parotid gland.
Lifelong immunity occurs after the infection.
Maternal antibody passes the placenta and provides
protection during the first 6 months.
Clinical findings
After an incubation period of 18-21 days, a prodromal
stage of fever, malaise, and anorexia is followed by tender
swelling of the parotid glands, either unilateral or
bilateral. The disease resolves spontaneously within 1
week.
Complications
1) Orchitis in postpupertal males, which is if bilateral, results
in sterility because of fibrous tunica albuginea, which
Unit 4: Virology
211
resist expansion, thereby causing pressure necrosis of the
spermatocytes. Unilateral orchitis don’t cause sterility.
2) Meningitis, which is usually benign, self-limited, and
without sequel.
Laboratory diagnosis
1) Clinical diagnosis.
2) Viral isolation in cell culture.
3) A 4 –fold rise in antibody titer in either hemagglutination
inhibition or the complement fixation test is diagnostic.
Treatment
No antiviral therapy.
Prevention
Immunization with live attenuated virus vaccine given at
15 months of age given subcutaneously combined with
measles and rubella vaccines.
The vaccine is contraindicated in immunocompromised
patient and in pregnant women.
Respiratory Syncytial virus
It is the most important cause of pneumonia and
bronchioloitis in infants. It is an important cause of otitis
media in children and of pneumonia in the elderly and in
patients with chronic cardiopulmonary diseases.
Important properties
The surface spikes are fusion proteins, not hemagglutinin
or neuraminidase. Humans are the natural host of RSV.
Two serotypes, A and B.
Replicative cycle
As for measles virus.
Transmission and epidemiology.
1) via respiratory droplets
2) Direct contact of contaminated hands with the nose or mouth
3) RSV causes outbreaks of respiratory infections every winter
4) RSV causes outbreaks of respiratory infections in
hospitalized infants.
Pathogenesis and immunity
RSV infection in infants is more sever and more often
involves the lower respiratory tract than in older children
and adults.
The infection is localized to the respiratory tract; viremia
doesn’t occur.
The sever disease in infants may have an
immunopathogenic mechanism. Maternal antibody passed
to the infant may react with the virus, form immune
complexes, and damage the respiratory tract cells. Trials
with a killed vaccine resulted in more severe disease; an
unexpected finding that supports such a mechanism.
Most individuals have multiple infections caused by RSV.
Laboratory diagnosis
1) Virus detection by immunofluorescence on smears of
respiratory epithelium or by isolation in cell culture.
2) A 4 –fold rise in antibody titer.
3) Reverse transcriptase PCR.
Treatment
Aerosolized ribavirin is recommended for severely ill
hospitalized infants.
Parainfluenza viruses
Diseases
These viruses cause croup, ( acute
laryngotracheobronchitis) in children younger than 5 years
of age (Croup is chartecterized by a harsh cough &
hoarseness), laryngitis, bronchiolitis, & pneumonia in
children & a disease resembling the common cold in adults.
Important properties:
The surface spikes consist of hemagglutinin,
neuraminidase, and fusion protein.
There are four serotypes. The virus is transmitted via
respiratory droplets.
Replicative cycle
As for measles.
Pathogenesis and immunity
These viruses cause lower and upper respiratory tract
disease without viremia.
A large proportion of infections are subclinical.
Parainfluenza 1 & 2 are the major cause of croup.
Parainfluenza 3 is the most commonly isolated from
children with lower respiratory tract infections.
Parainfluenza 4 rarely cause disease except for the
common cold.
Diagnosis:
Most cases are diagnosed clinically.
Treatment:
Neither antiviral therapy nor a vaccine is
available.

Unit 4: Virology
215
Coronaviruses
Diseases
Coronavirus is an important cause of common cold
In 2002, a new disease, an atypical pneumonia called
SARS (severe acute respiratory syndrome) emerged.
Important properties
1) Coronavirus is nonsegmented, SS, +ve polarity RNA
genome, enveloped with helical nucleocapsid.
2) In electron microscope, prominent club-shaped spikes in
the form of a Corona (halo) can be seen.
3) Two serotypes 229E and OC 43.
4) The corona virus recovered in 2002 that cause SARS
(CoV-SARS) is belonging to the second serotype (OC43).
5) The receptor for the SARS coronavirus is angiotensin-
converting enzyme -2.
Replicative cycle
The virus adsorbs to cells via its surface spikes
(hemagglutinin), after which it enters the cytoplasm,
where it is uncoated.
The positive polarity genome is translated into two large
polypeptides, which are self-cleaved by the virus-encoded
protease.
mRNA is synthesized, and then translated into the
structural proteins.
The virus is assembled and obtains its envelope from the
endoplasmic reticulum, not from the plasma membrane.
Transmission and epidemiology.
1) By the respiratory aerosol.
2) SARS originated in china in 2002. Human to human
transmission,
Pathogenesis and immunity
Viral infection is typically limited to the respiratory
mucosa.
50% of infections are asymptomatic
Reinfection can occur.
Pneumonia caused by SARS coronavirus is characterized
by diffuse edema, the binding of the virus to angiotensin
converting enzyme-2 on the surface of respiratory tract
epithelium may contribute to the dysregulation of fluid
balance and edema in the alveolar space.
Clinical findings
The common cold is characterized by coryza (rhinorrhea,
runny nose), scratchy sore throat, and low grade fever.
The illness lasts several days.
SARS is a severe atypical pneumonia characterized by
fever (38C), nonproductive cough, dyspnoea, hypoxia,
chills, rigor, malaise, and headache. Chest X-ray reveals
interstitial (ground-Glass) infiltrates.
Leucopenia and thrombocytopenia.
Laboratory diagnosis
1) Common cold diagnosed clinically.
2) If SARS is suspected, antibody –based and PCR-based
tests can be used.
Treatment and prevention
1) There is no antiviral therapy or vaccine available.
2) A combination of Ribavirin + steroid has been tried in
the treatment of SARS.
Rubella virus
This virus causes rubella (German measles) and
congenital rubella syndrome.
Important properties
1) Rubella virus is a member of togavirus family (not
paramyxovirus).
2) It is composed of one piece of SS RNA, positive polarity
RNA with icosahedral symmetry, and lipoprotein
envelope.
3) Its surface spikes contain hemagglutinin. The virus has a
single antigenic type.
4) Humans are the natural host.
Replicative cycle
The same as for any SS positive sense RNA virus.
Transmission:
1) respiratory droplets
2) From the mother to fetus transplacentally.
Pathogenesis and immunity:
1) Initial replication of the virus occurs in the nasopharynx
and local lymph nodes.
2) From there it spreads via the blood to the internal organs
and skin.
3) The origin of rash is thought to be due to antigen-antibody
mediated vasculitis
4) Natural infection leads to lifelong immunity
5) Antibody crosses the placenta and protects the newborn.
Unit 4: Virology
211
Clinical findings:
A. Rubella
Rubella is milder, shorter disease than measles. After an
incubation period of 14-21 days, a brief prodromal period
with fever , malaise, followed by maculopapular rash,
which starts on the face and progresses downward to
involve the extremities.
Posterior auricular lymphadenopathy is characteristic. The
rash lasts for 3 days.
B. Congenital Rubella Syndrome.
When a non-immune pregnant woman is infected during
the first trimester, especially the first month, significant
congenital malformation (heart leading to patent ductus
arteriosus, the eye leading to cataract, and the brain
leading to deafness and mental retardation) can occur as a
result of maternal viremia and fetal infection. The infected
new born continue to excrete rubella virus for months
following birth. Some shedders are asymptomatic and
without any congenital malformation.
Laboratory diagnosis:
1) Virus isolation by tissue culture.
2) 4 fold increase in antibody titer between acute and
convalescent phase.
3) In pregnant mother, IgM antibody indicates recent
infection.
4) An amniocentesis can reveal whether there is rubella virus
in the amniotic fluid, which indicates definite fetal
infection.
Treatment
No antiviral therapy.
Prevention
Immunization with live attenuated virus vaccine given at
15 months of age given subcutaneously combined with
measles and mumps vaccines. Also the vaccine given to
unimmunized young adult women if they are not pregnant
and will use contraception for the next 3 months.
The vaccine has caused a significant reduction in the
incidence of both rubella and congenital rubella
syndrome. It induces some respiratory IgA, thereby
interrupting the spread of virulent virus by nasal carriage.
Immune serum globulin can be given to pregnant mother
in the first trimester who have been exposed to a known
case of rubella.
Rhabdoviruses
Rabies virus
This virus causes rabies which is an acute infection of the
CNS that is almost always fatal.
Important properties
1) Rabies virus is the only medically important member of
the rhabdovirud family.
2) It is SS, negative polarity RNA virus with bullet-shaped
capsid and an envelope.
3) It has single antigenic type.
4) Rabies virus has a broad host range: it can infect all
mammals, but only certain mammals are important
sources of human infection.
Replicative cycle
1) Rabies virus attaches to the acetylcholine receptor on the
cell surface.
2) After entry into the cell, the virion RNA polymerase
synthesizes five mRNA that code for viral proteins.
3) After replication of the genome viral RNA by a virus-
encoded RNA polymerase, progeny RNA is assembled
with virion proteins to form the nucleocapsid, and the
envelope is acquired as the virion buds through the cell
membrane.
Transmission
1) The virus is transmitted by the bite of rabid animal that
manifests aggressive, bitting behavior induced by viral
encephalitis.
2) In certain developed countries, transmission is usually
from the bite of wild animals such as skunks, raccoons,
and bats.
3) Human rabies has also occurred in certain developed
countries in people who have not been bitten, so called
nonbite exposures, e.g. is the exposure to aerosols of bat
secretions containing rabies virus.
4) Another rare example is transmission in transplants of
corneas taken from patients who died of undiagnosed
rabies.

Unit 4: Virology
211
Pathogenesis:
The virus multiplies locally at the bite site, infects the
sensory neurons, and moves by axonal transport to the
central nervous system (CNS).
During its transport within the nerve, the virus is sheltered
from the immune system, and little, if any, immune
response occurs.
The virus multiplies in the CNS and then travels down the
peripheral nerves to the salivary glands and other organs.
From the salivary glands, it enters the saliva to be
transmitted by the bite.
There is no viremic stage.
Within the CNS, encephalitis develops, with the death of
neurons and demylination.
Infected neurons contain an eosinophilic cytoplasmic
inclusion called a Negri body, which is important in
laboratory diagnosis of rabies.
Clinical findings:
The incubation period varies, according to the location of
the bite, from as short as 2 weeks to 16 weeks or longer. It
is shorter when bites are sustained on the head rather than
on the leg, because the virus has a shorter distance to
travel to reach the CNS.
The patients exhibits a prodrome of non-specific
symptoms such as fever, anorexia, and changes in
sensation at the bite site.
Within a few days, signs such as confusion, lethargy, and
increased salivation develop. Painful spasm of the throat
muscles on swallowing, this result in hydrophobia.
The disease progress to seizure paralysis and coma
Death almost invariably ensues, but with the advent of life
support systems a few individuals have survived.
Laboratory diagnosis:
1) Rabies in humans can be diagnosed by fluorescent-
antibody staining of a biopsy specimen, usually taken
from the skin of the neck at the hairline.
2) Isolation of the virus from sources such as saliva, spinal
fluid, and brain tissue
3) Rise in titer of antibody to the virus.
4) Negri bodies can be demonstrated in corneal scrapings
and in autopsy specimens of the brain.
Treatment
There is no antiviral therapy. Only supportive treatment is
available.
Prevention:
There are two approaches to prevention of rabies in
humans: preexposure and postexposure.
Preexposure immunization:
Vaccine given to high risk individuals: veterinarians,
zookeepers, and travelers to areas of hyperendimic
infection. The schedule consist of three doses given on
days 0, 7, and 21 or 28 .Booster doses given as needed.
Types of rabies vaccine:
Human diploid cell vaccine (HDCV). Inactivated virus
grown in human diploid cells.
Rabies Vaccine, Adsorbed(RVA)
Purified Chick Embryo Cell Vaccine(PCEC)
Nerve Tissue Vaccine : low antigenicity, induce
postvaccinal encephalitis (allergic)
Duck Embryo Vaccine: Low antigenicity.
Live Attenuated Vaccine: for animals but not for humans.
Postexposure immunization:
Both the vaccine and human rabies immune globulin
(RIG) plus immediate cleaning of the wound and tetanus
immunization.
The decision to give immunization depends on many
factors:
1) The type of the animal (all wild animal attacks
demand immunization.
2) Whether an attack by a domestic animal was
provoked, whether the animal was immunized, and
whether the animal is available to be observed.
3) Whether rabies is endemic in the area.
If the decision is to immunize, both HDCV and RIG are
recommended. Five doses of HDCV are given (on days 0,
3, 7, 14, and 28), but RIG is given only once with the first
dose of HDCV (at a different site?).
It is advisable to give RIG as much as possible into the
bite site, and the remainder is given intramuscularly.
If the animal has been captured, it should be observed for
10 days.
