
Unit 2: Protozoa
40
Lecture 15+16 - Suborder Eimeriina
Genus Toxoplasm
Subkingdom Protozoa
Phylum Apicomplexa
Class Sporozoea
Subclass Coccidia
Order Eucoccidia
Suborder Eimeriina
Genus Toxoplasma
Cryptosporidium
Isospora
Sarcosystis
Toxoplasma gondii
is a coccidian parasite of
cosmopolitan distribution, able to develop in a wide
variety of vertebrate hosts, but the definitive host is the
house cat and certain felidae. The prevalence is highest in
hot, humid climates and lowest in dry, cold climates.
It was originally distributed in a North African rodent
called the gundii from which it derives its special name.
The definitive host is the house or domestic cat and other
felidae.
Intermediate host: man (considered as a dead end host)
and other mammals (cattle, sheep, mouse and pig).
Morphology
There are five forms in T. gondii life cycle:
1- Trophozoite (tachyzoite),
2- Tissue cyst (bradyzoite),
3- Schizont
4- Gametocyte
5- Oocyst .
Tachyzoites, tissue cysts and oocysts
are important stages seen during the life cycle of the
parasite, and all these stages are infectious to man.
Trophozoite (tachyzoite): Fig. 1-8
It is oval to crescent-shaped with a pointed anterior end
and a rounded posterior end. It is 4 -7 µ m long and 2 -4 µ
m wide. An ovoid nucleus is present in the posterior end
of the parasite. Tachyzoite is the multiplying form seen
during the acute stage of the infection. It can invade any
type of cell in a host and once inside a cell, it multiplies
within a vacuole by a process known as endodyogeny, or
by binary fission or schizogony. Tachyzoites divide until
they fill the host cell, which then liberates them, and they
reinvade (or ingested by) other macrophages, repeating
the process. The cell which contains them, when becomes
merely a bag full of tachyzoites, called(Pseudocyst).
Fig 1-8 tachyzoite
Tissue cyst:
It is spherical and may vary in size from 5 to 100 µ m in
diameter. This is the chronic stage of the infection. The
tissue cysts can be found in any organ of the body but are
commonly found in the brain ,skeletal and heart muscels.
An eosinophilic cyst wall surrounds each cyst. The cyst
contain hundreds of bradyzoite ( cystozoites). Bradyziotes
multiply slowly.
Oocyst:
This stage is only present in cats and other felines but not
in humans. It is oval and measures 10-12 µm diameters.
Each cyst is surrounded by a thick resistant wall which
encloses a spheroplast.The oocyst librated from the
intestinal epithelial cell while still immature; it completes
its development while passing down the gut and after
expulsion in the feces. Its contents are divided first into
two cells; these then secrete cyst walls to form 2
sporocyst. The content of each sporocyst then divide once
more to produce two infective sporozoites.
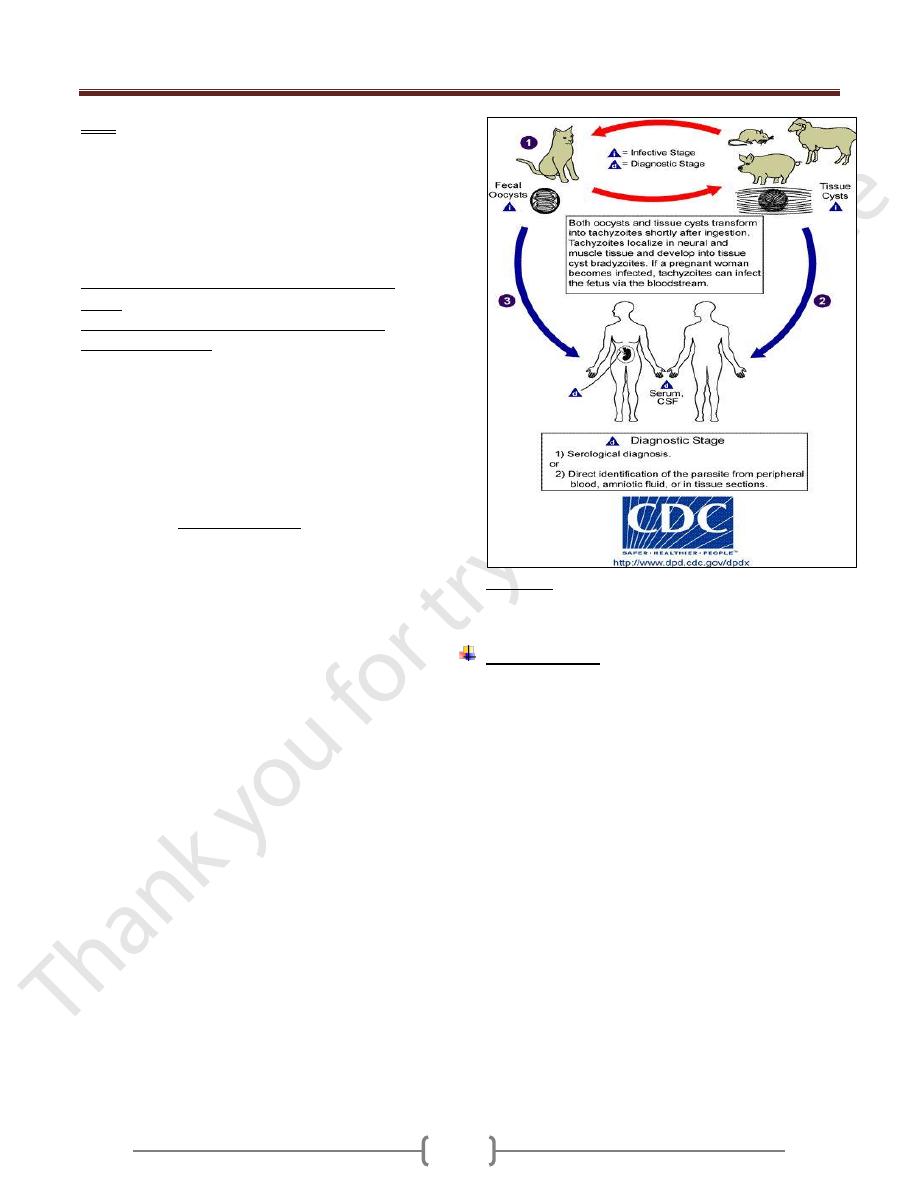
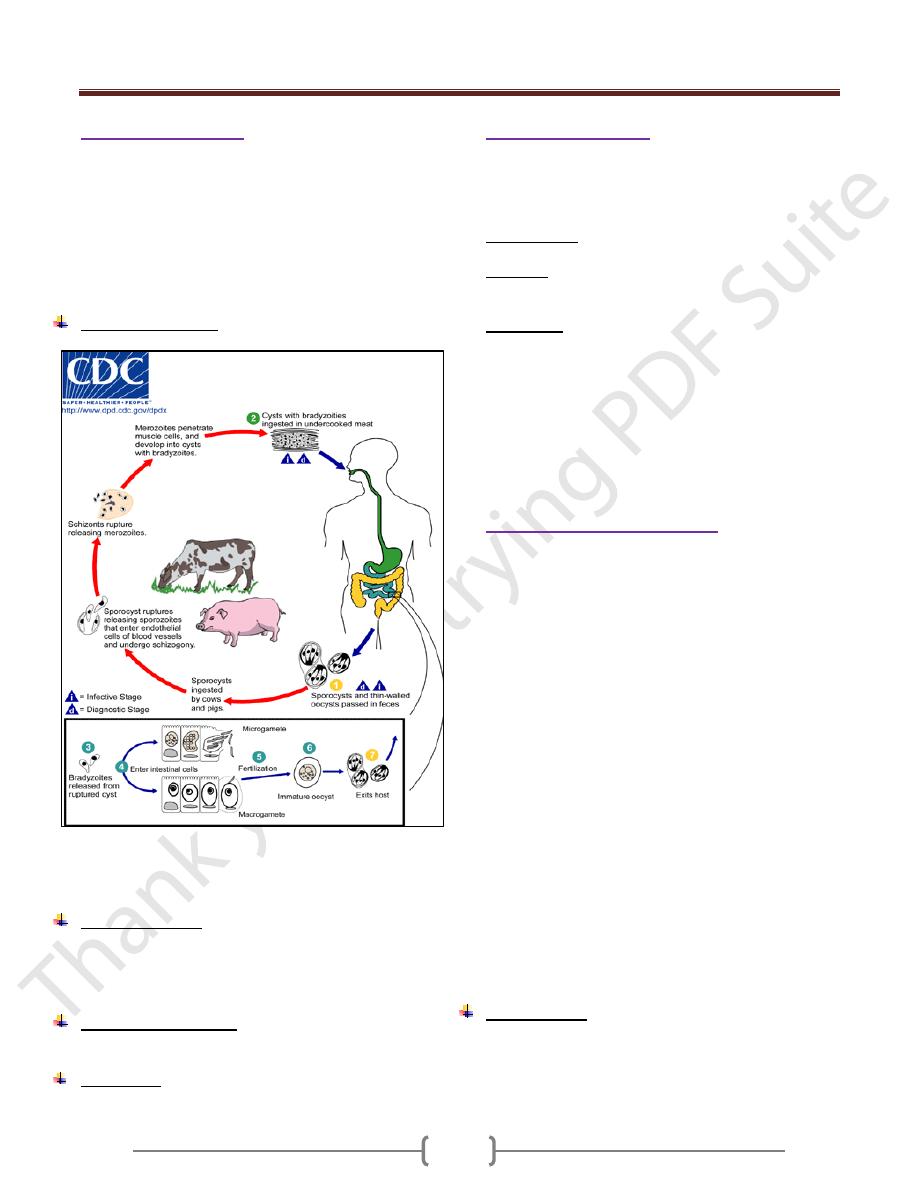
Life cycle (Figure 2-8 ):
In cat (definitive host):
Ingestion of the organism (either sporozoite from oocyst
or bradyzoite from tissue cyst) invades the mucosal cells
of the cat’s small intestine, and then they form schizont
(asexual) and gametocytes (sexual). After sexual fusion of
gametes, zygotes are formed and a thin wall protect the
zygote and an oocyst develops, then exit into the gut
lumen, then passes out via feces as an oocyst with single
sporoblast. Within each oocyst, 2 sporocysts form (zygote
divide into 2 sporoblast) in about 3-4 days, four
sporozoite forms within each sporocyst (i.e. 8 sporozoite)
(infective oocyst).
Unit 2: Protozoa
41
Note:
If the cat ingest infective oocyst and initiate intestinal
infection, the animal will pass oocyst within 21-24 days.
If the cat fed an acutely ill mouse with proliferative forms
in its tissue, oocyst will appear in the cat's feces within 9-
11 days, and if the cat fed on chronically ill mouse the
oocyst will appear in cat feces within 3-5 days.
In human and other mammals (intermediate
hosts)
Human infection may be acquired in several ways:
Mode of transmission
a) Ingestion of undercooked infected meat
b) Ingestion of the oocyst from fecally contaminated
hands (oocyst during the cleaning of cat litter box) or
food, milk and water;
c) Organ transplantation or blood transfusion;
d) Transplacental transmission;
e) Accidental inoculation of tachyzoites.
Swallowing the oocyst or tissue cyst initiate the
development of ASEXUAL CYCLE. This process occurs
mainly in macrophages.
The sporozoites from the ingested oocyst and bradyzoites
from the tissue cyst invade the mucosal epithelial cell of
the small intestine in which they multiply as tachyzoites
by endodyogeny. The tachyzoites divide until they fill the
host cell which then liberates them, and the tachyzoite
reinvade (or ingested by) other macrophages, repeating
the process and form pseudocyst.
The multiplying tachyzoites also spread to distant extra -
intestinal organ (e.g. brain, eye, liver, spleen, heart,
skeletal muscle and placenta of pregnant mother) by
invading lymphatics and blood.
The multiplication of tachyzoites constitutes the acute
phase of infection.
If the host lives, and the infection is untreated, the host’s
immune system becomes effective and tachyzoites are
destroyed, but the the parasite usually responds to this
by entering other cells (muscle cells, neurons, and
perhaps others) and secreting a thin but tough cyst
wall around itself form a tissue cyst and initiate the
chronic phase of the disease. A tissue cyst contains
hundreds of bradyzoites.
If another intermediate host eats uncooked meat
containing this tissue cyst , bradyzoites emerge in the
duodenum and repeat the cycle.
Figure 2-8 Life cycle of Toxoplasma gondii
Pathogenesis:
Upon the host’s ingestion of either tissue cysts containing
bradyzoites or oocysts containing sporozoites, the
parasites are released from the cysts by a digestive
process. Bradyzoites are resistant to the effect of pepsin
and invade the host’s gastrointestinal tract.
Within enterocytes (or other gut-associated cells), the
parasites undergo morphologic transformation, giving rise
to invasive tachyzoites. These tachyzoites induce a
parasite-specific secretory IgA response.
From the gastrointestinal tract, parasites are disseminated
to a variety of organs, particularly lymphatic tissue,
skeletal muscle, myocardium, retina, placenta, and the
CNS. At these sites, the parasite infects host cells,
replicates, and invades the adjoining cells. In this fashion,
the hallmarks of the infection develop: cell death and
focal necrosis surrounded by an acute inflammatory
response. In the normal immune host, both the humoral
and the cellular immune responses control infection. As
tachyzoites are cleared from the acutely infected host,
tissue cysts containing bradyzoites begin to appear,
usually within the CNS and the muscles initiating chronic
phase of the infection.
Unit 2: Protozoa
42
In the immunocompromised or fetal host, the immune
factors necessary to control the spread of tachyzoite
infection are lacking. This allows the persistence of
tachyzoites and gives rise to the progressive focal
destruction that result in organ failure (i.e., necrotizing
encephalitis, pneumonia, and myocarditis).
Persistence of infection with cysts containing bradyzoites
is common in the immunocompetent host. This lifelong
infection usually remains subclinical. Although
bradyzoites are in a slow metabolic phase, cysts do
degenerate and rupture within the CNS. This degenerative
process, with the development of new bradyzoite-
containing cysts, is the most probable source of
recrudescent infection in immunocompromised
individuals and the most likely stimulus for the
persistence of antibody titers in the immunocompetent
host.
In the brain, minute scattered necrotic areas may later
calcify.
Note:
Tachyzoites directly destroy cells, particularly
parenchymal and reticuloendothelial cells, whereas
bradyzoites released from ruptured tissue cysts cause local
inflammation with blockage of blood vessels and
necrosis.
Clinical features:
Toxoplasmosis is either acquired or congenital infections.
1) Acquired infection:
It is seen in adults
In immune competent, it is either:
a) Asymptomatic which is the majority of the cases or
b) It may persist as infectious mononucleosis like
symptom with negative heterophil antibody test.
In immunocompromised patient (as in AIDS patient):
a) Myocarditis, meningoencephalitis and atypical
pneumonia.
b) CNS involvement which is fatal.
c) Retinochoroiditis.
In immunocompromised patient, it is the tachyzoite form
rather than the bradyzoite form and cysts that commonly
are seen.
2) Congenital toxoplasmosis:
Congenital infection of the fetus occurs only when the
mother is infected during pregnancy.
If she is infected before pregnancy, the organism will be
in the cyst form and there will be no trophozoite to pass
through the placenta.
The mother who is reinfected during pregnancy but who
has immunity from previous infection will not transmit
the infection to her child.
One third of mother infected primarily during pregnancy,
give birth of infected infants, and only 10 % of these
infants are symptomatic.
Congenital infection leads to stillbirth, retinochoroiditis,
intracranial calcification, psychomotor disturbances and
hydrocephaly or microcephaly.
If the infection occurred in the first trimester, the
incidence of transplacental infection is lowest (_15%), but
the disease in the neonate is most severe. Stillbirth or
major C.N.S anomalies, encephalitis, retinochoroiditis,
hepatosplenomegaly, fever, jaundice, and intracranial
calcification.
Second- third trimester infection, the incidence of
transplacental infection is greatest (65%), but the infant is
usually asymptomatic at birth and the clinical
manifestation of these infections may be delayed long
after birth , even beyond childhood and manifested by
neurological problem or learning difficulties. They may
develop retinochoroiditis later.
Laboratory diagnosis
A. Pathogenic
1) Smears from Lymph node, bone marrow, spleen. For
organism detection.
2) Body fluids (in acute infection) or from tissue (chronic
infection) inoculated intraperitonealy into young
laboratory mice for demonstrations of the organisms.
B. Serological
Serology remains the primary approach for the diagnosis
of toxoplasmosis.
1) Indirect fluorescent antibody (IFA) test and ELISA
test including IgM and IgG kits.
In Immunocompetent adults.
Toxoplasmosis is normally diagnosed serologically by
detection of parasite-specific IgG and IgM antibodies.
IgM antibodies can be detected as early as one week after
the primary infection, peak within two to four weeks, then
drop to below the detection limit within a few weeks; in
some cases persistence at low titers lasts longer. IgG
antibodies appear somewhat later, peak after two to four
months and persist for many years. A high or rising IgG

Unit 2: Protozoa
43
titer with contemporal detection of IgM indicates an acute
primary infection.
Ocular toxoplasmosis normally cannot be diagnosed by
serological methods.
Serological findings are often not reliable indicators in
immunodeficient patients due to reduced antibody
production. The cerebral form of the infection seen
frequently in reactivated toxoplasmosis is therefore
usually diagnosed by means of clinical imaging method.
Prenatal toxoplasmosis in neonates is difficult, but highly
important. Since IgG antibodies are transmitted from
mother to child diaplacentally, detection of them in the
child cannot serve as a definitive diagnostic indicator.
IgM is only present in about 50% of prenatally infected
children. In suspected cases, the blood or cerebrospinal
fluid should be examined using the PCR.
Toxoplasmosis and pregnancy
Acute Toxoplasma gondii infection in early pregnancy
carries
the risk of transmitting the infection to the fetus
with serious
sequelae. Serological testing for IgG/IgM
anti-Toxoplasma
antibodies may fail to differentiate
between a recent and past
infection. IgM may remain
positive for up to 1 year. IgG Avidity test had been
developed based on the fact that following immune
response, the IgG antibodies produced initially bind
weakly to the antigen (low avidity). As the immune
response develops there is maturation of IgG antibody
response and the avidity increases progressively over
weeks or months (high avidity). The presence of high
avidity IgG excludes the possibility that infection
occurred within the previous five months.
2) sabin-feldman dye test
depends upon the appearance in 2-3 weeks of antibody
that will render the membrane of laboratory cultured
living Toxoplasma gondii impermeable to alkaline
methylene blue , so that the organism are unstained in
presence of positive specimen.
C. Molecular diagnosis by PCR
Treatment:
1) in adult with acute infection:
a) No symptoms, no treatment.
b) In case of severs symptom, active retinochoroiditis, and
immunocompromised patient, treatment should be given.
The regime include: sulphodiazine + pyrimethamine for 4
weeks.
Alternative drugs: spiramycin, clindamycin, trimethprim-
sulphamethoxazole
2)
In pregnancy
: Spiramycin (Rovamycin) ® continued
till delivery.
Prevention:
1) Meat cooked to 50-60 Cº for 4 – 6 minutes or freezing at
– 20 Cº for 48 hours.
2) Pregnant mother should avoid all contacts with cats.
Unit 2: Protozoa
44
Genus Cryptosporidium
It is a minute coccidian parasite with worldwide
distribution. Twenty species of the parasite have been
described from a variety of vertebrates including birds,
fish, mammals and reptiles.
The species that infects humans and most mammals is
Cryptosporidium parvum.
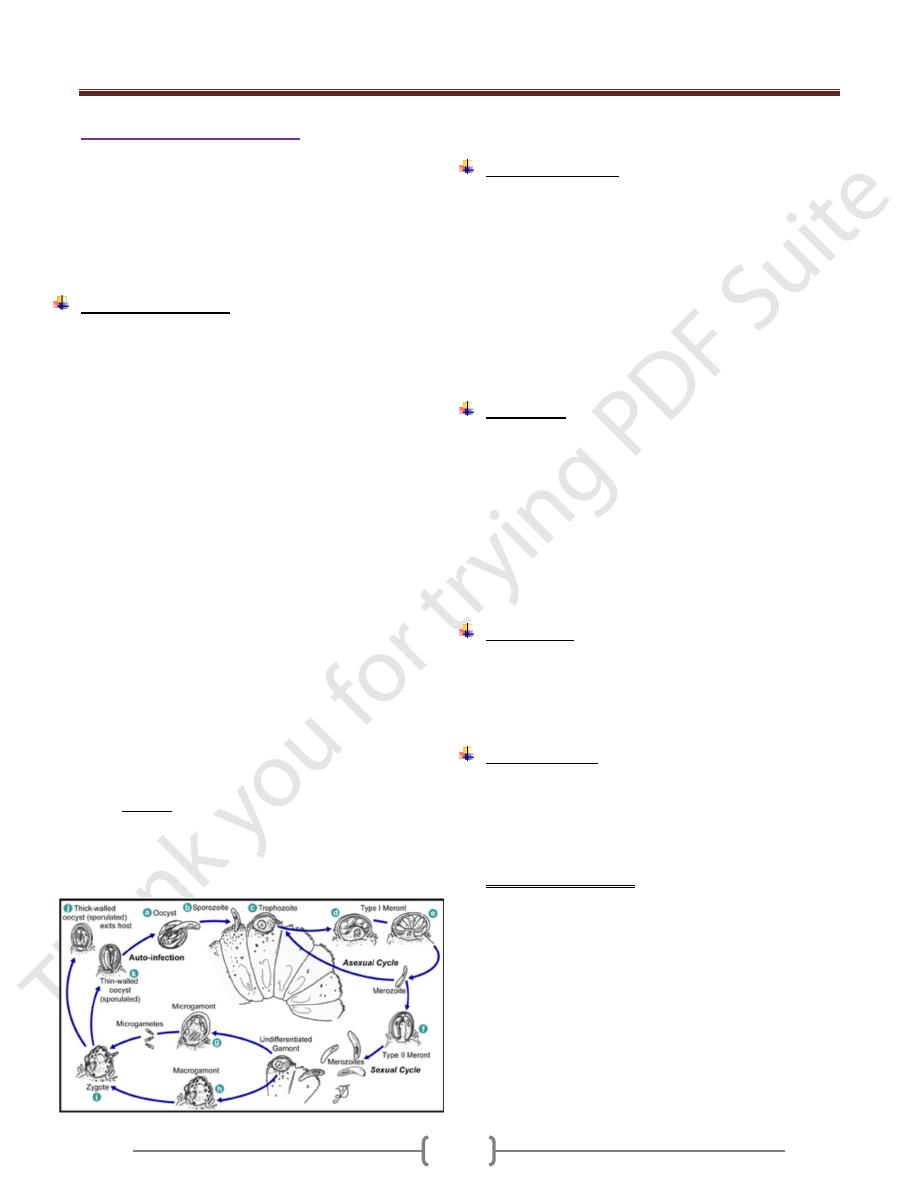
Life cycle (figure 1)
Cryptosporidium species use a single host in their life cycle.
Development of cryptosporidia occurs within the brush
border of the epithelial cells of the intestine. The parasite can
cause damage to the microvilli where it attaches.
The life cycle of Cryptosporidium parvum consists of an
asexual stage and a sexual stage. After being ingested, the
oocysts excyst in the small intestine. They release
sporozoites that attach to the microvilli of the epithelial
cells of the small intestine. From there they become
trophozoites that reproduce asexually by multiple fission,
a process known as schizogony. The trophozoites develop
into Type 1 meronts that contain 8 daughter cells. These
daughter cells are Type 1 merozoites, which get released
by the meronts. Some of these merozoites can cause
autoinfection by attaching to epithelial cells. Others of
these merozoites become Type II meronts, which contain
4 Type II merozoites.These merozoites get released and
they attach to the epithelial cells. From there they become
either macrogamonts or microgamonts. These are the
female and male sexual forms, respectively. This stage,
when sexual forms arise, is called gametogony. Zygotes
are formed by microgametes from the microgamont
penetrating the macrogamonts. The zygotes develop into
oocysts of two types. 20% of oocysts have thin walls and
so can reinfect the host by rupturing and releasing
sporozoites that start the process over again. The thick-
walled oocysts are excreted into the environment. The
oocysts are mature and infective upon being excreted.
They can survive in the environment for months.
Figure 1. Life cycle of C.parvum
Clinical features:
Incubation periods = 7-10 days.
Cryptosporidium can be transmitted from human to
human and from animals to human.
In immunocompetent patient, it is a self-limited diarrhea
lasts about 2 weeks accompanied by abdominal
discomfort, anorexia, fever, nausea, and weight loss.
In immunodeficient patient, severe diarrhea which may be
life threatening. In such cases, cryptosporidia can affects the
biliary tree leading to acalculous cholecystitis or sclerosing
cholangitis, pancreatitis & respiratory tract infection.
Diagnosis:
1) Detection of oocysts in fresh stool samples.
2) Stool concentration techniques using a modified acid fast
stain.
3) Identifying the organism (meronts containing merozoit
and gamonts containing micro and macrogametes) in
intestinal biopsy.
4) Monoclonal antibody for detection of low level of
infection.
5) ELISA for detection of fecal antigens.
Treatment:
1) Immunocompetent patient, no treatment.
2) Immunocompromised patient e.g. AIDS patient,
spiramycin, paromomycin (Humentin), Nitazoxanide
(Cryptaz), or combination therapy with azithromycin.
Epidemiology:
Person – person transmission occurs in child day –care
centers and among house hold contacts and medical
providers.
Oocyst is highly resistant to chlorination but removal can
be achieved by filtration of water.
For patient with AIDS:
1) Boiling of water for 1 minute.
2) Water filtration.
3) Pasteurization is sufficient to destroy infectivity in milk.
Unit 2: Protozoa
45
Genus Isospora
It is a coccidian parasite of the epithelial cells of the
intestine in which it may undergo repeated asexual
development (with consequent destruction of the surface
layer of considerable portion of the intestine) and sexual
stage which culminate in oocyst passed in the feces.
Isospora belli
It is a sporozoan of the human intestine leading to
coccidiosis (Isosporiosis) in human.
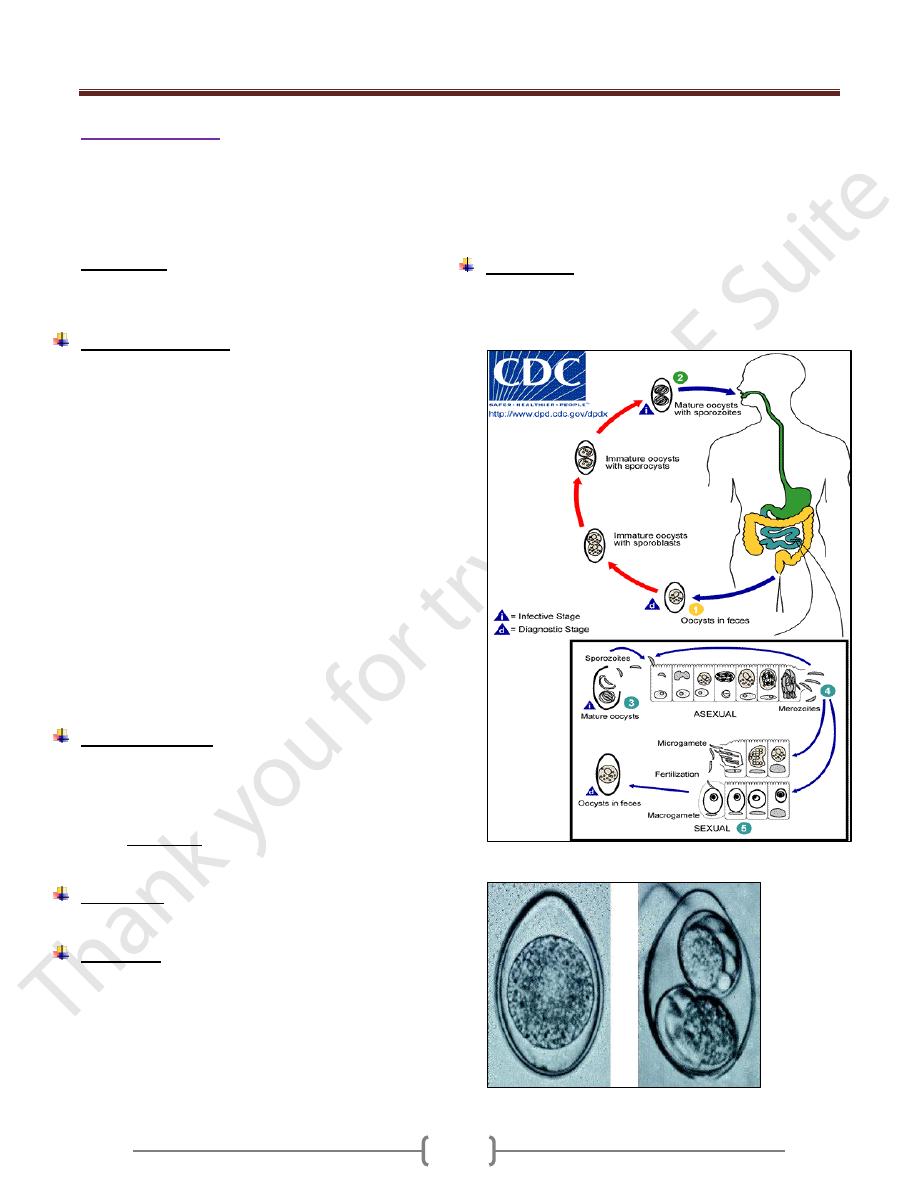
Life cycle (figure 2)
Infection occurs by ingestion of sporocyst-containing
oocysts: the sporocysts excyst in the small intestine and
release their sporozoites, which invade the epithelial cells
and initiate schizogony. Upon rupture of the schizonts, the
merozoites are released, invade new epithelial cells, and
continue the cycle of asexual multiplication. Trophozoites
develop into schizonts which contain multiple merozoites.
After a minimum of one week, the sexual stage begins
with the development of male and female gametocytes.
Fertilization results in the development of oocysts that are
excreted in the stool. At time of excretion, the immature
oocyst contains usually one sporoblast (more rarely two).
In further maturation after excretion, the sporoblast
divides in two, so the oocyst now contains two
sporoblasts. The sporoblasts secrete a cyst wall, thus
becoming sporocysts; and the sporocysts divide twice to
produce four sporozoites.
Isospora belli infects both humans and animals.
Clinical features:
Infection causes acute, non-bloody diarrhea with crampy
abdominal pain, which can last for weeks and result in
malabsorption and weight loss. In immunodepressed
patients, and in infants and children, the diarrhea can be
severe. Eosinophilia may be present (differently from
other protozoan infections).
Pathology:
Jejunal biopsy reveals villous atrophy.
Diagnosis:
1) Stool examination by modified acid fast staining for
oocyst (either immature or mature oocyst containing 2
sporocyst) (figure 3).
Immature oocyst is an ellipsoid or spindle shaped with
blunt ends. In the immature oocyst, is a spherical mass of
protoplasm which soon divide it form 2 sporoblasts which
in turn develop heavy cyst walls and are known as
sporocysts; within each sporocyst, 4 curved sausage
shaped sporozoites develop (mature oocyst).
The immature oocyst requires 4-5 days for maturation.
2) Duodenal biopsy if repeated stool examination is
negative.
3) Eosinophilia which is not found in other protozoal infection.
Treatment:
Trimethoprim – sulfamethoxasole for 3 weeks.
If the patient is hypersensitive to Trimethoprim –
sulfamethoxasole, pyrimethamine or ciprofloxacin can be used
Figure 2. Life cycle of Isospora belli
Figure 3. a immature oocyst, b mature oocyst .
Unit 2: Protozoa
46
Genus Sarcocystis
Sarcocystis species are coccidian with a biphasic life
cycle: An intestinal (sexual) stage in gut mucosal cells of
carnivores, and an encysted tissue (asexual) stage in
muscle or other cells of herbivores animals. Humans serve
as both intermediate and final host depending on the
species. Human sarcocystosis develops from ingestion of
undercooked beef and pork meat.
Life cycle (figure 4)
Figure 4. Life cycle of sarcocystis (Human can serve as
intermediate host (with tissue sarcocystis) and as
definitive host (oocyst formed in the intestinal mucosa))
Clinical findings:
The infection causes no symptoms or signs in humans, but
sever symptoms may be developed in immunocompressed
person.heart failure may be attributed to sarcocystis.
Laboratory diagnosis:
Complement fixation test.
Treatment
: No effective treatment.
Genus Cyclospora
Cyclospra cayetanensis is an intestinal protozoan that
causes watery diarrhea in immunocompetent and
immunocompromised individuals
Transmission: through feco-oral route.
Diagnosis: By demonstration of spherical oocysts by
modified acid-fast stain of a stool sample.
Treatment: Trimetheprim- sulfamethoxazol
) (غير مطلوبMicrosporidia
Microsporidia are obligate intracellular spore forming
protozoa that infect many animals and cause disease in
humans, especially as opportunistic pathogens in AIDS. It
reproduces through formation of minute spores that have
polar tubules or filaments. Tubules are used to inject the
infective material (sporoplasm) into the host cells.
Microsporidia are members of a distinct phylum,
Microspora, which contains dozens of genera and
hundreds of species. The various microsporidia are
differentiated by their developmental life cycles, by
ultrastructural features, and by molecular taxonomy based
on ribosomal RNA. The complex life cycles of the
organisms result in the production of infectious spores.
Currently, eight genera of microsporidia—
Encephalitozoon,
Pleistophora, Nosema, Vittaforma, Trachipleistophora,
Brachiola, and Enterocytozoon and
Microsporidium. ) (لالطالع
Microsporidiosis is most common among patients with
AIDS, less common among patients with other types of
immunocompromise, and rare among immunocompetent
hosts.
Transmission:
1) Ingestion of spores in food or water.
2) Transplacental transmission
3) Ocular
4) Sexual.

Unit 2: Protozoa
47
Life cycle:
Figure 4. Life cycle of Microsporidia: The infective form
of microsporidia is the resistant spore and it can survive
for a long time in the environment
. The spore
extrudes its polar tubule and infects the host cell
. The
spore injects the infective sporoplasm into the eukaryotic
host cell through the polar tubule
. Inside the cell, the
sporoplasm undergoes extensive multiplication either by
merogony (binary fission) or schizogony (multiple
. This development can occur either in direct
contact with the host cell cytoplasm (e.g., E. bieneusi) or
inside a vacuole termed parasitophorous vacuole (e.g., E.
intestinalis). Either free in the cytoplasm or inside a
parasitophorous vacuole, microsporidia develop by
sporogony to mature spores
. During sporogony, a
thick wall is formed around the spore, which provides
resistance to adverse environmental conditions. When the
spores increase in number and completely fill the host cell
cytoplasm, the cell membrane is disrupted and releases
the spores to the surroundings
. These free mature
spores can infect new cells thus continuing the cycle.
Clinical features:
Depends on the site of infection, which includes
intestinal, ocular, muscular and systemic.
In patients with AIDS, intestinal infections with
Enterocytozoon bieneusi and Encephalitozoon (formerly
Septata) intestinalis are increasingly recognized to
contribute to chronic diarrhea and wasting; these
infections are found in 10 to 40% of patients with chronic
diarrhea.
Both organisms have been found in the biliary tracts of
patients with cholecystitis. E. intestinalis may also
disseminate to cause fever, diarrhea, sinusitis, cholangitis,
and bronchiolitis.
In patients with AIDS, Encephalitozoon hellem has
caused superficial keratoconjunctivitis as well as sinusitis,
respiratory tract disease, and disseminated infection.
Myositis due to Pleistophora has been documented.
Nosema, Vittaforma, and Microsporidium have caused
stromal keratitis associated with trauma in
immunocompetent patients.
Diagnosis:
Visualization of spores in stool samples or intestinal
biopsy samples.
Treatment:
Albendazole in treating intestinal and disseminated
infection.
Topical fumagillin for treatment of ocular infection
caused by Encephalitozoon hellem.
Unit 2: Protozoa
48
Pneumocystis ( )غير مطلوب
Pneumocystis jirovecii (jirovecii pronounced: yee row vet
zee) causes pneumonia in immunocompromised patients.
Before 2002, P.jirovecii was called P. carinii but after
2002, taxonomists renamed the human species of
pneumocystis as P.jirovecii and recommended that
P.carinii be used only to describe the rat species of
pneumocystis.
Until recently, P.jirovecii was thought to be a protozoan,
but molecular biologic studies have proved that it is a
fungus with a close relationship to ascomycetes.
Pneumocystis species are found in domestic animals such
as horses and sheep and in a variety of rodents, but it is
thought that these animals are not a reservoir for human
infection.
P.jirovecii has morphologically distinct forms (figure 5):
thin-walled trophozoites and cysts, which are thick,
walled, spherical to elliptical, and contain 4-8 nuclei.
P.jirovecii contains a surface glycoprotein that exhibit
antigenic variation.
It is an extracellular pathogen. Growth in the lung is
limited to the surfactant layer above the alveolar
epithelium.
Transmission: through inhalation.
Figure 5. Life cycle of P.jirovecii
Clinical features
In absence of immunosupression, P. jirovecii does not
cause disease. Pneumocystis is commonly found in the
lungs of healthy people. Most individual are infected in
early childhood.
In immunosupressed patient, it is presented with sudden
onset of
Fever, nonproductive cough, dyspnoea and tachypnoea
X-ray gives ground glass appearance (Figure 6).
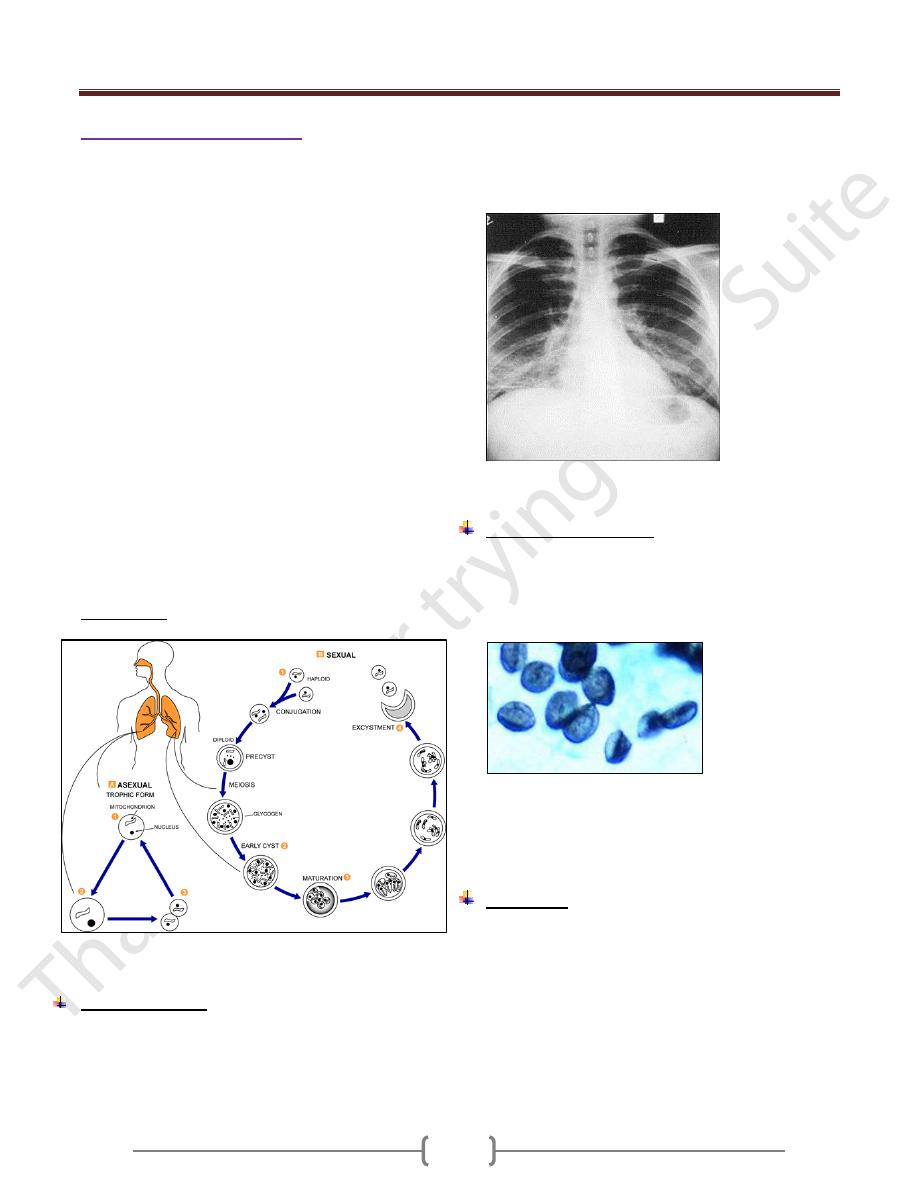
Figure 6 .Chest x-ray showing ground glass appearance
Laboratory Diagnosis
1) Cysts by microscopic examination of lung tissue or fluids
obtained by bronchoscopy, bronchial lavage, or open lung
biopsy. The cysts resemble crushed ping-pong balls and
are present in aggregates of 2 to 8 (Figure 7)
2)
Figure 7. Cysts of P.jirovecii
3) Fluorescent – antibody staining.
4) Polymerase chain reaction (PCR).
Treatment
Trimetheprim- sulfamethoxazole.
