
mild narcosis appear is about 120 feet. At this level the diver begins to exhibit
more and is breathing compressed air, the depth at which the first symptoms of
degrees of narcosis. When the diver remains beneath the sea for an hour or
no significant effect on bodily function, but at high pressures it can cause varying
About four fifths of the air is nitrogen. At sea-level pressure, the nitrogen has
physiologic effects at high pressures.
carbon dioxide;
, and
oxygen
The individual gases to which a diver is exposed when breathing air are
Effect of High Partial Pressures of Individual
10 liters.
a depth of 300 feet; this is the same
. For instance, we might speak of an actual volume of 1 liter at
causes serious damage.
can collapse the air chambers of the diver’s body, especially the lungs, and often
Boyle’s law
proportional to the pressure. This is a principle of physics called
liter. Thus, the volume to which a given quantity of gas is compressed is inversely
pressed to only one-half liter, and at 8 atmospheres (233 feet) to one-eighth
beneath the sea, where the pressure is 2 atmospheres, the volume has been com-
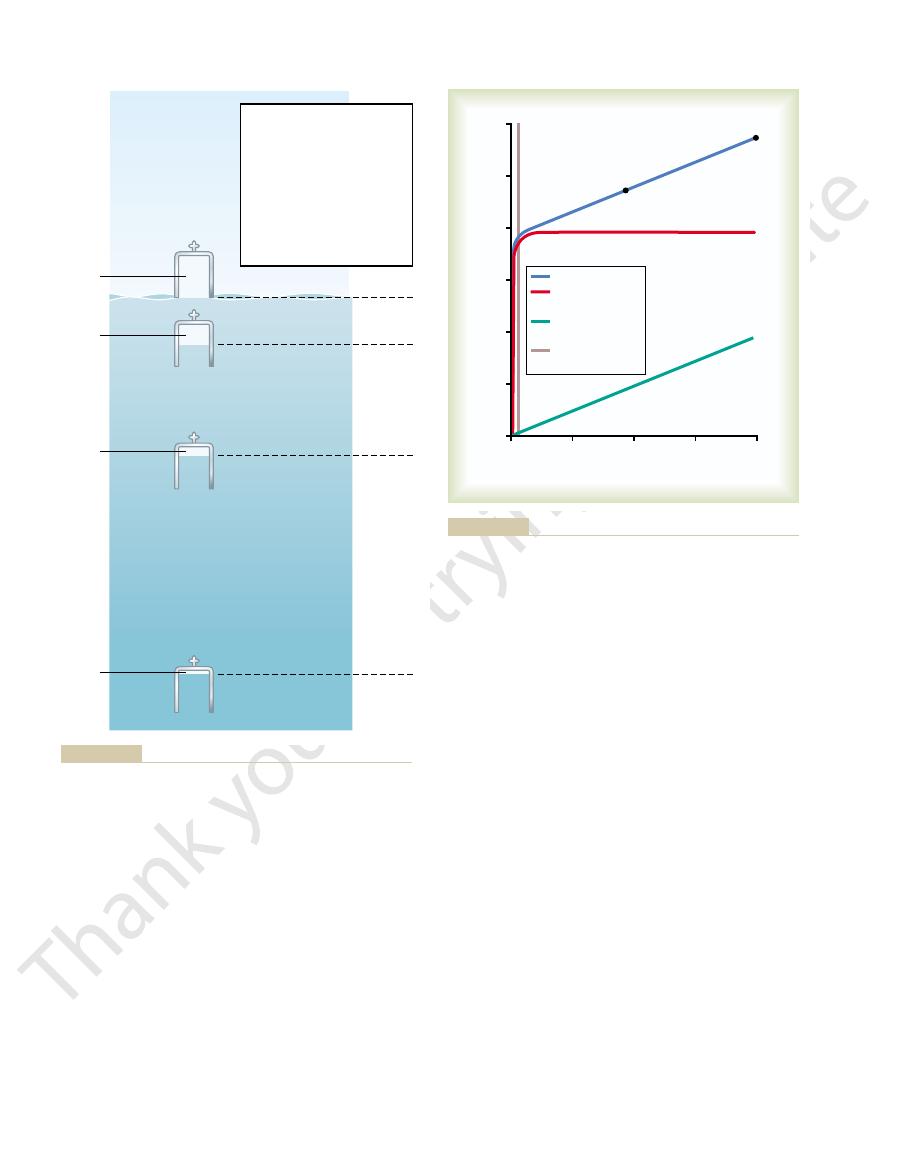
of Figure 44–1 shows a bell jar at sea level containing 1 liter of air. At 33 feet
depth is compression of gases to smaller and smaller volumes. The lower part
Effect of Sea Depth on the Volume of Gases-Boyle’s Law.
Figure 44–1.
feet the pressure is 3 atmospheres, and so forth, in accord with the table in
the water and the second atmosphere by the weight of the water itself. At 66
pheres pressure, 1 atmosphere of pressure caused by the weight of the air above
Therefore, a person 33 feet beneath the ocean surface is exposed to 2 atmos-
. Beyond certain limits, these high pressures
high alveolar gas pressure, a condition called
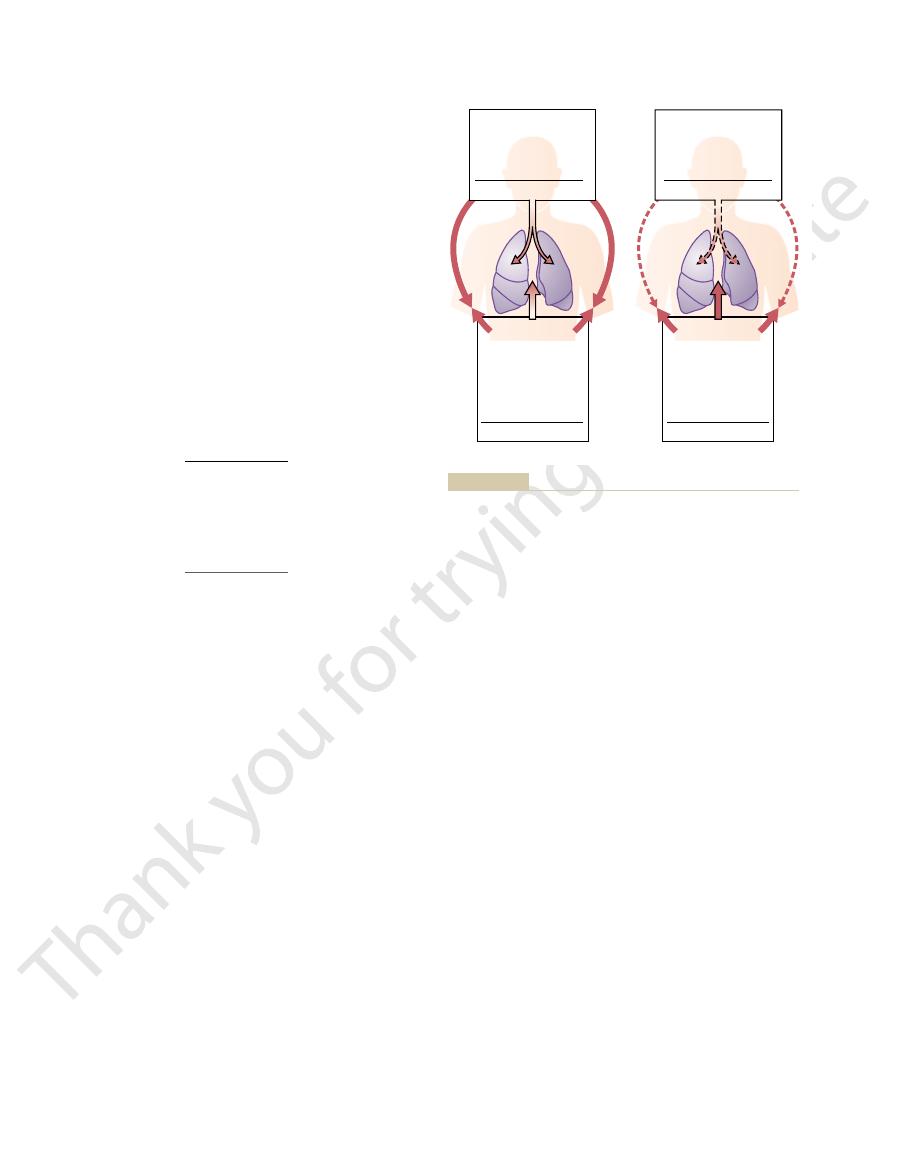
at very high pressure to keep them inflated. This
keep the lungs from collapsing, air must be supplied
pressure around them increases tremendously. To
When human beings descend beneath the sea, the
C
H
A
P
T
E
R
4
4
545
Physiology of Deep-Sea Diving
and Other Hyperbaric Conditions
exposes the blood in the lungs also to extremely
hyper-
barism
can cause tremendous alterations in body physiol-
ogy and can be lethal.
Relationship of Pressure to Sea Depth.
A column of seawater 33 feet deep exerts the
same pressure at its bottom as the pressure of the atmosphere above the sea.
Another important effect of
,
which is extremely important in diving physiology because increased pressure
Many times in this chapter it is necessary to refer to actual volume versus
sea-level volume
quantity of air as a sea-level volume of
Gases on the Body
nitro-
gen,
each of these at times can cause significant
Nitrogen Narcosis at High Nitrogen Pressures
5 milliliters from each 100 milliliters of blood, the
tissues use their normal amount of oxygen, about
volumes per cent dissolved in the blood water. As this
demonstrated by point A in the figure—this means
milliliters of blood of about 29 volumes per cent, as
pheres pressure). Referring to Figure 44–2, one finds
in the lungs is about 3000 mm Hg (4 atmos-
on Tissue P
then dissolved in the water of the blood, in addition to
ters of mercury, a large portion of the total oxygen is
blood is accounted for by dissolved oxygen, but as the
120 mm Hg), almost none of the total oxygen in the
volume of oxygen
extended to more than 3000 mm Hg. Also depicted by
markedly. This is shown in Figure 44–2, which depicts
in the blood rises above 100 mm Hg, the amount
When the
on Blood Oxygen Transport.
Effect of Very High P
Oxygen Toxicity at High Pressures
neuronal excitability.
ionic conductance through the membranes, reduces
branes and, because of its
same as that of most other gas anesthetics. That is, it
frequently been called “raptures of the depths.” The
of alcohol intoxication, and for this reason it has
too long.
required. Beyond 250 feet (8.5 atmospheres pressure),
feet, his or her strength wanes considerably, and the
to 200 feet, the diver becomes drowsy. At 200 to 250
joviality and to lose many of his or her cares. At 150
Aviation, Space, and Deep-Sea Diving Physiology
546
Unit VIII
diver often becomes too clumsy to perform the work
the diver usually becomes almost useless as a result of
nitrogen narcosis if he or she remains at these depths
Nitrogen narcosis has characteristics similar to those
mechanism of the narcotic effect is believed to be the
dissolves in the fatty substances in neuronal mem-
physical effect on altering
O
2
Po
2
of oxygen dissolved in the water of the blood increases
the same oxygen-hemoglobin dissociation curve as
that shown in Chapter 40 but with the alveolar Po
2
the lowest curve in the figure is the
dissolved in the fluid of the blood at each Po
2
level.
Note that in the normal range of alveolar Po
2
(below
oxygen pressure rises into the thousands of millime-
that bound with hemoglobin.
Effect of High Alveolar P
O
2
O
2
.
Let us assume that
the Po
2
that this represents a total oxygen content in each 100
20 volumes per cent bound with hemoglobin and 9
blood passes through the tissue capillaries and the
Depth (feet)
Sea level
33
66
100
133
166
200
300
400
500
Atmosphere(s)
1 liter
Sea level
33 ft
1
/
2
liter
1
/
4
liter
1
/
8
liter
1
2
3
4
5
6
7
10
13
16
100 ft
233 ft
Effect of sea depth on pressure (
Figure 44–1
top table) and on gas volume
(bottom).
1560
2280
3040
Total O
0
760
2
in blood
Oxygen
poisoning
Combined with
hemoglobin
Oxygen-hemoglobin dissociation curve
B
A
Dissolved in
water of blood
Normal alveolar
oxygen pressure
Oxygen partial pressure in lungs (mm Hg)
Oxygen in blood (volumes per cent)
0
5
10
15
20
25
30
bination with hemoglobin at very high P
Figure 44–2
Quantity of oxygen dissolved in the fluid of the blood and in com-
O
2
s.

And over several more hours, enough nitrogen is
high pressure as that in the alveolar breathing mixture.
lowing: Blood flowing through the pulmonary capil-
body fluids increases. The reason for this is the fol-
long time, the amount of nitrogen dissolved in the
When a person breathes air under high pressure for a
Decompression of the Diver After
lethargy, narcosis, and finally even anesthesia, as dis-
severe respiratory acidosis, and varying degrees of
than to compensate. In addition, the diver develops
. The diver’s respiration then begins to fail rather
center begins to be depressed, rather than excited,
becomes intolerable, and eventually the respiratory
, the situation
to compensate for the increased carbon dioxide.
about 80 mm Hg, twice that in normal alveoli, the
air of the apparatus and be rebreathed by the diver.
tuses, carbon dioxide can build up in the dead space
In certain types of diving gear, however, such as the
normal value.
and expires the carbon dioxide as it is formed, alveo-
carbon dioxide production in the body, and as long as
the carbon dioxide partial pressure in the alveoli. This
properly, the diver has no problem due to carbon
Depths in the Sea
Carbon Dioxide Toxicity at Great
are directly exposed to the high oxygen pressure, but
to develop. The reason for this effect in the lungs but
, and
lung passageway con-
1 atmosphere oxygen exposure,
just described. However, after only about 12 hours of
ity.
Therefore, most of the acute lethal effects of acute
metabolic systems. The nervous tissues are especially
cellular enzymes, thus damaging severely the cellular
membranes. Another effect is to oxidize some of the
tive and even lethal effects on the cells. One of the
remove them, and now they can have serious destruc-
these high levels, the amounts of oxidizing free radi-
hundreds or thousands of millimeters of mercury. At
mechanism fails, and the tissue P
), the hemoglobin-oxygen buffering
(above about 2
that they have little or no effect in the tissues.
. Therefore, so long as the hemoglobin-oxygen
superoxide dis-
, and
peroxidases
enzymes that rapidly remove these free radicals,
oxygen. Fortunately, the tissues also contain multiple
40 mm Hg, small amounts of free radicals are contin-
ide. Even when the tissue P
, and another
. One of the most important
oxygen free radicals
oxygen. There are several forms of active oxygen
must first be converted into an “active” form of
of oxidizing other chemical compounds. Instead, it
Nervous System Oxygen Toxicity—“Oxidizing Free
oxygen toxicity, causing symptoms to appear much
Exercise greatly increases the diver’s susceptibility to
disturbances of vision, irritability, and disorientation.
soning include nausea, muscle twitchings, dizziness,
occur without warning and, for obvious reasons, are
people within 30 to 60 minutes. The seizures often
in most
seizures followed by coma
3040 mm Hg)
the body’s tissues. For instance, breathing oxygen at
The extremely high tissue P
between 20 and 60 mm Hg.
in the normal, safe range
a critical level, the hemoglobin-oxygen buffer mecha-
of 40 mm Hg. Thus, once the alveolar P
is approximately 1200 mm Hg, which
point, the P
24 volumes per cent (point B in the figure). At this
Chapter 44
Physiology of Deep-Sea Diving and Other Hyperbaric Conditions
547
oxygen content on leaving the tissue capillaries is still
o
2
means that oxygen is delivered to the tissues at this
extremely high pressure instead of at the normal value
o
2
rises above
nism (discussed in Chapter 40) is no longer capable
of keeping the tissue Po
2
Acute Oxygen Poisoning.
o
2
that occurs when oxygen is breathed at very high alve-
olar oxygen pressure can be detrimental to many of
4 atmospheres pressure of oxygen (Po
2
=
will cause brain
likely to be lethal to divers submerged beneath the
sea.
Other symptoms encountered in acute oxygen poi-
earlier and with far greater severity than in the resting
person.
Excessive Intracellular Oxidation as a Cause of
Radicals.”
Molecular oxygen (O
2
) has little capability
called
of these is the superoxide free radical O
2
_
is the peroxide radical in the form of hydrogen perox-
o
2
is normal at the level of
ually being formed from the dissolved molecular
including
, catalases
mutases
buffering mechanism maintains a normal tissue Po
2
,
the oxidizing free radicals are removed rapidly enough
Above a critical alveolar Po
2
atmospheres Po
2
o
2
can then rise to
cals literally swamp the enzyme systems designed to
principal effects is to oxidize the polyunsaturated fatty
acids that are essential components of many of the cell
susceptible because of their high lipid content.
oxygen toxicity are caused by brain dysfunction.
Chronic Oxygen Poisoning Causes Pulmonary Disabil-
A person can be exposed to only 1 atmosphere
pressure of oxygen almost indefinitely without devel-
oping the acute oxygen toxicity of the nervous system
gestion, pulmonary edema
atelectasis caused by
damage to the linings of the bronchi and alveoli begin
not in other tissues is that the air spaces of the lungs
oxygen is delivered to the other body tissues at almost
normal Po
2
because of the hemoglobin-oxygen buffer
system.
If the diving gear is properly designed and functions
dioxide toxicity because depth alone does not increase
is true because depth does not increase the rate of
the diver continues to breathe a normal tidal volume
lar carbon dioxide pressure will be maintained at a
diving helmet and some types of rebreathing appara-
Up to an alveolar carbon dioxide pressure (Pco
2
) of
diver usually tolerates this buildup by increasing the
minute respiratory volume a maximum of 8- to 11-fold
Beyond 80-mm Hg alveolar Pco
2
because of the negative tissue metabolic effects of high
Pco
2
cussed in Chapter 42.
Excess Exposure to High Pressure
laries becomes saturated with nitrogen to the same
coalesce, progressively larger vessels are affected.
are blocked by minute bubbles, but as the bubbles
different tissues. At first, only the smallest vessels
The symptoms of decompression sickness are caused
state for hours before bubbling.
gases can remain dissolved in the “supersaturated”
for many minutes to hours, because sometimes the
many small blood vessels. The bubbles may not appear
actual bubbles, composed almost entirely of nitrogen,
Hg pressure on the outside of the body. Therefore, the
value of 4065 mm Hg is far greater than the 760 mm
which is caused by the nitrogen. Obviously, this total
nitrogen, or a total of 4065 mm Hg, 97 per cent of
pressures of water vapor, carbon dioxide, oxygen, and
becomes only 1 atmosphere (760 mm Hg), while the
), the pressure on the outside of the body
when the diver suddenly rises to sea level (Figure
ciently to keep the excess nitrogen gas dissolved. But
(5000 mm Hg) compresses all the body tissues suffi-
As long as the diver remains deep beneath the sea, the
times the normal amount of nitrogen in the tissues.
3918 mm Hg), about 6.5
in water.
little more than one half in the fat of the body. This is
is dissolved in the entire body. Slightly less than one
At sea level, almost exactly 1 liter of nitrogen
Volume of Nitrogen Dissolved in the Body Fluids at Different
sickness
by the reverse respiratory process; however, this
lower level, at which time the nitrogen can be removed
Because nitrogen is not metabolized by the body, it
in the breathing air.
Aviation, Space, and Deep-Sea Diving Physiology
548
Unit VIII
carried to all the tissues of the body to raise their tissue
Pn
2
also to equal the Pn
2
remains dissolved in all the body tissues until the nitro-
gen pressure in the lungs is decreased back to some
removal often takes hours to occur and is the source
of multiple problems collectively called decompression
.
Depths.
half of this is dissolved in the water of the body and a
true because nitrogen is five times as soluble in fat as
After the diver has become saturated with nitrogen,
the sea-level volume of nitrogen dissolved in the body
at different depths is as follows:
nitrogen pressure (Pn
2
=
pressure against the outside of his or her body
44–3B
gas pressure inside the body fluids is the sum of the
gases can escape from the dissolved state and form
both in the tissues and in the blood where they plug
Symptoms of Decompression Sickness (“Bends”).
by gas bubbles blocking many blood vessels in
200
7
100
4
33
2
Feet
Liters
0
1
, the diver’s
shown in Figure 44–3. In Figure 44–3
The principles underlying bubble formation are
decompression sickness
number and sizes of bubbles formed; this is called
in almost any area of the body, depending on the
of the sea, significant quantities of nitrogen bubbles
Sickness, Caisson Disease, Diver’s Paralysis, Dysbarism).
deep level for several hours, both the body water and
fluids and tissues, whereas if the person remains at a
few minutes, not much nitrogen dissolves in the body
reason, if a person remains at deep levels for only a
reaches equilibrium only after several hours. For this
nitrogen and having a relatively poor blood supply,
the fat tissue, requiring five times as much transport of
to almost complete equilibrium in less than 1 hour, but
The nitrogen dissolved in the water of the body comes
The reason for this is that the blood does not flow
300
10
Several hours are required for the gas pressures of
nitrogen in all the body tissues to come nearly to equi-
librium with the gas pressure of nitrogen in the alveoli.
rapidly enough and the nitrogen does not diffuse
rapidly enough to cause instantaneous equilibrium.
body fat become saturated with nitrogen.
Decompression Sickness (Synonyms: Bends, Compressed Air
If a
diver has been beneath the sea long enough that large
amounts of nitrogen have dissolved in his or her body
and the diver then suddenly comes back to the surface
can develop in the body fluids either intracellularly or
extracellularly and can cause minor or serious damage
.
A
tissues have become equilibrated to a high dissolved
= 3918
= 60
= 3918
= 60
Before
decompression
Pressure Outside Body
A
B
O
2
= 1044 mm Hg
N
2
= 3956
Total = 5000 mm Hg
After sudden
decompression
O
2
= 159 mm Hg
N
2
= 601
Total = 760 mm Hg
Total = 4065
Body
Gaseous pressure
in the body fluids
H
2
O = 47 mm Hg
CO
2
= 40
O
2
N
2
Total = 4065
Body
Gaseous pressure
in the body fluids
H
2
O = 47 mm Hg
CO
2
= 40
O
2
N
2
760 mm Hg.
suddenly returned from 5000 mm Hg to normal pressure of
the tissues when the lung intra-alveolar pressure body is
intra-body pressures that are responsible for bubble formation in
) the great excesses of
at a total pressure of 5000 mm Hg, and (
saturation of the body to high gas pressures when breathing air
Gaseous pressures both inside and outside the body, showing (
Figure 44–3
A)
B

flow continually into the mask. Instead, with each
water pressure. The breathing mixture does not
sure only a few mm Hg greater than the surrounding
The demand system operates as follows: The first-
small “dead space.”
water pressure, and (4) a mask and tube system with
tion “demand” valve and exhalation valve that allows
tanks to a low pressure level, (3) a combination inhala-
ing” valve for reducing the very high pressure from the
some other breathing mixture, (2) a first-stage “reduc-
ponents: (1) one or more tanks of compressed air or
Figure 44–4. This system consists of the following com-
apparatus. The type of SCUBA apparatus used in
, known as the SCUBA
1943, Jacques Cousteau popularized a
was pumped to the diver from the surface. Then, in
Before the 1940s, almost all diving was done using a
Underwater Breathing
30 minutes.
to the lungs of more than 4 atmospheres,
the oxygen required by the diver, whereas a 21 per
pressure), a 1 per cent oxygen mixture will provide all
instance, at a depth of 700 feet (22 atmospheres of
because otherwise oxygen toxicity would result. For
Finally, in very deep dives it is important to reduce
breathing beyond endurance.
can become extreme, sometimes making the work of
at a minimum, which is very important because highly
reducing the problem of decompression sickness; and
sion several times as rapidly as does nitrogen, thus
the body tissues as nitrogen, and the volume that does
about one fifth the narcotic effect of nitrogen; (2) only
instead of nitrogen for three reasons: (1) it has only
diving, helium is usually used in the gas mixture
In very deep dives, especially during saturation
so that decompression bubbles do not occur.
working, there are no significant changes in pressure,
Then, when they return to the same tank after
the gases to which they will be exposed while diving.
level near that at which they will be working. This
weeks at a time, remaining compressed at a pressure
When divers must work at very deep levels—
is recompressed immediately to a deep level. Then
have returned to the surface. In this case, the diver
Tank decompression is even more important for
essentially the same time schedule as noted above.
ually back to normal atmospheric pressure, using
Tank Decompression and Treatment of Decompression Sick-
3 hours.
1 hour, the total time for decompression is about
Thus, for a work period on the bottom of only
process, a diver who has been breathing air and has
sion. To give the student an idea of the decompression
U.S. Navy that detail procedures for safe decompres-
erated in 1 hour and about 90 per cent in 6 hours.
sickness. About two thirds of the total nitrogen is lib-
a diver is brought to the surface slowly, enough of the
Nitrogen Elimination from the Body; Decompression Tables.
and, occasionally, death.
breath, often followed by severe pulmonary edema
the lungs; this is characterized by serious shortness of
sion sickness develop “the chokes,” caused by massive
Finally, about 2 per cent of people with decompres-
The paralysis may be temporary, but in some instances,
sickness, nervous system symptoms occur, ranging
accounts for the term “bends” that is often applied to
who develop decompression sickness. The joint pain
and arms, affecting 85 to 90 per cent of those persons
In most people with decompression sickness, the
Tissue ischemia and sometimes tissue death are the
Chapter 44
Physiology of Deep-Sea Diving and Other Hyperbaric Conditions
549
result.
symptoms are pain in the joints and muscles of the legs
this condition.
In 5 to 10 per cent of people with decompression
from dizziness in about 5 per cent to paralysis or col-
lapse and unconsciousness in as many as 3 per cent.
damage is permanent.
numbers of microbubbles plugging the capillaries of
If
dissolved nitrogen can usually be eliminated by expi-
ration through the lungs to prevent decompression
Decompression tables have been prepared by the
been on the sea bottom for 60 minutes at a depth of
190 feet is decompressed according to the following
schedule:
10 minutes at 50 feet depth
17 minutes at 40 feet depth
19 minutes at 30 feet depth
50 minutes at 20 feet depth
84 minutes at 10 feet depth
ness.
Another procedure widely used for decompres-
sion of professional divers is to put the diver into a
pressurized tank and then to lower the pressure grad-
treating people in whom symptoms of decompression
sickness develop minutes or even hours after they
decompression is carried out over a period several
times as long as the usual decompression period.
“Saturation Diving” and Use of Helium-Oxygen Mixtures in Deep
Dives.
between 250 feet and nearly 1000 feet—they fre-
quently live in a large compression tank for days or
keeps the tissues and fluids of the body saturated with
about one half as much volume of helium dissolves in
dissolve diffuses out of the tissues during decompres-
(3) the low density of helium (one seventh the density
of nitrogen) keeps the airway resistance for breathing
compressed nitrogen is so dense that airway resistance
the oxygen concentration in the gaseous mixture
cent mixture of oxygen (the percentage in air) deliv-
ers a Po
2
a level very likely to cause seizures in as little as
Scuba (Self-Contained
Apparatus) Diving
diving helmet connected to a hose through which air
self-contained
underwater breathing apparatus
more than 99 per cent of all sports and commercial
diving is the open-circuit demand system shown in
air to be pulled into the lungs with slight negative pres-
sure of breathing and then to be exhaled into the sea
at a pressure level slightly positive to the surrounding
stage reducing valve reduces the pressure from the
tanks so that the air delivered to the mask has a pres-

include decompression sickness, arterial gas embolism,
instances by early treatment with hyperbaric therapy.
70 mm Hg. Therefore, hyperbaric oxygenation of the
, grow best under anaerobic conditions and
. The
been especially beneficial follow.
at least some of the therapeutic benefits. Some of the
tracheal tube, whereas the gas around the body is
The oxygen is usually administered at P
conditions. Therefore, large pressure tanks are now
hyperbaric oxygen
The intense oxidizing properties of high-pressure
toxicity.
sion, even freon gas has been found to diffuse out of
to cause carbon monoxide poisoning. And, on occa-
enough carbon monoxide, if not removed rapidly,
gence, cigarette smoking by the crew can liberate
rapidly. For instance, during several weeks’ submer-
Second, poisonous gases on occasion escape into the
received above the surface of the sea from cosmic rays.
diation hazards, but with appropriate shielding, the
atomic submarines, there exists the problem of ra-
hazards out of the internal environment. First, in
Except for escape, submarine medicine generally
to exhale continually.
person ascends, he or she must make a special effort
cause air embolism of the circulation. Therefore, as the
blood vessel, forcing the gases to enter the vessel and
of air embolism. As the person ascends, the gases in
more.
devices, especially when using helium, theoretically
apparatus. However, proper use of rebreathing
to escape from a submerged submarine. Escape is
tion to submarines, especially when it is necessary
Special Physiologic Problems
have been compressed to small sizes.
air per minute that is required, because the
depth, the greater the airflow in terms of
ble at a 200-foot depth. The reason for this is that
sea surface; for instance, only a few minutes are possi-
The most important problem in use of the self-
Then, on expiration, the air cannot go back into the
the tank into the mask and lungs. In this way, only the
valve open, and this automatically releases air from
inspiration, slight extra negative pressure in the
Aviation, Space, and Deep-Sea Diving Physiology
550
Unit VIII
demand valve of the mask pulls the diaphragm of the
amount of air needed for inhalation enters the mask.
tank but instead is expired into the sea.
contained underwater breathing apparatus is the
limited amount of time one can remain beneath the
tremendous airflow from the tanks is required to wash
carbon dioxide out of the lungs—the greater the
quantity of
volumes
in Submarines
Escape from Submarines.
Essentially the same problems
encountered in deep-sea diving are often met in rela-
possible from as deep as 300 feet without using any
can allow escape from as deep as 600 feet or perhaps
One of the major problems of escape is prevention
the lungs expand and sometimes rupture a pulmonary
Health Problems in the Submarine Internal Environment.
centers around several engineering problems to keep
amount of radiation received by the crew submerged
beneath the sea has been less than normal radiation
atmosphere of the submarine and must be controlled
refrigeration systems in sufficient quantity to cause
Hyperbaric Oxygen Therapy
oxygen (
) can have valuable
therapeutic effects in several important clinical
available in many medical centers into which patients
can be placed and treated with hyperbaric oxygen.
o
2
s of 2 to
3 atmospheres of pressure through a mask or intra-
normal air compressed to the same high-pressure
level.
It is believed that the same oxidizing free radicals
responsible for oxygen toxicity are also responsible for
conditions in which hyperbaric oxygen therapy has
Probably the most successful use of hyperbaric
oxygen has been for treatment of gas gangrene
bacteria that cause this condition, clostridial organ-
isms
stop growing at oxygen pressures greater than about
tissues can frequently stop the infectious process
entirely and thus convert a condition that formerly was
almost 100 per cent fatal into one that is cured in most
Other conditions in which hyperbaric oxygen
therapy has been either valuable or possibly valuable
Mask
Demand valve
First stage
valve
Hose
Air cylinders
Open-circuit demand type of SCUBA apparatus.
Figure 44–4

keling in adult life. Respir Physiol Neurobiol 138:325,
West JB, Fu Z, Gaeth AP, Short RV: Fetal lung development
48:226, 2002
agement of musculoskeletal disorders. J Postgrad Med
Wang J, Li F, Calhoun JH, Mader JT: The role and effective-
literature. Arch Surg 138:272, 2003.
oxygen for treating wounds: a systematic review of the
Wang C, Schwaitzberg S, Berliner E, et al: Hyperbaric
53(Suppl 2):S20, 1998.
Russi EW: Diving and the risk of barotrauma. Thorax
Physiol Sci 16:217, 2001.
Nilsson GE: Surviving anoxia with the brain turned on. News
ness. News Physiol Sci 17:77, 2002.
Neuman TS: Arterial gas embolism and decompression sick-
therapy. BMJ 317:1140, 1998.
Leach RM, Rees PJ, Wilmshurst P: Hyperbaric oxygen
to depth: birds and mammals. Annu Rev Physiol 60:19,
Kooyman GL, Ponganis PJ: The physiological basis of diving
Butler PJ: Diving beyond the limits. News Physiol Sci 16:222,
carbon monoxide poisoning,
osteomyelitis,
and
Chapter 44
Physiology of Deep-Sea Diving and Other Hyperbaric Conditions
551
myocardial infarction.
References
2001.
1998.
ness of adjunctive hyperbaric oxygen therapy in the man-
in the elephant reflects the adaptations required for snor-
2003.
