
secretion is associated with energy abundance. That is, when there is great
As we discuss insulin in the next few pages, it will become apparent that insulin
Insulin Is a Hormone Associated with Energy Abundance
cellular functional disorders. Therefore, it is clear that insulin affects fat and
of death in diabetic patients. Also, in patients with prolonged diabetes, dimin-
causing such conditions as acidosis and arteriosclerosis, that are the usual causes
effects on carbohydrate metabolism. Yet it is abnormalities of fat metabolism,
has been associated with “blood sugar,” and true enough, insulin has profound
of rapid decline and death to that of a nearly normal person. Historically, insulin
Insulin was first isolated from the pancreas in 1922 by Banting and Best, and
Insulin and Its Metabolic Effects
inhibits insulin secretion, and somatostatin inhibits the secretion of both insulin and
by the other hormones. For instance, insulin inhibits glucagon secretion, amylin
The close interrelations among these cell types in the islets of Langerhans allow
pancreatic polypeptide.
the PP cell, is present in small numbers in the islets and secretes a hormone of uncer-
In addition, at least one other type of cell,
cent of the total, secrete
And the delta cells, about 10 per
about 25 per cent of the total, secrete
secreted in parallel with insulin, although its function is unclear. The alpha cells,
, a hormone that is often
The beta cells, constituting about 60 per cent of all the cells of the islets, lie mainly
staining characteristics.
cells, which are distinguished from one another by their morphological and
, and
secrete their hormones. The islets contain three major types of cells,
The human pancreas has 1 to 2 million islets of Langerhans, each only about 0.3
directly into the blood. The digestive secretions of the pancreas are discussed in
, which secrete insulin and glucagon
duodenum, and (2) the
, which secrete digestive juices into the
tissues, as shown in Figure 78–1: (1) the
The pancreas is composed of two major types of
, caused by abnormal secretion or activity of these hormones.
insulin and glucagon and the pathophysiology of diseases, especially
tions are not as well established. The main purpose
, their func-
, and
pancreas secretes other hormones, such as
glucose, lipid, and protein metabolism. Although the
, that are crucial for normal regulation of
secretes two important hormones,
The pancreas, in addition to its digestive functions,
Insulin, Glucagon, and
C
H
A
P
T
E
R
7
8
961
Diabetes Mellitus
insulin and
glucagon
amylin,
somatostatin
pancreatic polypeptide
of this chapter is to discuss the physiologic roles of
diabetes
mellitus
Physiologic Anatomy of the Pancreas.
acini
islets of Langerhans
Chapter 64.
millimeter in diameter and organized around small capillaries into which its cells
alpha, beta
delta
in the middle of each islet and secrete insulin and amylin
glucagon.
somatostatin.
tain function called
cell-to-cell communication and direct control of secretion of some of the hormones
glucagon.
almost overnight the outlook for the severely diabetic patient changed from one
ished ability to synthesize proteins leads to wasting of the tissues as well as many
protein metabolism almost as much as it does carbohydrate metabolism.

cussed in Chapter 74. Autophosphorylation of the beta
, dis-
become autophosphorylated. Thus, the insulin recep-
but because of the linkages with the beta subunits, the
brane, protruding into the cell cytoplasm. The insulin
The insulin receptor is a combination of four sub-
the subsequent effects.
It is the activated receptor, not the insulin, that causes
has a molecular weight of about 300,000 (Figure 78–3).
To initiate its effects on target cells, insulin first binds
and the Resulting Cellular Effects
Activation of Target Cell Receptors by Insulin
the plasma is important, because, at times, it is as
slightly in most other tissues. This rapid removal from
liver, to a lesser extent in the kidneys and muscles, and
der is degraded by the enzyme
combines with receptors in the target cells, the remain-
minutes. Except for that portion of the insulin that
half-life that averages only about 6 minutes, so that it
almost entirely in an unbound form; it has a plasma
When insulin is secreted into the blood, it circulates
tually no insulin activity.
is still in the form of proinsulin. The proinsulin has vir-
However, about one sixth of the final secreted product
before being packaged in the secretory granules.
of about 9000; most of this is further cleaved in the
11,500, but it is then cleaved in the endoplasmic retic-
. This
RNA by ribosomes attached to the endoplasmic re-
Chapter 3, beginning with translation of the insulin
cell machinery for protein synthesis, as explained in
are split apart, the functional activity of the insulin
by disulfide linkages. When the two amino acid chains
chains, shown in Figure 78–2, connected to each other
ular weight of 5808. It is composed of two amino acid
Insulin is a small protein; human insulin has a molec-
Insulin Chemistry and Synthesis
down of the proteins that are already in the cells.
acids into protein. In addition, it inhibits the break-
teins, insulin has a direct effect in promoting amino
fats and stored in the adipose tissue. In the case of pro-
glycogen mainly in the liver and muscles. Also, all the
of excess carbohydrates, it causes them to be stored as
important role in storing the excess energy. In the case
secreted in great quantity. In turn, the insulin plays an
cially excess amounts of carbohydrates, insulin is
abundance of energy-giving foods in the diet, espe-
962
Unit XIV
Endocrinology and Reproduction
excess carbohydrates that cannot be stored as glyco-
gen are converted under the stimulus of insulin into
acid uptake by cells and conversion of these amino
molecule is lost.
Insulin is synthesized in the beta cells by the usual
ticulum to form an insulin preprohormone
initial preprohormone has a molecular weight of about
ulum to form a proinsulin with a molecular weight
Golgi apparatus to form insulin and peptide fragments
is mainly cleared from the circulation within 10 to 15
insulinase mainly in the
important to turn off rapidly as to turn on the control
functions of insulin.
with and activates a membrane receptor protein that
units held together by disulfide linkages: two alpha
subunits that lie entirely outside the cell membrane
and two beta subunits that penetrate through the mem-
binds with the alpha subunits on the outside of the cell,
portions of the beta subunits protruding into the cell
tor is an example of an enzyme-linked receptor
subunits of the receptor activates a local tyrosine
Islet of
Langerhans
Pancreatic
acini
Delta cell
Alpha cell
Beta cell
Red blood cells
Physiologic anatomy of an islet of Langerhans in the pancreas.
Figure 78–1
Lys
Tyr
Val
Tyr
Val
Val
Tyr
Tyr
Val
Gly•Ileu•
•Glu•Glu•Cy•Cy•Thr•Ser•Ileu•Cy•Ser•Leu•
•Glu•Leu•Glu•Asp•
•Cy•Asp
Phe•
•Asp•Glu•His•Leu•Cy•Gly•Ser•His•Leu•
•Glu•Ala•Leu•
•Leu•
•Cy•Gly•Glu•Arg•Gly•Phe•Phe•
•Thr•Pro•
•Thr
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
S
S
S
S
S
Figure 78–2
Human insulin molecule.

into the muscle cells in abundance, then most of the
over fatty acids, as we discuss later.
glucose into the muscle cells. This causes the muscle
insulin. The extra insulin causes rapid transport of
meal. At this time the blood glucose concentration is
The second condition for muscle usage of large
process itself.
require large amounts of insulin, because exercising
erate or heavy exercise. This usage of glucose does not
large amounts of glucose. One of these is during mod-
However, under two conditions the muscles do use
into the muscle cells.
meals, the amount of insulin that is secreted is too
when the muscle fiber is stimulated by insulin; between
brane is only slightly permeable to glucose, except
glucose for its energy but on fatty acids. The principal
During much of the day, muscle tissue depends not on
Insulin Promotes Muscle Glucose Uptake
liver.
body, but especially by the muscles, adipose tissue, and
storage, and use of glucose by almost all tissues of the
in the chapter. The insulin in turn causes rapid uptake,
secretion of insulin, which is discussed in detail later
Immediately after a high-carbohydrate meal, the
Effect of Insulin on Carbohydrate
to achieve its metabolic goals.
DNA in the cell nucleus. In this way, insulin
rates of translation of messenger RNAs at the
and even several days. They result from changed
4. Much slower effects continue to occur for hours
phosphorylation of the enzymes.
more intracellular metabolic enzymes. These
3. Slower effects occur during the next 10 to 15
phosphate ions, causing increased transport of
many of the amino acids, potassium ions, and
2. The cell membrane becomes more permeable to
the cells. When insulin is no longer available, these
of glucose transport proteins, which bind with the
vesicles to the cell membranes; these vesicles
the usual carbohydrate metabolic functions. The
. The increased glucose
their uptake of glucose. This is especially true of
80 per cent of the body’s cells markedly increase
membrane receptors, the membranes of about
1. Within seconds after insulin binds with its
protein metabolism. The end effects of insulin stimu-
produce the desired effects on carbohydrate, fat, and
of these enzymes while inactivating others. In this way,
in different tissues. The net effect is to activate some
types of IRS (e.g. IRS-1, IRS-2, IRS-3) are expressed
. Different
, which in turn causes phosphorylation of multi-
Insulin, Glucagon, and Diabetes Mellitus
Chapter 78
963
kinase
ple other intracellular enzymes including a group
called insulin-receptor substrates (IRS)
insulin directs the intracellular metabolic machinery to
lation are the following:
muscle cells and adipose cells but is not true of
most neurons in the brain
transported into the cells is immediately
phosphorylated and becomes a substrate for all
increased glucose transport is believed to result
from translocation of multiple intracellular
carry in their own membranes multiple molecules
cell membrane and facilitate glucose uptake into
vesicles separate from the cell membrane within
about 3 to 5 minutes and move back to the cell
interior to be used again and again as needed.
these substances into the cell.
minutes to change the activity levels of many
effects result mainly from the changed states of
ribosomes to form new proteins and still slower
effects from changed rates of transcription of
remolds much of the cellular enzymatic machinery
Metabolism
glucose that is absorbed into the blood causes rapid
and Metabolism
reason for this is that the normal resting muscle mem-
small to promote significant amounts of glucose entry
muscle fibers become more permeable to glucose even
in the absence of insulin because of the contraction
amounts of glucose is during the few hours after a
high and the pancreas is secreting large quantities of
cell during this period to use glucose preferentially
Storage of Glycogen in Muscle.
If the muscles are not
exercising after a meal and yet glucose is transported
glucose is stored in the form of muscle glycogen
Tyrosine
Tyrosine
S S
S
b
a
a
b
S
S
Cell membrane
Glucose
Insulin
receptor
Insulin
kinase
kinase
S
Insulin receptor substrates (IRS)
Phosphorylation of enzymes
Glucose
transport
Fat
synthesis
Protein
synthesis
Glucose
synthesis
Growth
and gene
expression
porters are moved to the cell membrane to facilitate glucose entry
glucose, fat, and protein metabolism. For example, glucose trans-
insulin receptor substrates, that mediate the effects of glucose on
tion that increases or decreases the activity of enzymes, including
tor tyrosine kinase activity begins a cascade of cell phosphoryla-
receptor, which in turn induces tyrosine kinase activity. The recep-
its receptor, which causes autophosphorylation of the
Schematic of the insulin receptor. Insulin binds to the
Figure 78–3
a-subunit of
b-subunit
into the cell.

. These fatty
cyte metabolism,
When the quan-
and Inhibits Gluconeogenesis in the Liver.
and then returned later.
falls between meals. Ordinarily, about 60 per cent of
Thus, the liver removes glucose from the blood
split away from the glucose; this allows the free
inhibited by insulin, now becomes activated by the
, which had been
4. The enzyme
, which causes the splitting
glucagon, which is discussed later) activates the
3. The lack of insulin (along with increase of
listed earlier for glycogen storage, essentially
2. The lack of insulin then reverses all the effects
1. The decreasing blood glucose causes the pancreas
between meals, several events transpire that cause the
When the
glycogen in the whole liver.
mass, which is equivalent to almost 100 grams of stored
amount of glycogen in the liver. The glycogen can
The net effect of all these actions is to increase the
form the glycogen molecules.
, which is responsible
that promote glycogen synthesis, including
3. Insulin also increases the activities of the enzymes
through the cell membrane.
the liver cells. Once phosphorylated, the glucose is
the blood by the liver cells. It does this by
2. Insulin causes
glycogen that has been stored in the liver cells.
split into glucose. This prevents breakdown of the
, the
1. Insulin
The mechanism by which insulin causes glucose
centration from falling too low.
the liver glycogen is split back into glucose, which is
begins to fall, insulin secretion decreases rapidly and
form of glycogen. Then, between meals, when food is
Insulin Promotes Liver Uptake, Storage, and
when insulin was added. Thus, it is clear that insulin
glucose concentration rose to as high as 400 mg/100 ml
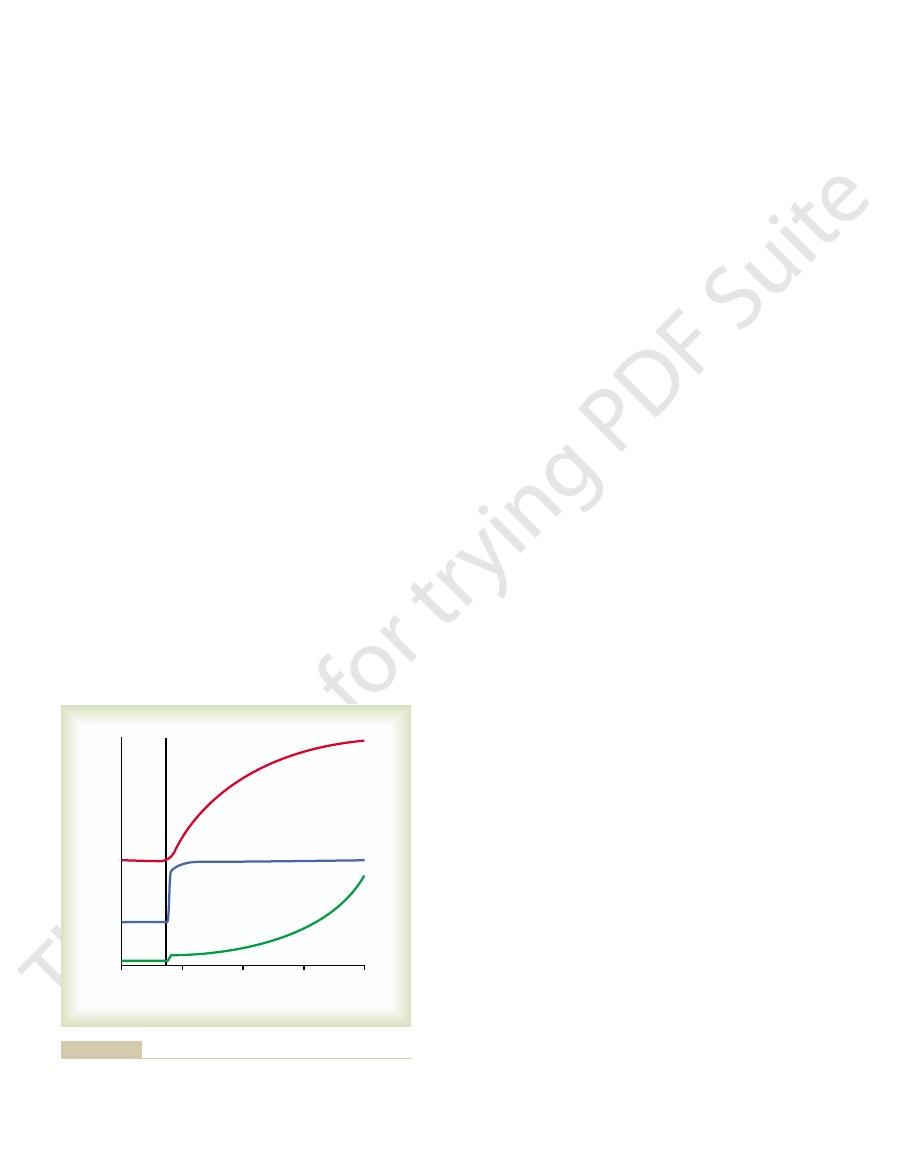
labeled “insulin” demonstrates that the intracellular
up to as high as 750 mg/100 ml. In contrast, the curve
free glucose measured inside the cell, demonstrating
curve labeled “control” shows the concentration of
experimental results shown in Figure 78–4. The lower
The quantitative
Quantitative Effect of Insulin to Facilitate Glucose Transport
absence of oxygen.
glycogen to lactic acid, which can occur even in the
used for energy by the muscle. It is especially useful
3 per cent concentration. The glycogen can later be
instead of being used for energy, up to a limit of 2 to
964
Unit XIV
Endocrinology and Reproduction
for short periods of extreme energy use by the muscles
and even to provide spurts of anaerobic energy for a
few minutes at a time by glycolytic breakdown of the
Through the Muscle Cell Membrane.
effect of insulin to facilitate glucose transport through
the muscle cell membrane is demonstrated by the
that the glucose concentration remained almost zero
despite increased extracellular glucose concentration
can increase the rate of transport of glucose into the
resting muscle cell by at least 15-fold.
Use of Glucose
One of the most important of all the effects of insulin
is to cause most of the glucose absorbed after a meal
to be stored almost immediately in the liver in the
not available and the blood glucose concentration
released back into the blood to keep the glucose con-
uptake and storage in the liver includes several almost
simultaneous steps:
inactivates liver phosphorylase
principal enzyme that causes liver glycogen to
enhanced uptake of glucose from
increasing the activity of the enzyme glucokinase,
which is one of the enzymes that causes the initial
phosphorylation of glucose after it diffuses into
temporarily trapped inside the liver cells because
phosphorylated glucose cannot diffuse back
especially glycogen synthase
for polymerization of the monosaccharide units to
increase to a total of about 5 to 6 per cent of the liver
Glucose Is Released from the Liver Between Meals.
blood glucose level begins to fall to a low level
liver to release glucose back into the circulating blood:
to decrease its insulin secretion.
stopping further synthesis of glycogen in the liver
and preventing further uptake of glucose by the
liver from the blood.
enzyme phosphorylase
of glycogen into glucose phosphate.
glucose phosphatase
insulin lack and causes the phosphate radical to
glucose to diffuse back into the blood.
when it is present in excess after a meal and returns it
to the blood when the blood glucose concentration
the glucose in the meal is stored in this way in the liver
Insulin Promotes Conversion of Excess Glucose into Fatty Acids
tity of glucose entering the liver cells is more than can
be stored as glycogen or can be used for local hepato-
insulin promotes the conversion
of all this excess glucose into fatty acids
acids are subsequently packaged as triglycerides in
0
900
0
400
Insulin
Control
300
200
100
600
300
Intracellular glucose
(mg/100 ml)
Extracellular glucose
(mg/100 ml)
Brown, 1964.)
AB: The Biochemical Aspects of Hormone Action, Boston, Little,
high extracellular glucose concentrations. (Data from Eisenstein
intracellular glucose concentration remains near zero, despite
muscle cells. Note that in the absence of insulin (control), the
Effect of insulin in enhancing the concentration of glucose inside
Figure 78–4

available, even storage of the large amounts of
in adipose cells. Therefore, when insulin is not
-glycerol phosphate. This substance supplies the
more important, it also forms large quantities of
synthesize minute amounts of fatty acids, but
muscle cells. Some of this glucose is then used to
Therefore, the release of fatty acids from the
the triglycerides already stored in the fat cells.
. This is the enzyme that causes hydrolysis of
for them to be absorbed into the adipose cells,
triglycerides again into fatty acids, a requirement
walls of the adipose tissue, which splits the
the liver cells to the blood in the lipoproteins.
usual form of storage fat. They are released from
, the
the liver itself and used to form triglycerides
Most of the fatty acids are then synthesized within
of fatty acid synthesis.
, the first stage
carboxylase,
. These ions then
by the citric acid cycle when excess amounts of
(acetyl-CoA), the substrate from which fatty acids
subsequently is converted to acetyl coenzyme A
in the glycolytic pathway, and the pyruvate
to form fat. The glucose is first split to pyruvate
further glycogen synthesis. Then all the additional
reaches 5 to 6 per cent, this in itself inhibits
. After the liver glucogen concentration
to the adipose cells to be stored. The different factors
in the liver cells, and the fatty acids are then trans-
strate for fat synthesis. Almost all this synthesis occurs
used for immediate energy, thus providing the sub-
promotes fatty acid synthesis. This is especially true
functioning as a fat sparer. However, insulin also
automatically decreases the utilization of fat, thus
of glucose by most of the body’s tissues, which
adipose tissue. First, insulin increases the utilization
Insulin Promotes Fat Synthesis and Storage
vascular accidents. But first, let us discuss the acute
leading to heart attacks, cerebral strokes, and other
in causing extreme atherosclerosis, often
insulin lack
tant. Especially dramatic is the long-term effect of
on fat metabolism are, in the long run, equally impor-
insulin on carbohydrate metabolism, insulin’s effects
Effect of Insulin on Fat Metabolism
deposition of fat in these cells.
ecule. Therefore, in this indirect way, insulin promotes
The transport of glucose into adipose cells mainly pro-
it affects glucose transport and usage in muscle cells.
tion of the brain cells, as noted) in the same way that
in Other Cells
Effect of Insulin on Carbohydrate Metabolism
nervous irritability that leads to fainting, seizures, and
develop, characterized by progressive
glycemic shock
the range of 20 to 50 mg/100 ml, symptoms of
system. When the blood glucose falls too low, into
tained above a critical level, which is one of the most
strates, such as fats, only with difficulty. Therefore, it is
The brain cells are also quite different from most
use of glucose. Instead,
The brain is quite different from most other tissues of
Lack of Effect of Insulin on Glucose Uptake
required for gluconeogenesis. This is discussed
However, part of the effect is caused by an action
the liver enzymes required for gluconeogenesis.
. It does this
Insulin, Glucagon, and Diabetes Mellitus
Chapter 78
965
very-low-density lipoproteins and transported in this
form by way of the blood to the adipose tissue and
deposited as fat.
Insulin also inhibits gluconeogenesis
mainly by decreasing the quantities and activities of
of insulin that decreases the release of amino acids
from muscle and other extrahepatic tissues and in
turn the availability of these necessary precursors
further in relation to the effect of insulin on protein
metabolism.
and Usage by the Brain
the body in that insulin has little effect on uptake or
the brain cells are permeable to
glucose and can use glucose without the intermediation
of insulin.
other cells of the body in that they normally use only
glucose for energy and can use other energy sub-
essential that the blood glucose level always be main-
important functions of the blood glucose control
hypo-
even coma.
Insulin increases glucose transport into and glucose
usage by most other cells of the body (with the excep-
vides substrate for the glycerol portion of the fat mol-
Although not quite as visible as the acute effects of
effects of insulin on fat metabolism.
Insulin has several effects that lead to fat storage in
when more carbohydrates are ingested than can be
ported from the liver by way of the blood lipoproteins
that lead to increased fatty acid synthesis in the liver
include the following:
1. Insulin increases the transport of glucose into the
liver cells
glucose entering the liver cells becomes available
are synthesized.
2. An excess of citrate and isocitrate ions is formed
glucose are being used for energy
have a direct effect in activating acetyl-CoA
the enzyme required to carboxylate
acetyl-CoA to form malonyl-CoA
3.
Insulin activates lipoprotein lipase in the capillary
where they are again converted to triglycerides
and stored.
Role of Insulin in Storage of Fat in the Adipose Cells.
Insulin
has two other essential effects that are required for fat
storage in adipose cells:
1. Insulin inhibits the action of hormone-sensitive
lipase
adipose tissue into the circulating blood is
inhibited.
2. Insulin promotes glucose transport through the cell
membrane into the fat cells in exactly the same
ways that it promotes glucose transport into
a
glycerol that combines with fatty acids to form
the triglycerides that are the storage form of fat
fatty acids transported from the liver in the
lipoproteins is almost blocked.
ones.
the uptake of amino acids into cells. However, the
. Thus, insulin shares
, and
. Among the amino acids most
and fat storage. Some of the facts follow.
the tissues; insulin is required for this to occur. The
ents are available in the circulating blood, not only car-
Effect of Insulin on Protein
, which often leads to death.
. We see later that in severe diabetes the ace-
, and their pres-
toacetic acid, are called
. These two substances, along with the ace-
As explained in Chapter 68, some of the acetoacetic
body fluid acidosis.
tions of 10 mEq/L or more, which is a severe state of
of insulin secretion, sometimes reaching concentra-
lized by the tissues. Therefore, as shown in Figure 78–5,
peripheral tissues. Thus, so much acetoacetic acid is
At the same time, the absence of insulin also
used for energy in the usual manner.
cells, where it is again converted into acetyl-CoA and
culating blood. Most of this passes to the peripheral
acetoacetic acid, which in turn is released into the cir-
releasing extreme amounts of acetyl-CoA.A large part
dation of the fatty acids then proceeds very rapidly,
increasingly activated. In the mitochondria, beta oxi-
liver cells, the carnitine transport mechanism for trans-
results from the following effect: In the absence of
to be formed in the liver cells. This
sclerosis in people with serious diabetes.
per cent rather than the normal 0.6 per cent. This high
lipoproteins. Occasionally the plasma lipoproteins
the liver, are then discharged into the blood in the
ucts of fat metabolism. These two substances, along
phospholipids and cholesterol, two of the major prod-
plasma associated with insulin deficiency also pro-
The excess of fatty acids in the
than the concentration of glucose.
tion in the plasma begins to rise, more rapidly even
removal of the pancreas, the free fatty acid concentra-
acetoacetic acid. Note that almost immediately after
plasma concentrations of free fatty acids, glucose, and
Figure 78–5 shows the effect of insulin lack on the
acids begins to rise within minutes. This free fatty acid
Consequently, the plasma concentration of free fatty
the stored triglycerides, releasing large quantities of
becomes strongly activated. This causes hydrolysis of
fat are reversed. The most important effect is that
In the absence of insulin, all the
zero. The resulting effects are as follows.
tion of insulin is minimal, but it becomes extreme in
This occurs even normally between meals when secre-
for Energy
Insulin Deficiency Increases Use of Fat
966
Unit XIV
Endocrinology and Reproduction
All aspects of fat breakdown and use for providing
energy are greatly enhanced in the absence of insulin.
diabetes mellitus when secretion of insulin is almost
Insulin Deficiency Causes Lipolysis of Storage Fat and Release
of Free Fatty Acids.
effects of insulin noted earlier that cause storage of
the enzyme hormone-sensitive lipase in the fat cells
fatty acids and glycerol into the circulating blood.
then becomes the main energy substrate used by
essentially all tissues of the body besides the brain.
Insulin Deficiency Increases Plasma Cholesterol and Phospho-
lipid Concentrations.
motes liver conversion of some of the fatty acids into
with excess triglycerides formed at the same time in
increase as much as threefold in the absence of insulin,
giving a total concentration of plasma lipids of several
lipid concentration—especially the high concentration
of cholesterol—promotes the development of athero-
Excess Usage of Fats During Insulin Lack Causes Ketosis and
Acidosis.
Insulin lack also causes excessive amounts of
acetoacetic acid
insulin but in the presence of excess fatty acids in the
porting fatty acids into the mitochondria becomes
of this excess acetyl-CoA is then condensed to form
depresses the utilization of acetoacetic acid in the
released from the liver that it cannot all be metabo-
its concentration rises during the days after cessation
acid is also converted into
b-hydroxybutyric acid and
acetone
ketone bodies
ence in large quantities in the body fluids is called
ketosis
toacetic acid and the
b-hydroxybutyric acid can cause
severe acidosis and coma
Metabolism and on Growth
Insulin Promotes Protein Synthesis and Storage.
During the
few hours after a meal when excess quantities of nutri-
bohydrates and fats but proteins as well are stored in
manner in which insulin causes protein storage is not
as well understood as the mechanisms for both glucose
1. Insulin stimulates transport of many of the amino
acids into the cells
strongly transported are valine, leucine, isoleucine,
tyrosine
phenylalanine
with growth hormone the capability of increasing
amino acids affected are not necessarily the same
0
Free fatty acids
Depancreatized
Control
Removal of pancreas
Acetoacetic acid
2
3
1
4
Days
0
Free fatty acids
Blood glucose
Depancreatized
Control
Removal of pancreas
Acetoacetic acid
2
3
1
4
Concentration
Days
tions of blood glucose, plasma free fatty acids, and acetoacetic
Effect of removing the pancreas on the approximate concentra-
Figure 78–5
acid.

blocking their activity. This results in a depolarizing
binding to the ATP-sensitive potassium channels and
tors), inhibit exocytosis of insulin.
of glucose. Other hormones, including somatostatin
enhance the effect of glucose, although they do not
peptide, as well as acetylcholine increase intracellular
hormones, such as glucagon and gastric inhibitory
lular ATP levels and stimulate insulin secretion. Some
Other nutrients, such as certain amino acids, can also
exocytosis.
voltage. This produces an influx of calcium that stimu-
, which are sensitive to changes in membrane
voltage-gated calcium chan-
brane, thereby opening
of the cell. Closure
ATP-sensitive potassium channels
form adenosine triphosphate (ATP), which inhibits the
The glucose-6-phosphate is subsequently oxidized to
secreted insulin to the blood glucose levels.
. This step
range. Once inside the cells, glucose is phosphorylated
glucose transporters (GLUT-
have a large number of
primary controller of insulin secretion. The beta cells
response to increased blood glucose concentration, the
Figure 78–7 shows the basic cellular mechanisms for
Mechanisms of Insulin Secretion
of amino acids, all of which are required if growth is
from that of the other. Perhaps a small part of this
dramatic growth. Thus, it appears that the two hor-
growth. Yet a combination of these hormones causes
Furthermore, the administration of either growth
78–6, which shows that a depancreatized, hypophysec-
growth hormone is. This is demonstrated in Figure
proteins, it is as essential for growth of an animal as
tions of the organs.
all the effects of severe diabetes mellitus. It can lead
leads to enhanced urea excretion in the urine. The
neogenesis. This degradation of the amino acids also
ably, and most of the excess amino acids are used
The plasma amino acid concentration rises consider-
proteins increases, protein synthesis stops, and large
halt when insulin is not available. The catabolism of
Virtually all protein storage comes to a
prevents the degradation of proteins.
In summary, insulin promotes protein formation and
protein stores of the body.
the plasma amino acids, this suppression of
gluconeogenesis. Because the substrates most used
. It does this by decreasing
lysosomes.
cells, especially from the muscle cells. Presumably
, thus
fats, and proteins.
array of enzymes for storage of carbohydrates,
increased quantities of RNA and still more
in the cell nuclei, thus forming
increases the rate of transcription of selected DNA
3. Over a longer period of time,
insulin operates an “on-off” mechanism.
ribosomes simply stop working, almost as if
machinery. In the absence of insulin, the
unexplained way, insulin “turns on” the ribosomal
, thus forming new proteins. In some
RNA
Insulin, Glucagon, and Diabetes Mellitus
Chapter 78
967
2. Insulin increases the translation of messenger
insulin also
genetic sequences
protein synthesis—especially promoting a vast
4. Insulin inhibits the catabolism of proteins
decreasing the rate of amino acid release from the
this results from the ability of insulin to diminish
the normal degradation of proteins by the cellular
5. In the liver, insulin depresses the rate of
gluconeogenesis
the activity of the enzymes that promote
for synthesis of glucose by gluconeogenesis are
gluconeogenesis conserves the amino acids in the
Insulin Lack Causes Protein Depletion and Increased Plasma
Amino Acids.
quantities of amino acids are dumped into the plasma.
either directly for energy or as substrates for gluco-
resulting protein wasting is one of the most serious of
to extreme weakness as well as many deranged func-
Insulin and Growth Hormone Interact Synergistically to Promote
Growth.
Because insulin is required for the synthesis of
tomized rat without therapy hardly grows at all.
hormone or insulin one at a time causes almost no
mones function synergistically to promote growth,
each performing a specific function that is separate
necessity for both hormones results from the fact that
each promotes cellular uptake of a different selection
to be achieved.
insulin secretion by the pancreatic beta cells in
2) that permit a rate of glucose influx that is propor-
tional to the blood concentration in the physiologic
to glucose-6-phosphate by glucokinase
appears to be the rate limiting for glucose metabolism
in the beta cell and is considered the major mechanism
for glucose sensing and adjustment of the amount of
of the potassium channels depolarizes the cell mem-
nels
lates fusion of the docked insulin-containing vesicles
with the cell membrane and secretion of insulin into
the extracellular fluid by
be metabolized by the beta cells to increase intracel-
calcium levels through other signaling pathways and
have major effects on insulin secretion in the absence
and norepinephrine (by activating
a-adrenergic recep-
Sulfonylurea drugs stimulate insulin secretion by
100
150
200
0
50
Depancreatized and
hypophysectomized
Growth
hormone
Insulin
Growth hormone
and insulin
0
250
200
150
50
100
250
Weight (grams)
Days
on growth in a depancreatized and hypophysectomized rat.
Effect of growth hormone, insulin, and growth hormone plus insulin
Figure 78–6
centrations between 400 and 600 mg/100 ml, as shown
of insulin secretion rises rapidly, reaching a peak some
glucose rises above 100 mg/100 ml of blood, the rate
Feedback Relation Between Blood Glucose Concentration and
releases new insulin from the cells.
phase. This secretion results both from additional
2 to 3 hours, this time usually at a rate of
2. Beginning at about 15 minutes, insulin secretion
minutes.
instead, the insulin concentration decreases about
beta cells of the islets of Langerhans. However,
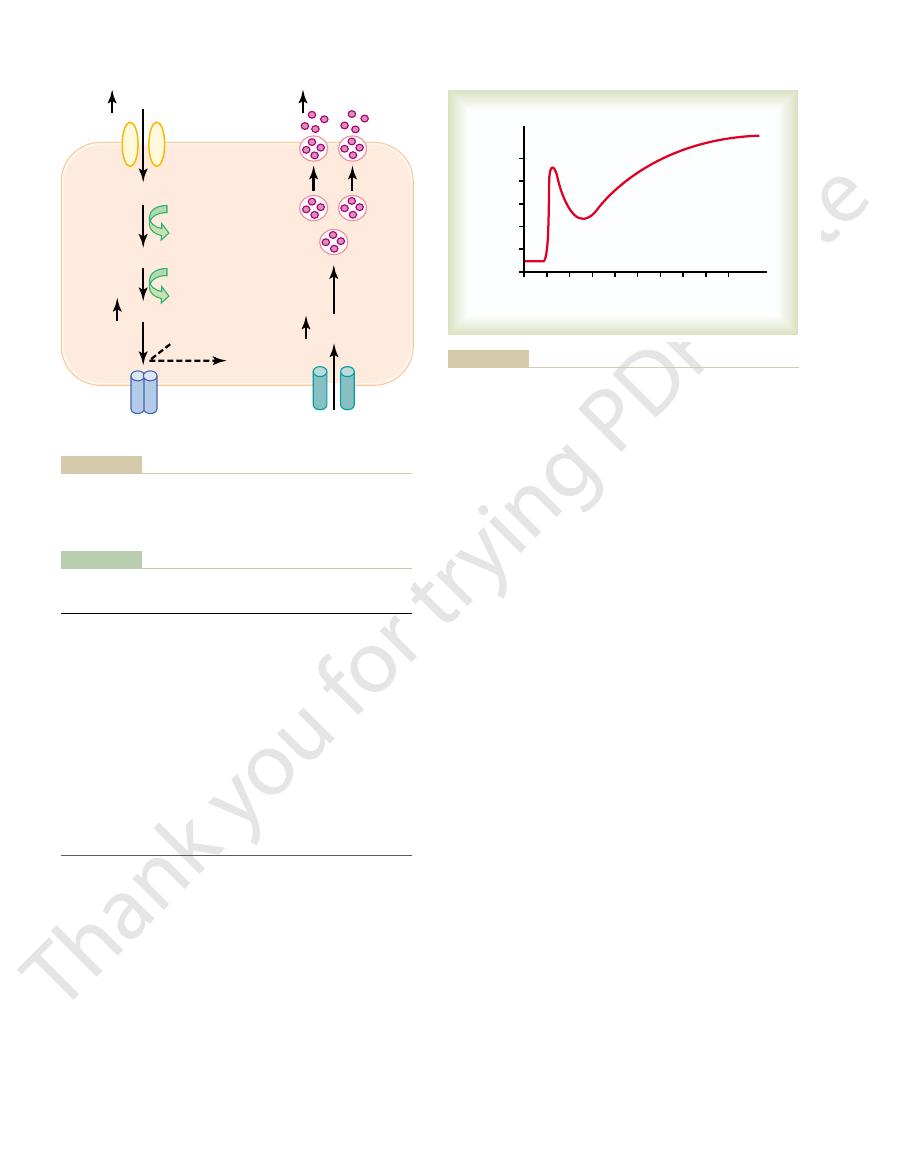
elevation of the blood glucose; this results from
1. Plasma insulin concentration increases almost
centration seen in Figure 78–8.
stages, as shown by the changes in plasma insulin con-
thereafter, insulin secretion increases markedly in two
that has only slight physiologic activity. If the blood
on the order of 25 ng/min/kg of body weight, a level
90 mg/100 ml, the rate of insulin secretion is minimal—
controlling insulin secretion (see Table 78–1).
metabolism, it has become apparent that blood amino
centration. However, as more has been learned about
Formerly, it was believed that insulin secretion was
Control of Insulin Secretion
Table 78–1 summarizes some of the factors that can
patients with type II diabetes, as we will discuss later.
effect that triggers insulin secretion, making these
968
Unit XIV
Endocrinology and Reproduction
drugs very useful in stimulating insulin secretion in
increase or decrease insulin secretion.
controlled almost entirely by the blood glucose con-
the metabolic functions of insulin for protein and fat
acids and other factors also play important roles in
Increased Blood Glucose Stimulates Insulin Secretion.
At
the normal fasting level of blood glucose of 80 to
glucose concentration is suddenly increased to a level
two to three times normal and kept at this high level
10-fold within 3 to 5 minutes after the acute
immediate dumping of preformed insulin from the
the initial high rate of secretion is not maintained;
halfway back toward normal in another 5 to 10
rises a second time and reaches a new plateau in
secretion even greater than that in the initial
release of preformed insulin and from activation
of the enzyme system that synthesizes and
Insulin Secretion Rate.
As the concentration of blood
10 to 25 times the basal level at blood glucose con-
ATP
GLUT 2
ATP
Glucose
Glucose-6-phosphate
Ca
++
Depolarization
K
+
Glucokinase
Oxidation
Ca
++
channel
(open)
+
K
+
channel
(closed)
Insulin
Glucose
beta cells of the pancreas. GLUT, glucose transporter.
Basic mechanisms of glucose stimulation of insulin secretion by
Figure 78–7
Table 78–1
Factors and Conditions That Increase or Decrease
• Sulfonylurea drugs (glyburide,
• Insulin resistance; obesity
• Parasympathetic stimulation;
• Glucagon, growth hormone,
(gastrin, cholecystokinin, secretin,
• Leptin
• Gastrointestinal hormones
•
• Increased blood amino acids
• Somatostatin
• Increased blood free fatty acids
• Fasting
• Increased blood glucose
• Decreased blood glucose
Increase Insulin Secretion
Decrease Insulin Secretion
Insulin Secretion
a-Adrenergic activity
gastric inhibitory peptide)
cortisol
acetylcholine
•
b-Adrenergic stimulation
tolbutamide)
10
0
10 20 30 40 50 60 70
-
0
250
80
60
40
20
80
Plasma insulin (
m
U/ml)
Minutes
20 minutes later.
higher and continuing increase in concentration beginning 15 to
in blood glucose to two to three times the normal range. Note an
Increase in plasma insulin concentration after a sudden increase
Figure 78–8
initial rapid surge in insulin concentration and then a delayed but

in the next section of this chapter. Both growth
of Langerhans in the pancreas. Glucagon is discussed
medulla, and
growth
cells for energy.
fore, one of the most important functional roles of
of liver glycogen, liver fat, and muscle glycogen. There-
fat, and the excess blood glucose is stored in the form
When the glucose concentration is high, insulin secre-
concentration. When the glucose concentration is low,
tissue. Furthermore, the signal that controls this
to the exclusion of glucose utilization, except by brain
Conversely, lack of insulin causes fat utilization mainly
energy, whereas it depresses the utilization of fats.
From the preceding discussions, it should be clear that
Role of Insulin (and Other Hormones)
secretion. However, it is doubtful that this effect is of
Under some conditions, stimulation of the parasym-
excess glucocorticoids.
tumors, or in people whose adrenal glands secrete
hormones. Diabetes is particularly common in giants or
Indeed, diabetes often occurs in people who are main-
increase the risk for developing diabetes mellitus.
The impor-
, and, to a
growth hormone
Other Hormones and the Autonomic Nervous System.
insulin secretion as the blood glucose level rises.
increased blood glucose, almost doubling the rate of
acids to be absorbed from the meal. These gastroin-
a meal. They then cause an “anticipatory” increase in
increase in insulin secretion. These hormones are
, and
cholecystokinin
A mixture of several impor-
of carbohydrates.
intracellular formation of protein. That is, insulin is
is important, because the insulin in turn promotes
The stimulation of insulin secretion by amino acids
in the presence of the excess amino acids. Thus, the
blood glucose concentration is elevated, the glucose-
However, when administered at the same time that the
ulation of insulin secretion in the following way: Amino
This effect differs from glucose stim-
lysine.
acids have a similar effect. The most potent of these are
secretion by excess blood glucose, some of the amino
Insulin Secretion
Other Factors That Stimulate
concentration back toward the normal value.
and other cells, thereby reducing the blood glucose
turn increases transport of glucose into liver, muscle,
glucose increases insulin secretion, and the insulin in
glucose concentration. That is, any rise in blood
This response of insulin secretion to an elevated
equally as rapid, occurring within 3 to 5 minutes after
Furthermore, the turn-off of insulin secretion is almost
in Figure 78–9. Thus, the increase in insulin secretion
Insulin, Glucagon, and Diabetes Mellitus
Chapter 78
969
under a glucose stimulus is dramatic both in its rapid-
ity and in the tremendous level of secretion achieved.
reduction in blood glucose concentration back to the
fasting level.
blood glucose concentration provides an extremely
important feedback mechanism for regulating blood
Amino Acids.
In addition to the stimulation of insulin
arginine and
acids administered in the absence of a rise in blood
glucose cause only a small increase in insulin secretion.
induced secretion of insulin may be as much as doubled
amino acids strongly potentiate the glucose stimulus for
insulin secretion.
transport of amino acids into the tissue cells as well as
important for proper utilization of excess amino acids
in the same way that it is important for the utilization
Gastrointestinal Hormones.
tant gastrointestinal hormones—gastrin,
secretin,
gastric inhibitory peptide (which
seems to be the most potent)—causes a moderate
released in the gastrointestinal tract after a person eats
blood insulin in preparation for the glucose and amino
testinal hormones generally act the same way as amino
acids to increase the sensitivity of insulin response to
Other hor-
mones that either directly increase insulin secretion or
potentiate the glucose stimulus for insulin secretion
include glucagon,
, cortisol
lesser extent, progesterone and estrogen.
tance of the stimulatory effects of these hormones is
that prolonged secretion of any one of them in large
quantities can occasionally lead to exhaustion of the
beta cells of the islets of Langerhans and thereby
tained on high pharmacological doses of some of these
acromegalic people with growth hormone-secreting
pathetic nerves to the pancreas can increase insulin
physiologic significance for regulating insulin secretion.
in “Switching” Between Carbohydrate
and Lipid Metabolism
insulin promotes the utilization of carbohydrates for
switching mechanism is principally the blood glucose
insulin secretion is suppressed and fat is used almost
exclusively for energy everywhere except in the brain.
tion is stimulated and carbohydrate is used instead of
insulin in the body is to control which of these two
foods from moment to moment will be used by the
At least four other known hormones also play
important roles in this switching mechanism:
hormone from the anterior pituitary gland, cortisol
from the adrenal cortex, epinephrine from the adrenal
glucagon from the alpha cells of the islets
hormone and cortisol are secreted in response to
0
100
200
300
400
500
X
0
20
15
10
5
600
Insulin secretion
(times normal)
Plasma glucose concentration
(mg/100 ml)
Approximate insulin secretion at different plasma glucose levels.
Figure 78–9

importance in the normal function of the body.
secretion. All these effects are probably of minimal
enhances bile secretion; and (4) inhibits gastric acid
flow in some tissues, especially the kidneys; (3)
enhances the strength of the heart; (2) increases blood
available for the other tissues of the body.
the liver from removing fatty acids from the blood; this
the storage of triglycerides in the liver, which prevents
energy systems of the body. Glucagon also inhibits
, making
found in the blood. Perhaps the most important effect
Other Effects of Glucagon
enolpyruvate, a rate-limiting step in gluconeogenesis.
and gluconeogenesis, especially activation of the
gluconeogenesis. This is achieved by activating multi-
hormone still causes continued hyperglycemia. This
influence of glucagon, continued infusion of this
more within a few minutes.
as much as a millionfold amplification in response. This
if not most, cellular metabolic systems, often causing
widely used throughout the body for controlling many,
mechanism; this type of amplifying mechanism is
. Therefore, it represents a potent
each succeeding product
adenosine monophosphate. Second, it demonstrates
functions of cyclic
several reasons. First, it is one of the most thoroughly
This sequence of events is exceedingly important for
is released from the liver cells.
8. Which then is dephosphorylated; and the glucose
glucose-1-phosphate,
7. Which promotes the degradation of glycogen into
6. Which converts
5. Which activates
4. Which activates
3. Which activates
2. Which causes the formation of
cell membrane,
1. Glucagon activates
minutes.
its ability to cause glycogenolysis in the liver, which in
The most dramatic effect of glucagon is
to the other organs of the body.
in the liver. Both of
The major effects of glucagon on glucose metabolism
reason, glucagon is also called the
25 per cent increase) in about 20 minutes. For this
glucose concentration about 20 mg/100 ml of blood (a
occurs. Only 1
into an animal, a profound
of 29 amino acids. On injection of purified glucagon
Like insulin, glucagon is a large polypeptide. It has
centration, an effect that is exactly the opposite that of
cally opposed to those of insulin. Most important of
tration falls, has several functions that are diametri-
Glucagon, a hormone secreted by the
and anxiety.
in such stressful states as exercise, circulatory shock,
than the enhancement of blood glucose. Therefore,
tively, the enhancement of fatty acids is far greater
blood concentration of fatty acids as well. Quantita-
hormone-sensitive lipase, thus greatly enhancing the
into the blood; (2) it also has a direct lipolytic effect
effect of causing glycogenolysis in the liver, thus
effects are as follows: (1) epinephrine has the potent
concentration at the same time. The reasons for these
However, epinephrine acts differently from the other
slowly, usually requiring many hours for maximal
glucose while promoting fat utilization. However,
hypoglycemia, and both inhibit cellular utilization of
970
Unit XIV
Endocrinology and Reproduction
the effects of both of these hormones develop
expression.
Epinephrine is especially important in increasing
plasma glucose concentration during periods of stress
when the sympathetic nervous system is excited.
hormones in that it increases the plasma fatty acid
releasing within minutes large quantities of glucose
on the adipose cells because it activates adipose tissue
epinephrine especially enhances the utilization of fat
Glucagon and Its Functions
alpha cells of the
islets of Langerhans when the blood glucose concen-
these functions is to increase the blood glucose con-
insulin.
a molecular weight of 3485 and is composed of a chain
hyperglycemic effect
mg/kg of glucagon can elevate the blood
hyperglycemic
hormone.
Effects on Glucose Metabolism
are (1) breakdown of liver glycogen (glycogenolysis)
and (2) increased gluconeogenesis
these effects greatly enhance the availability of glucose
Glucagon Causes Glycogenolysis and Increased Blood Glucose
Concentration.
turn increases the blood glucose concentration within
It does this by the following complex cascade of
events:
adenylyl cyclase in the hepatic
cyclic adenosine
monophosphate,
protein kinase regulator protein,
protein kinase,
phosphorylase b kinase,
phosphorylase b into
phosphorylase a,
studied of all the second messenger
a cascade system in which
is produced in greater quantity than the preceding
product
amplifying
explains how only a few micrograms of glucagon can
cause the blood glucose level to double or increase even
Infusion of glucagon for about 4 hours can cause such
intensive liver glycogenolysis that all the liver stores of
glycogen become depleted.
Glucagon Increases Gluconeogenesis.
Even after all the
glycogen in the liver has been exhausted under the
results from the effect of glucagon to increase the rate
of amino acid uptake by the liver cells and then the
conversion of many of the amino acids to glucose by
ple enzymes that are required for amino acid transport
enzyme system for converting pyruvate to phospho-
Most other effects of glucagon occur only when its
concentration rises well above the maximum normally
is that glucagon activates adipose cell lipase
increased quantities of fatty acids available to the
also helps make additional amounts of fatty acids
Glucagon in very high concentrations also (1)
control have been presented in this chapter. Let us
The mechanisms for achieving this high degree of
absorption of carbohydrates. Conversely, in starvation,
to the control level, usually within 2 hours after the last
meal, but the feedback systems for control of blood
to 140 mg/100 ml during the first hour or so after a
before breakfast. This concentration increases to 120
narrowly controlled, usually between 80 and 90 mg/
In a normal person, the blood glucose concentration is
Summary of Blood
hormone,
growth hormone inhibitory
a longer period of time.
nutrients by the tissues, thus preventing rapid exhaus-
are assimilated into the blood. At the same time, the
Putting all this information together, it has been sug-
3. Somatostatin decreases both secretion and
duodenum, and gallbladder.
2. Somatostatin decreases the motility of the stomach,
1. Somatostatin acts locally within the islets of
In turn, somatostatin has multiple inhibitory effects
tract in response to food intake.
increased amino acids, (3) increased fatty acids, and (4)
secretion. They include (1) increased blood glucose, (2)
3 minutes in the circulating blood. Almost all factors
, a polypeptide containing only 14
The
Secretion
of the islets of Langerhans, may also play a role.
acids. Other factors, such as
glucagon is that it prevents a decrease in blood glucose.
does not necessarily fall. A beneficial effect of the
understood, because the blood glucose concentration
increases fourfold to fivefold. What causes this is not
exercise, the blood concentration of glucagon often
even more glucose available to the tissues.
conversion of the amino acids to glucose, thus making
The importance of amino acid stimulation of glucagon
opposites.
glucagon. This is the same effect that amino acids have
High concentrations of amino acids, as occur in the
glucagon is secreted in large amounts; it then greatly
decreases plasma glucagon. Thus, in hypoglycemia,
concentration of glucagon severalfold. Conversely,
normal fasting level of about 90 mg/100 ml of blood
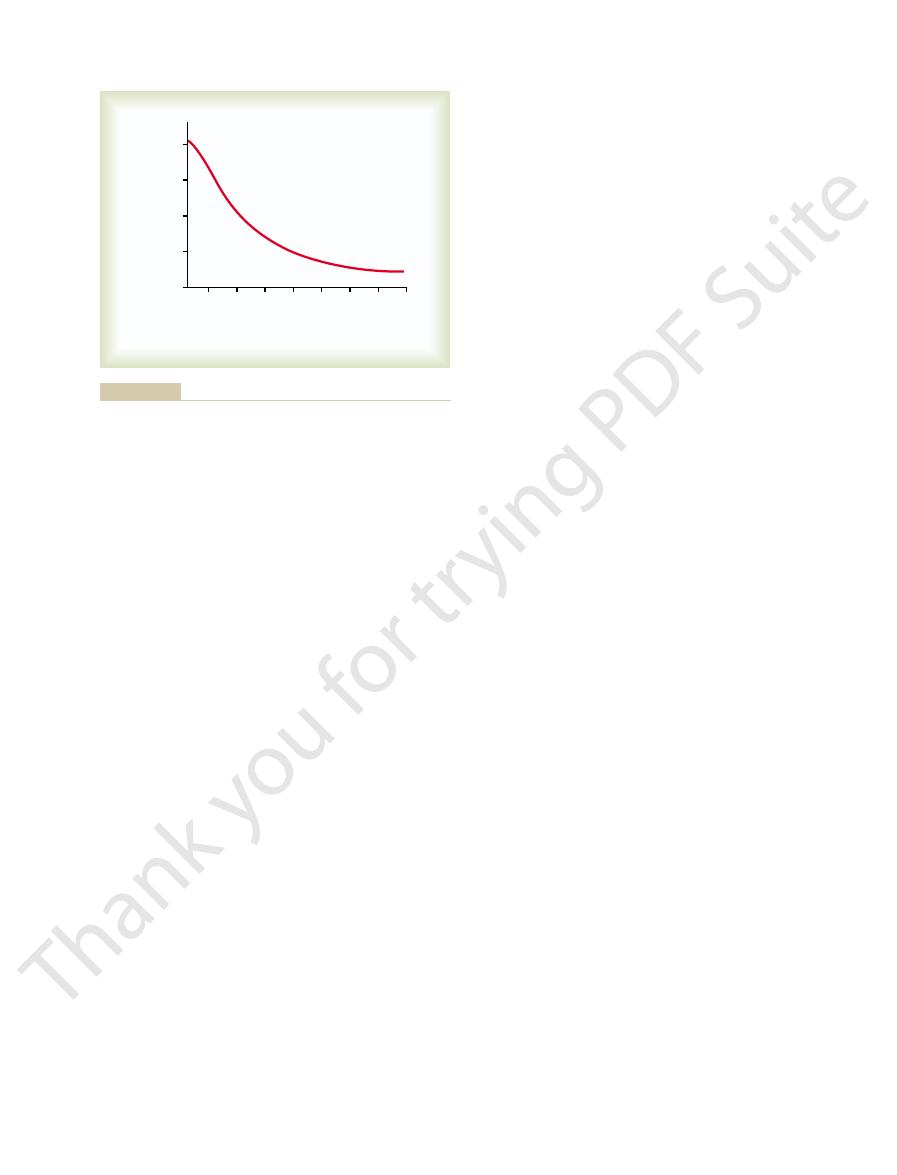
This is demonstrated in Figure 78–10, showing that
cally, however, that
factor that controls glucagon secretion. Note specifi-
The
Increased Blood Glucose Inhibits Glucagon Secretion.
Regulation of Glucagon Secretion
Insulin, Glucagon, and Diabetes Mellitus
Chapter 78
971
blood glucose concentration is by far the most potent
the effect of blood glucose concen-
tration on glucagon secretion is in exactly the opposite
direction from the effect of glucose on insulin secretion.
a decrease in the blood glucose concentration from its
down to hypoglycemic levels can increase the plasma
increasing the blood glucose to hyperglycemic levels
increases the output of glucose from the liver and
thereby serves the important function of correcting the
hypoglycemia.
Increased Blood Amino Acids Stimulate Glucagon Secretion.
blood after a protein meal (especially the amino acids
alanine and arginine), stimulate the secretion of
in stimulating insulin secretion. Thus, in this instance,
the glucagon and insulin responses are not
secretion is that the glucagon then promotes rapid
Exercise Stimulates Glucagon Secretion.
In exhaustive
One of the factors that might increase glucagon
secretion in exercise is increased circulating amino
b-adrenergic stimulation
Somatostatin Inhibits
Glucagon and Insulin
delta cells of the islets of Langerhans secrete the
hormone somatostatin
amino acids that has an extremely short half-life of only
related to the ingestion of food stimulate somatostatin
increased concentrations of several of the gastrointesti-
nal hormones released from the upper gastrointestinal
as follows:
Langerhans themselves to depress the secretion of
both insulin and glucagon.
absorption in the gastrointestinal tract.
gested that the principal role of somatostatin is to
extend the period of time over which the food nutrients
effect of somatostatin to depress insulin and glucagon
secretion decreases the utilization of the absorbed
tion of the food and therefore making it available over
It should also be recalled that somatostatin is the
same chemical substance as
which is secreted in the hypothalamus and
suppresses anterior pituitary gland growth hormone
secretion.
Glucose Regulation
100 ml of blood in the fasting person each morning
glucose return the glucose concentration rapidly back
the gluconeogenesis function of the liver provides the
glucose that is required to maintain the fasting blood
glucose level.
summarize them.
60
80
100
120
0
4
3
2
1
Plasma glucagon
(times normal)
Blood glucose
(mg/100 ml)
Approximate plasma glucagon concentration at different blood
Figure 78–10
glucose levels.

blood glucose, (2) increased utilization of fats for energy
or weeks, with three principal sequelae: (1) increased
may develop very abruptly, over a period of a few days
. Type I diabetes
years of age in the United States, and for this reason it
The usual onset of type I diabetes occurs at about 14
disorders.
there may be a hereditary tendency for beta cell degen-
cells to destruction by these insults. In some instances,
with type I diabetes, although heredity also plays a
autoimmune disorders
Viral infections
impair insulin production can lead to type I diabetes.
Type I Diabetes
utilization of fats and proteins increases.
utilization of glucose falls increasingly lower, and
a result, blood glucose concentration increases, cell
by most cells of the body, except those of the brain. As
the main foodstuffs is altered. The basic effect of insulin
In both types of diabetes mellitus, metabolism of all
of insulin. This reduced sensitivity to insulin is often
, is caused by decreased
, also called
Type II diabetes
, is caused by lack of
, also called
Type I diabetes
tissues to insulin. There are two general types of dia-
drate, fat, and protein metabolism caused by either lack
disease, and blindness.
increased risk for heart attack, stroke, end-stage renal
associated with uncontrolled diabetes mellitus, leads to
tissues, especially to blood vessels. Vascular injury,
the body of its fluids and electrolytes.(4) Long-term
osmotic diuresis by the kidneys, which can deplete
the urine. (3) Loss of glucose in the urine also causes
cellular dehydration.(2) An excessively high level of
rises to excessive values, this can cause considerable
extracellular fluid, and if the glucose concentration
tration not rise too high for four reasons: (1) Glucose
leaving the brain without a nutritive source.
go into the muscles and other peripheral tissues,
not secrete any insulin during this time; otherwise, the
in the brain. Indeed, it is important that the pancreas
optimally with their required energy. Therefore, it is
, and
for energy in the absence of glucose? The answer is
blood glucose concentration, particularly because
question: Why is it so important to maintain a constant
greater amounts of fat utilization. This, too, helps
most cells of the body, converting instead to
response to prolonged hypoglycemia, and they
4. And finally, over a period of hours and days, both
too, helps protect against severe hypoglycemia.
still further release of glucose from the liver. This,
the sympathetic nervous system. In turn, the
3. Also, in severe hypoglycemia, a direct effect of
valuable.
situations, the glucagon mechanism also becomes
important than the glucagon mechanism, but in
toward normal. Under most normal conditions,
glucagon secretion; the glucagon then functions in
Conversely, a decrease in blood glucose stimulates
concentration rises too high, insulin is secreted;
. When the glucose
feedback control systems for maintaining a normal
severe liver disease, it becomes almost impossible
would otherwise be. In fact, in patients with
the glucose back into the blood. In this way, the
the rate of insulin secretion fall, the liver releases
of glycogen. Then, during the succeeding hours,
rate of insulin secretion also increases, as much as
. That is, when the blood glucose
972
Unit XIV
Endocrinology and Reproduction
1. The liver functions as an important blood glucose
buffer system
rises to a high concentration after a meal and the
two thirds of the glucose absorbed from the gut is
almost immediately stored in the liver in the form
when both the blood glucose concentration and
liver decreases the fluctuations in blood glucose
concentration to about one third of what they
to maintain a narrow range of blood glucose
concentration.
2. Both insulin and glucagon function as important
blood glucose concentration
the insulin in turn causes the blood glucose
concentration to decrease toward normal.
the opposite direction to increase the glucose
the insulin feedback mechanism is much more
instances of starvation or excessive utilization of
glucose during exercise and other stressful
low blood glucose on the hypothalamus stimulates
epinephrine secreted by the adrenal glands causes
growth hormone and cortisol are secreted in
both decrease the rate of glucose utilization by
return the blood glucose concentration toward
normal.
Importance of Blood Glucose Regulation.
One might ask the
most tissues can shift to utilization of fats and proteins
that glucose is the only nutrient that normally can be
used by the brain, retina
germinal epithelium
of the gonads in sufficient quantities to supply them
important to maintain the blood glucose concentration
at a sufficiently high level to provide this necessary
nutrition.
Most of the glucose formed by gluconeogenesis
during the interdigestive period is used for metabolism
scant supplies of glucose that are available would all
It is also important that the blood glucose concen-
can exert a large amount of osmotic pressure in the
blood glucose concentration causes loss of glucose in
increases in blood glucose may cause damage to many
Diabetes Mellitus
Diabetes mellitus is a syndrome of impaired carbohy-
of insulin secretion or decreased sensitivity of the
betes mellitus:
1.
insulin-dependent
diabetes mellitus (IDDM)
insulin secretion.
2.
non–insulin-dependent
diabetes mellitus (NIDDM)
sensitivity of target tissues to the metabolic effect
called insulin resistance.
lack or insulin resistance on glucose metabolism is to
prevent the efficient uptake and utilization of glucose
—Lack of Insulin
Production by Beta Cells
of the Pancreas
Injury to the beta cells of the pancreas or diseases that
or
may be
involved in the destruction of beta cells in many patients
major role in determining the susceptibility of the beta
eration even without viral infections or autoimmune
is often called juvenile diabetes mellitus

acidosis are shown in Figure 78–11.
death can occur within hours. The overall changes in the
severe instances of uncontrolled diabetes, when the pH
bicarbonate stores. The kidneys compensate by decreas-
causes increased expiration of carbon dioxide; this
, which
They include
in metabolic acidosis take place in diabetic acidosis.
This leads rapidly to
excessive urine formation, can cause severe acidosis.
acids, which, in association with dehydration due to the
oxidized by the tissue cells. As a result, the patient
-hydroxybutyric acid, into
olism in diabetes increases the release of keto acids,
The shift from carbohydrate to fat metab-
glucose.
metabolism, often develop in patients with diabetes and
, secondary to abnormal lipid
injury, and
, secondary to renal
tissues. In addition,
lial and vascular smooth muscle cells, as well as other
The precise mechanisms that cause tissue injury in
other symptoms of peripheral nerve damage.
control, decreased sensation in the extremities, and
impaired cardiovascular reflexes, impaired bladder
betes mellitus. These abnormalities can result in
frequent complications of chronic, uncontrolled dia-
nerves, and
, which is abnormal function of peripheral
damage to many other tissues. For example,
gangrene of the limbs.
disease, retinopathy and blindness, and ischemia and
increased risk for heart attack, stroke, end-stage kidney
blood supply to the tissues. This in turn leads to
diabetes mellitus, blood vessels in multiple tissues
When
Chronic High Glucose Concentration Causes Tissue Injury
classic symptoms of diabetes.
, and
Thus,
intracellular fluid, for reasons discussed in Chapter 26.
urine, causing dehydration of the extracellular fluid,
of fluid. The overall effect is massive loss of fluid in the
That is, the osmotic effect of glucose in
osmotic diuresis.
excessive glucose, the loss of glucose in the urine causes
causes osmotic transfer of water out of the cells.
through the pores of the cell membrane, and the
severe cell dehydration throughout the body. This
The very high
the urine each day.
the blood glucose level rises to 300 to 500 mg/100 ml—
old” for the appearance of glucose in the urine. When
180 mg/100 ml, a level that is called the blood “thresh-
excess glucose spills into the urine. This normally occurs
into the renal tubules than can be reabsorbed, and the
The high blood glucose causes more glucose to filter
multiple effects throughout the body.
1200 mg/100 ml. The increased plasma glucose then has
glucose production, raising plasma glucose to 300 to
The lack of insulin decreases the efficiency
Blood Glucose Concentration Rises to Very High Levels in Diabetes
depletion of the body’s proteins.
and for formation of cholesterol by the liver, and (3)
Insulin, Glucagon, and Diabetes Mellitus
Chapter 78
973
Mellitus.
of peripheral glucose utilization and augments
Increased Blood Glucose Causes Loss of Glucose in the Urine
when the blood glucose concentration rises above
common values in people with severe untreated dia-
betes—100 or more grams of glucose can be lost into
Increased Blood Glucose Causes Dehydration
levels of blood glucose (sometimes as high as 8 to 10
times normal in severe untreated diabetes) can cause
occurs partly because glucose does not diffuse easily
increased osmotic pressure in the extracellular fluids
In addition to the direct cellular dehydrating effect of
the renal tubules greatly decreases tubular reabsorption
which in turn causes compensatory dehydration of the
polyuria (excessive urine excretion), intracellular
and extracellular dehydration
increased thirst are
blood glucose is poorly controlled over long periods in
throughout the body begin to function abnormally and
undergo structural changes that result in inadequate
Chronic high glucose concentration also causes
peripheral
neuropathy
autonomic nervous system dysfunction are
diabetes are not well understood but probably involve
multiple effects of high glucose concentrations and
other metabolic abnormalities on proteins of endothe-
hypertension
atherosclerosis
amplify the tissue damage caused by the elevated
Diabetes Mellitus Causes Increased Utilization of Fats and Meta-
bolic Acidosis.
such as acetoacetic acid and
b
the plasma more rapidly than they can be taken up and
develops severe metabolic acidosis from the excess keto
diabetic coma and death unless the
condition is treated immediately with large amounts of
insulin.
All the usual physiologic compensations that occur
rapid and deep breathing
buffers the acidosis but also depletes extracellular fluid
ing bicarbonate excretion and generating new bicar-
bonate that is added back to the extracellular fluid.
Although extreme acidosis occurs only in the most
of the blood falls below about 7.0, acidotic coma and
electrolytes of the blood as a result of severe diabetic
Excess fat utilization in the liver occurring over a long
time causes large amounts of cholesterol in the circu-
lating blood and increased deposition of cholesterol in
Total cations
Glucose
Keto acids
pH
Cholesterol
HCO
3
Cl
-
180 mg/dL
360 mg/dL
100 mg/dL
400
+
mg/dL
7.4
6.9
103 mEq
90 mEq
155 mEq
130 mEq
155 mEq
30 mEq
27 mEq
5 mEq
values (lavender bars) and diabetic coma values (red bars).
Changes in blood constituents in diabetic coma, showing normal
Figure 78–11

drates in the early stages of the disease.
maintain normal glucose regulation. As a result, mod-
With prolonged and severe insulin resistance,
Development of Type II Diabetes During Prolonged Insulin Resis-
including cardiovascular disease.
as many other features of the metabolic syndrome,
severe enough, also can lead to type II diabetes as well
litus. Genetic causes of obesity and insulin resistance, if
growth hormone
Cushing’s syn-
cardiovascular disease.
risk for diabetes mellitus, increased blood lipids, and
women. The long-term consequences include increased
remains uncertain, insulin resistance and hyperinsuline-
productive life. Although the pathogenesis of PCOS
common endocrine disorders in women, affecting ap-
, for example, is
Polycystic ovary syndrome (PCOS)
(Table 78–2).
fat, severe insulin resistance and type II diabetes can
Other Factors That Can Cause Insulin Resistance and Type II Dia-
of cardiovascular disease.
opment of type II diabetes mellitus, also a major cause
disease, and insulin resistance predisposes to the devel-
body. Several of the metabolic abnormalities associated
syndrome is cardiovascular disease, including athero-
tion. The major adverse consequence of the metabolic
unclear, although it is clear that insulin resistance is the
The role of insulin resistance in contributing to some
ceral organs.
and (5) hypertension. All of the features of the meta-
resistance; (3) fasting hyperglycemia; (4) lipid abnor-
especially accumulation of abdominal fat; (2) insulin
features of the metabolic syndrome include: (1) obesity,
.” Some of the
is often called the “
link receptor activation with multiple cellular effects.
However, most of the insulin resistance appears to be
liver, and adipose tissue, in obese than in lean subjects.
fewer insulin receptors, especially in the skeletal muscle,
are still uncertain. Some studies suggest that there are
nisms that link obesity with insulin resistance, however,
ning with excess weight gain and obesity. The mecha-
glucose metabolism is usually a gradual process, begin-
carbohydrate utilization and storage, raising blood
The decrease in insulin sensitivity impairs
resistance.
bolic effects of insulin, a condition referred to as
). This occurs as
contrast to type I, is associated with
Type II diabetes, in
Precede Development of Type II Diabetes.
Obesity, Insulin Resistance, and “Metabolic Syndrome” Usually
dren as well as in adults.
years old, with type II diabetes. This trend appears to be
in the number of younger individuals, some less than 20
recent years, however, there has been a steady increase
adult-onset diabetes.
years, and the disease develops gradually. Therefore, this
occurs after age 30, often between the ages of 50 and 60
mellitus. In most cases, the onset of type II diabetes
Type II diabetes is far more common than type I,
Metabolic Effects of Insulin
Type II Diabetes
few weeks.
treatment, these metabolic abnormalities can cause
). Without
decreased storage of proteins as well as fat. Therefore,
Failure to
Diabetes Causes Depletion of the Body’s Proteins.
and other vascular lesions, as discussed earlier.
the arterial walls. This leads to severe
974
Unit XIV
Endocrinology and Reproduction
arteriosclerosis
use glucose for energy leads to increased utilization and
a person with severe untreated diabetes mellitus suffers
rapid weight loss and asthenia (lack of energy) despite
eating large amounts of food (polyphagia
severe wasting of the body tissues and death within a
—Resistance to the
accounting for about 90 per cent of all cases of diabetes
syndrome is often referred to as
In
related mainly to the increasing prevalence of obesity,
the most important risk factor for type II diabetes in chil-
increased plasma
insulin concentration (hyperinsulinemia
a compensatory response by the pancreatic beta cells
for diminished sensitivity of target tissues to the meta-
insulin
glucose and stimulating a compensatory increase in
insulin secretion.
Development of insulin resistance and impaired
caused by abnormalities of the signaling pathways that
Impaired insulin signaling appears to be closely related
to toxic effects of lipid accumulation in tissues such as
skeletal muscle and liver secondary to excess weight
gain.
Insulin resistance is part of a cascade of disorders that
metabolic syndrome
malities such as increased blood triglycerides and
decreased blood high-density lipoprotein-cholesterol;
bolic syndrome are closely related to excess weight gain,
especially when it is associated with accumulation of
adipose tissue in the abdominal cavity around the vis-
of the components of the metabolic syndrome is
primary cause of increased blood glucose concentra-
sclerosis and injury to various organs throughout the
with the syndrome are risk factors for cardiovascular
betes.
Although most patients with type II diabetes are
overweight or have substantial accumulation of visceral
also occur as a result of other acquired or genetic con-
ditions that impair insulin signaling in peripheral tissues
associated with marked increases in ovarian androgen
production and insulin resistance and is one of the most
proximately 6 per cent of all women during their re-
mia are found in approximately 80 per cent of affected
Excess formation of glucocorticoids (
drome) or
(acromegaly) also decreases
the sensitivity of various tissues to the metabolic effects
of insulin and can lead to development of diabetes mel-
tance.
even the increased levels of insulin are not sufficient to
erate hyperglycemia occurs after ingestion of carbohy-
Table 78–2
• Hemochromatosis (a hereditary disease that causes tissue iron
• Mutations that cause genetic obesity (e.g., melanocortin
(PPAR
• Mutations of the peroxisome proliferators’ activator receptor
• Mutations of insulin receptor
• Autoantibodies to the insulin receptor
• Lipodystrophy (acquired or genetic; associated with lipid
• Polycystic ovary disease
• Pregnancy, gestational diabetes
• Excess growth hormone (acromegaly)
• Excess glucocorticoids (Cushing’s syndrome or steroid therapy)
• Obesity/overweight (especially excess visceral adiposity)
Some Causes of Insulin Resistance
accumulation in liver)
g
g)
receptor mutations)
accumulation)

increased in type II diabetes.
measurements of plasma insulin, with plasma insulin
lished on the basis of such a curve, and type I and type
the control level. The
below
furthermore, it fails to fall
the upper curve in Figure 78–12, and the glucose level
normal rise in blood glucose level, as demonstrated by
glucose, these people exhibit a much greater than
ance test is almost always abnormal. On ingestion of
and often above 140 mg/100 ml. Also, the glucose toler-
concentration is almost always above 110 mg/100 ml
In a person with diabetes, the fasting blood glucose
about 2 hours.
140 mg/100 ml and falls back to below normal in
glucose level rises from about 90 mg/100 ml to 120 to
of glucose per kilogram of body weight, the blood
curve,” when a normal, fasting person ingests 1 gram
curve in Figure 78–12, called a “glucose tolerance
Glucose Tolerance Test.
type II diabetes, plasma insulin concentration may be
or undetectable during fasting and even after a meal. In
In type I diabetes, plasma insulin levels are very low
least marked insulin resistance.
upper limit of normal. A fasting blood glucose level
90 mg/100 ml, and 110 mg/100 ml is considered to be the
The fasting blood
and the intake of carbohydrates.
large amounts, in proportion to the severity of disease
normal person loses undetectable amounts of glucose,
the quantity of glucose lost in the urine. In general, a
Urinary Glucose.
and type II diabetes mellitus. The usual methods for
Table 78–3 compares some of clinical features of type I
Physiology of Diagnosis
glucose.
However, in the later stages of type II diabetes, insulin
, may also be used.
by the pancreas, such as
, or drugs that cause additional release of insulin
thiazolidinediones
insulin sensitivity, such as
insulin administration is required. Drugs that increase
restriction, and weight reduction, and no exogenous
treated, at least in the early stages, with exercise, caloric
In many instances, type II diabetes can be effectively
type II diabetes.
whether an individual’s pancreas can sustain the high
diabetes mellitus occurs. Some studies suggest that
from secreting large amounts of insulin, and full-blown
however, the pancreas gradually becomes exhausted
abnormalities of glucose metabolism. In others,
diabetes mellitus; apparently, the pancreas in these
glucose after a meal, never develop clinically significant
Some obese people, although having marked insulin
hyperglycemia, especially after the person ingests a
beta cells become “exhausted” and are unable to
In the later stages of type II diabetes, the pancreatic
Insulin, Glucagon, and Diabetes Mellitus
Chapter 78
975
produce enough insulin to prevent more severe
carbohydrate-rich meal.
resistance and greater than normal increases in blood
people produces enough insulin to prevent severe
genetic factors play an important role in determining
output of insulin over many years that is necessary to
avoid the severe abnormalities of glucose metabolism in
and met-
formin
sulfonylureas
administration is usually required to control plasma
of Diabetes Mellitus
diagnosing diabetes are based on various chemical tests
of the urine and the blood.
Simple office tests or more complicated
quantitative laboratory tests may be used to determine
whereas a person with diabetes loses glucose in small to
Fasting Blood Glucose and Insulin Levels.
glucose level in the early morning is normally 80 to
above this value often indicates diabetes mellitus or a
severalfold higher than normal and usually increases to
a greater extent after ingestion of a standard glucose
load during a glucose tolerance test (see the next
paragraph).
As demonstrated by the bottom
falls back to the control value only after 4 to 6 hours;
slow fall of this curve and its failure to fall below the
control level demonstrate that either (1) the normal
increase in insulin secretion after glucose ingestion does
not occur or (2) there is decreased sensitivity to insulin.
A diagnosis of diabetes mellitus can usually be estab-
II diabetes can be distinguished from each other by
being low or undetectable in type I diabetes and
Clinical Characteristics of Patients with Type I and Type II
Table 78–3
sulfonylureas,
thiazolidinediones,
Therapy
Insulin
Weight loss,
Insulin sensitivity
Normal
Reduced
Plasma glucose
Increased
Increased
suppressed
suppression
Plasma glucagon
High, can be
High, resistant to
Plasma insulin
Low or absent
Normal to high
Body mass
Low (wasted) to
Obese
20 years
Usually
Age at onset
Usually
Feature
Type I
Type II
Diabetes Mellitus
<
>30 years
normal
initially
metformin,
insulin
2
3
4
0
1
Normal
Diabetes
80
200
180
160
140
120
100
5
Blood glucose level
(mg/100 ml)
Hours
Glucose tolerance curve in a normal person and in a person with
Figure 78–12
diabetes.

Metab 285:E685, 2003.
of hepatic gluconeogenesis. Am J Physiol Endocrinol
Barthel A, Schmoll D: Novel concepts in insulin regulation
indirect? J Clin Invest 111:434, 2003.
Barrett EJ: Insulin’s effect on glucose production: direct or
nervous system often occurs.
rapidly. If treatment is not effected immediately, per-
the administration of glucagon (or, less effectively,
the patient out of shock within a minute or more. Also,
of large quantities of glucose. This usually brings
insulin. The acetone breath and the rapid, deep breath-
only a state of coma remains. Indeed, at times it is dif-
the glucose level falls still lower, the seizures cease and
seizures and loss of consciousness are likely to occur. As
blood glucose level falls to 20 to 50 mg/100 ml, clonic
trembles all over, and breaks out in a sweat. As the
the patient simply experiences extreme nervousness,
various forms of hallucinations result, but more often
hypoglycemia sensitizes neuronal activity. Sometimes
usually becomes quite excitable, because this degree of
of 50 to 70 mg/100 ml, the central nervous system
may occur as follows.
insulin shock
too much insulin to themselves, the syndrome called
depressed. Consequently, in patients with insulin-secret-
of insulin cause blood glucose to fall to low values, the
necessary for this use of glucose. However, if high levels
all its energy from glucose metabolism, and insulin is not
these patients.
the primary and the metastatic cancers. Indeed, more
body, causing tremendous production of insulin by both
adenomas are malignant, and occasionally metastases
islet of Langerhans. About 10 to 15 per cent of these
Although much rarer than diabetes, excessive insulin
prevent these disturbances.
level of blood lipids as well as the level of blood glucose,
diabetic retinopathy, cataracts, hypertension, and
erosclerosis, greatly increased susceptibility to infection,
This decreases the rate of fat metabolism and depresses
large enough insulin to metabolize the carbohydrates.
olism. Consequently, the current tendency is to allow the
high and attenuated loss of glucose in the urine, but it
that the insulin requirements would be minimized. This
In the early days of treating diabetes, the tendency
people. Indeed, those who have poorly controlled dia-
riosclerosis, severe coronary heart disease, and multiple
lesterol and other lipids, develop atherosclerosis, arte-
Diabetic patients,
Relation of Treatment to Arteriosclerosis.
ing its effectiveness.
nity and sensitization against animal insulin, thus limit-
duced by the recombinant DNA process has become
from animal pancreata. However, human insulin pro-
In the past, the insulin used for treatment was derived
insulin must be used to regulate blood glucose.
by the pancreas. In many persons, however, exogenous
fails, drugs may be administered to increase insulin sen-
weight loss and to reverse the insulin resistance. If this
In persons with type II diabetes, dieting and exercise
Thus, each patient is provided with an individualized
glucose level tends to rise too high, such as at mealtimes.
out the day. Then additional quantities of regular insulin
single dose of one of the longer-acting insulins each day
narily, a patient with severe type I diabetes is given a
fore have effects that last as long as 10 to 48 hours. Ordi-
from 3 to 8 hours, whereas other forms of insulin (pre-
“Regular” insulin has a duration of action that lasts
normal as possible. Insulin is available in several forms.
carbohydrate, fat, and protein metabolism that is as
The theory of treatment of type I diabetes mellitus is to
Treatment of Diabetes
type II diabetes.
energy, keto acids are then produced in persons with
However, when insulin resistance becomes very severe
keto acids are usually not produced in excess amounts.
betes. In the early stages of type II diabetes, however,
detected by chemical means in the urine, and their
on the breath of a patient. Also, keto acids can be
air. Consequently, one can frequently make a diagnosis
acetone. This is volatile and vaporized into the expired
increase greatly in severe diabetes, are converted to
quantities of acetoacetic acid in the blood, which
As pointed out in Chapter 68, small
976
Unit XIV
Endocrinology and Reproduction
Acetone Breath.
of type I diabetes mellitus simply by smelling acetone
quantitation aids in determining the severity of the dia-
and there is greatly increased utilization of fats for
administer enough insulin so that the patient will have
cipitated with zinc or with various protein derivatives)
are absorbed slowly from the injection site and there-
to increase overall carbohydrate metabolism through-
are given during the day at those times when the blood
pattern of treatment.
are usually recommended in an attempt to induce
sitivity or to stimulate increased production of insulin
more widely used because some patients develop immu-
mainly because of their high levels of circulating cho-
microcirculatory lesions far more easily than do normal
betes throughout childhood are likely to die of heart
disease in early adulthood.
was to severely reduce the carbohydrates in the diet so
procedure kept the blood glucose from increasing too
did not prevent many of the abnormalities of fat metab-
patient an almost normal carbohydrate diet and to give
the high level of blood cholesterol.
Because the complications of diabetes—such as ath-
chronic renal disease—are closely associated with the
most physicians also use lipid-lowering drugs to help
Insulinoma—Hyperinsulinism
production occasionally occurs from an adenoma of an
from the islets of Langerhans spread throughout the
than 1000 grams of glucose have had to be administered
every 24 hours to prevent hypoglycemia in some of
Insulin Shock and Hypoglycemia.
As already emphasized,
the central nervous system normally derives essentially
metabolism of the central nervous system becomes
ing tumors or in patients with diabetes who administer
As the blood glucose level falls into the range
ficult by simple clinical observation to distinguish
between diabetic coma as a result of insulin-lack acido-
sis and coma due to hypoglycemia caused by excess
ing of diabetic coma are not present in hypoglycemic
coma.
Proper treatment for a patient who has hypoglycemic
shock or coma is immediate intravenous administration
epinephrine) can cause glycogenolysis in the liver and
thereby increase the blood glucose level extremely
manent damage to the neuronal cells of the central
References

108:1422, 2003.
guide to origins and treatment: Part I. Circulation
Wilson PW, Grundy SM: The metabolic syndrome: practical
J Clin Endocrinol Metab 89:2526, 2004.
Ten S, Maclaren N: Insulin resistance syndrome in children.
24:91, 2003.
meets metabolism: multiple links to diabetes. Endocr Rev
Shi Y, Taylor SI, Tan SL, Sonenberg N: When translation
Med 349:2560, 2003.
Saltiel AR: Putting the brakes on insulin signaling. N Engl J
human skeletal muscle. News Physiol Sci 19:92, 2004.
Roden M: How free fatty acids inhibit glucose utilization in
106:165, 2000.
molecular targets of insulin resistance. J Clin Invest
Pessin JE, Saltiel AR: Signaling pathways in insulin action:
J Obes Relat Metab Disord 27(Suppl 3):S6, 2003.
of insulin resistance: potential links with inflammation. Int
Perseghin G, Petersen K, Shulman GI: Cellular mechanism
muscle cells. Physiol Rev 83:183, 2003.
Mann GE, Yudilevich DL, Sobrevia L: Regulation of amino
J Physiol Endocrinol Metab 286:E875, 2004.
the development and growth of pancreatic beta-cells. Am
List JF, Habener JF: Glucagon-like peptide 1 agonists and
phia: WB Saunders Co, 2003.
Williams Textbook of Endocrinology, 10th ed. Philadel-
Larsen PR, Kronenberg HM, Melmed S, Polonsky KS:
285:E669, 2003.
insulin secretion. Am J Physiol Endocrinol Metab
pancreatic beta-cell: lessons from models of impaired
Kowluru A: Regulatory roles for small G proteins in the
litus. Lancet 364:203, 2004.
Hussain MA, Theise ND: Stem-cell therapy for diabetes mel-
humans. Am J Physiol Endocrinol Metab 287:E199, 2004.
Holst JJ, Gromada J: Role of incretin hormones in the reg-
cell: man and mouse provide the key. J Clin Invest 114:314,
Hattersley AT: Unlocking the secrets of the pancreatic beta
Am J Hypertens 7:772, 1994.
Hall JE, Summers RL, Brands MW, et al: Resistance to the
109:433, 2004.
ence on scientific issues related to definition. Circulation
and Blood Institute/American Heart Association confer-
metabolic syndrome: Report of the National Heart, Lung,
Grundy SM, Brewer HB Jr, Cleeman JI, et al: Definition of
Endocrinol Metab 88:2412, 2003
receptor gamma and its therapeutic modulation. J Clin
metabolic syndrome: peroxisome proliferator-activated
Gurnell M, Savage DB, Chatterjee VK, O’Rahilly S: The
neered beta-cell lines. Ann N Y Acad Sci 1014:88, 2004.
Efrat S: Regulation of insulin secretion: insights from engi-
Physiol Rev 84:239, 2004.
sulinism in infancy: from basic science to clinical disease.
Dunne MJ, Cosgrove KE, Shepherd RM, et al: Hyperin-
289:2254, 2003.
and type 2 diabetes mellitus: scientific review. JAMA
DeWitt DE, Hirsch IB: Outpatient insulin therapy in type 1
Physiol Endocrinol Metab 287:E371, 2004.
in real life? Considerations on shape and function. Am J
Caumo A, Luzi L: First-phase insulin secretion: does it exist
glucose transporter GLUT4. Nat Rev Mol Cell Biol 3:267,
Bryant NJ, Govers R, James DE: Regulated transport of the
Limited, 2002.
crinology, 3rd ed. Philadelphia: Mosby, Elsevier Science
Besser GM, Thorner MO: Comprehensive Clinical Endo-
Insulin, Glucagon, and Diabetes Mellitus
Chapter 78
977
2002.
metabolic actions of insulin and its role in hypertension.
2004.
ulation of insulin secretion in diabetic and nondiabetic
acid and glucose transporters in endothelial and smooth
