HEART FAILURE
Prof. Dr. Najah R HadiMRCP,FRCP, FACP,FACC, PhD
Overview
Heart failure develops when the heart, via an abnormality of cardiac function (detectable or not), fails to pump enough blood to meet the metabolic requirements of the metabolizing tissues or is able to do so only with an elevated diastolic filling pressureEtiology
Causes of heart failure fall into the following 4 broad categories :• Underlying causes: Underlying causes of heart failure include structural abnormalities (congenital or acquired) that affect the peripheral and coronary arterial circulation, pericardium, myocardium, or cardiac valves, thus leading to increased hemodynamic burden or myocardial or coronary insufficiency
• Fundamental causes: Fundamental causes include the biochemical and physiologic mechanisms, through which either an increased hemodynamic burden or a reduction in oxygen delivery to the myocardium results in impairment of myocardial contraction
• Precipitating causes: Overt heart failure may be precipitated by progression of the underlying heart disease (eg, further narrowing of a stenotic aortic valve or mitral valve) or various conditions (fever, anemia, infection) or medications (chemotherapy, NSAIDs) that alter the homeostasis of heart failure patients
• Genetics of cardiomyopathy: Dilated, arrhythmic right ventricular and restrictive cardiomyopathies are known genetic causes of heart failure.
Underlying causes:
Underlying causes of systolic heart failure include the following:• Coronary artery disease
• Diabetes mellitus
• Hypertension
• Valvular heart disease (stenosis or regurgitant lesions)
• Arrhythmia (supraventricular or ventricular)
• Infections and inflammation (myocarditis)
• Peripartum cardiomyopathy
• Congenital heart disease
• Drugs (either recreational, such as alcohol and cocaine, or therapeutic drugs with cardiac side effects, such as doxorubicin)
• Idiopathic cardiomyopathy
• Rare conditions (endocrine abnormalities, rheumatologic disease, neuromuscular conditions)
Underlying causes of diastolic heart failure include the following:
• Coronary artery disease
• Diabetes mellitus
• Hypertension
• Valvular heart disease (aortic stenosis)
• Hypertrophic cardiomyopathy
• Restrictive cardiomyopathy (amyloidosis, sarcoidosis)
• Constrictive pericarditis
Underlying causes of acute heart failure include the following:
• Acute valvular (mitral or aortic) regurgitation
• Myocardial infarction
• Myocarditis
• Arrhythmia
• Drugs (eg, cocaine, calcium channel blockers, or beta-blocker overdose)
• Sepsis
Underlying causes of high-output heart failure include the following:
• Anemia• Systemic arteriovenous fistulas
• Hyperthyroidism
• Beriberi heart disease
• Paget disease of bone
• Albright syndrome (fibrous dysplasia)
• Multiple myeloma
• Pregnancy
• Glomerulonephritis
• Polycythemia vera
• Carcinoid syndrome
Underlying causes of right heart failure include the following:
• Left ventricular failure
• Coronary artery disease (ischemia)
• Pulmonary hypertension
• Pulmonary valve stenosis
• Pulmonary embolism
• Chronic pulmonary disease
• Neuromuscular disease
Precipitating causes of heart failure
• Profound anemia• Thyrotoxicosis
• Myxedema
• Paget disease of bone
• Albright syndrome
• Multiple myeloma
• Glomerulonephritis
• Cor pulmonale
• Polycythemia vera• Obesity
• Carcinoid syndrome
• Pregnancy
• Nutritional deficiencies (eg, thiamine deficiency, beriberi)
Compensatory mechanisms
The Frank-Starling mechanism, in which an increased preload helps to sustain cardiac performance
Alterations in myocyte regeneration and death
Myocardial hypertrophy with or without cardiac chamber dilatation, in which the mass of contractile tissue is augmented
Activation of neurohumoral systems
Pathophysiology
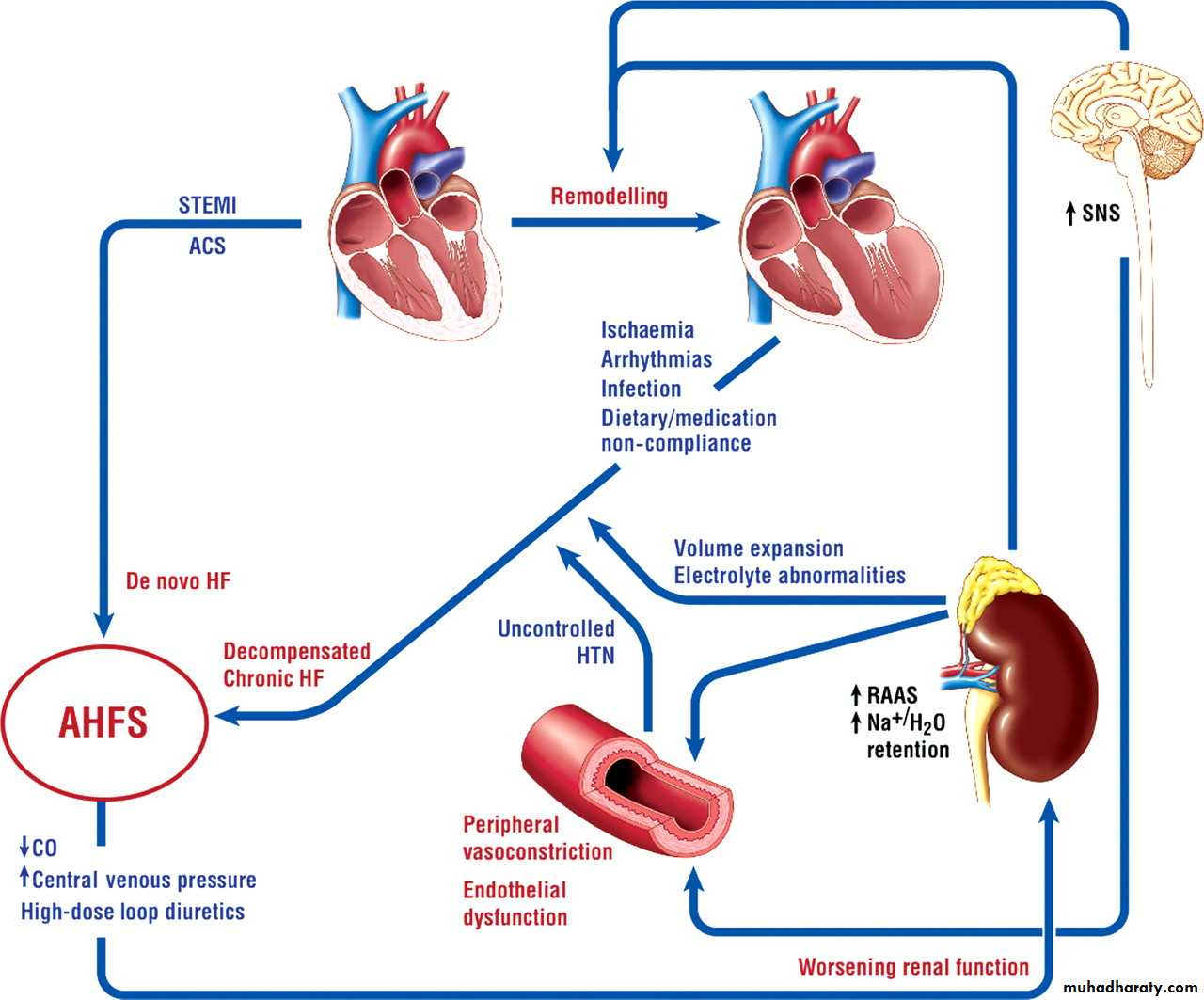
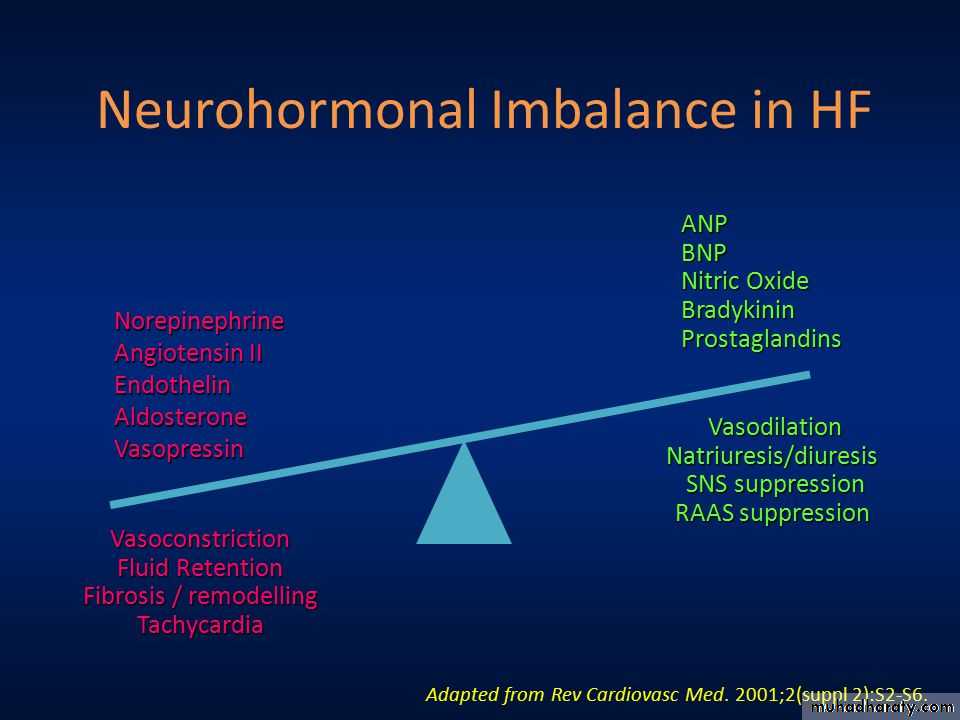
Neurohormonal imbalance contributes to the pathophysiology of chronic heart failure
In patients with heart failure, left ventricular remodeling leads to changes in cardiac volume and wall thickness. The abnormality in cardiac structure or function contributes to:• Inadequate cardiac output
• Poor organ perfusion
• Activation of the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system (SNS)
Activation of the RAAS gives rise to:
• Vasoconstriction
• Increased sodium and water retention
• Fibrosis
• Hypertrophy
• Increased blood pressure
Activation of the SNS leads to:
Vasoconstriction
Increased heart rate and myocardial contractility
Sodium retention
Renin release
Natriuretic Peptides
Natriuretic peptides help counter the effects of the RAAS and SNS in chronic heart failure but this balancing effect is diminished as heart failure progresses.Sustained overactivation of the RAAS and SNS with attenuation of the effects of the natriuretic peptide system leads to neurohormonal imbalance in heart failure
Only when compensatory mechanisms overwhelmed symptoms and signs appear.
Clinical presentationBreathlessness, a cardinal symptom of LV failure, may manifest with progressively increasing severity as the following:
Exertional dyspnea
Orthopnea
Paroxysmal nocturnal dyspnea
Dyspnea at rest
Acute pulmonary edema
Other cardiac symptoms of heart failure include chest pain/pressure and palpitations.
Common noncardiac signs and symptoms of heart failure include anorexia, nausea, weight loss, bloating, fatigue, weakness, oliguria, nocturia, and cerebral symptoms of varying severity, ranging from anxiety to memory impairment and confusion.
Distention of neck veins
Weak, rapid, and thready pulseRales, wheezing
S 3 gallop and/or pulsus alternans
Increased intensity of P 2 heart sound
Hepatojugular reflux
Ascites, hepatomegaly, and/or anasarca
Central or peripheral cyanosis, pallor
Left Side Heart Failure
BreathlessnessBasal lung crackles
Acute pulmonary oedema
Cold extremities
Easy fatigability
Right Side Heart Failure
Ascites, congestive hepatomegaly, and anasarca due to elevated right-sided heart pressures transmitted backward into the portal vein circulation may result in increased abdominal girth and epigastric and right upper quadrant (RUQ) abdominal painBilateral leg oedema.
The New York Heart Association (NYHA) classification
Class I patients have no limitation of physical activity
Class II patients have slight limitation of physical activity
Class III patients have marked limitation of physical activity
Class IV patients have symptoms even at rest and are unable to carry on any physical activity without discomfort
Differential Diagnosis
Acute Kidney InjuryAcute Respiratory Distress Syndrome
Bacterial Pneumonia
Cardiogenic Pulmonary Edema
Chronic Obstructive Pulmonary Disease (COPD)
Cirrhosis
Community-Acquired Pneumonia (CAP)
Emphysema
Goodpasture Syndrome
Idiopathic Pulmonary FibrosisInterstitial (Nonidiopathic) Pulmonary Fibrosis
Myocardial Infarction
Nephrotic Syndrome
Neurogenic Pulmonary Edema
Pneumothorax Imaging
Pulmonary Embolism
Respiratory Failure
Venous Insufficiency
Viral Pneumonia
• Work Up: The American College of Cardiology/American Heart Association (ACC/AHA\, Heart Failure Society of America (HFSA )and European Society of Cardiology (ESC) recommend the following basic laboratory tests and studies in the initial evaluation of patients with suspected heart failure:
• Complete blood count (CBC), which may indicate anemia or infection as potential causes of heart failure
• Urinalysis (UA), which may reveal proteinuria, which is associated with cardiovascular disease
• Serum electrolyte levels, which may be abnormal owing to causes such as fluid retention or renal dysfunction
• Blood urea nitrogen (BUN) and creatinine levels, which may indicate decreased renal blood flow
• Fasting blood glucose levels, because elevated levels indicate a significantly increased risk for heart failure (diabetic and nondiabetic patients)
• Liver function tests (LFTs), which may show elevated liver enzyme levels and indicate liver dysfunction due to heart failure
• B-type natriuretic peptide (BNP) and N-terminal pro-B-type (NT-proBNP) natriuretic peptide levels, which are increased in heart failure; these measurements are closely correlated with the NYHA heart failure classification
• Electrocardiogram (ECG) (12-lead), which may reveal arrhythmias, ischemia/infarction, and coronary artery disease as possible causes of heart failure
The ACC/AHA, HFSA, and ESC also recommend the following imaging studies and procedures :
• Chest radiography (posterior-anterior, lateral), which may show pulmonary congestion, an enlarged cardiac silhouette, or other potential causes of the patient's symptoms• 2-D echocardiographic and Doppler flow ultrasonographic studies, which may reveal ventricular dysfunction and/or valvular abnormalities [65, 66]
• Coronary arteriography in patients with a history of exertional angina or suspected ischemic LV dysfunction, which may reveal coronary artery disease
• Maximal exercise testing with/without respiratory gas exchange and/or blood oxygen saturation, which assesses cardiac and pulmonary function with activity, the inability to walk more than short distances, and a decreased peak oxygen consumption reflect more severe disease
• Other studies may be indicated in selected patients , such as the following:
• Screening for hemochromatosis, in which iron overload affects cardiac function• Screening for sleep-disturbed breathing, which affects neurohormonal activation
• Screening for human immunodeficiency virus (HIV), which may result in heart failure from possible direct infectious effects, from disease treatment effects causing CAD, or from other causes
• Testing for rheumatologic diseases, amyloidosis, or pheochromocytoma, all of which may cause cardiomyopathy
• Serum and urine electrophoresis for light-chain disease
• Genetic testing for at-risk patients with a first-degree relative who has been diagnosed with a cardiomyopathy leading to heart failure, which may aid in detecting early disease onset and guide treatment
• Holter monitoring, which may reveal arrhythmias or abnormal electrical activity (eg, in patients with heart failure and a history of MI who are being considered for electrophysiologic study to document ventricular tachycardia [VT] inducibility)
Treatment
Non-pharmacological
Pharmacological
Invasive proceduresNon-pharmacological
• Dietary salt (sodium) restriction• Aerobic regular exercise
• Omega-3 supplementation
Medications
• Diuretics (to reduce edema by reduction of blood volume and venous pressures) and salt restriction (to reduce fluid retention) in patients with current or previous heart failure symptoms and reduced left ventricular ejection fraction (LVEF) for symptomatic relief• Angiotensin-converting enzyme inhibitors (ACEIs) for neurohormonal modification, vasodilatation, improvement in LVEF, and survival benefit
• Angiotensin receptor blockers (ARBs) for neurohormonal modification, vasodilatation, improvement in LVEF, and survival benefit
• Hydralazine and nitrates to improve symptoms, ventricular function, exercise capacity, and survival in patients who cannot tolerate an ACEI/ARB or as an add-on therapy to ACEI/ARB and beta-blockers in the black population for survival benefit
Medications
Beta-adrenergic blockers for neurohormonal modification, improvement in symptoms and LVEF, survival benefit, arrhythmia prevention, and control of ventricular rateAldosterone antagonists, as an adjunct to other drugs for additive diuresis, heart failure symptom control, improved heart rate variability, decreased ventricular arrhythmias, reduction in cardiac workload, improved LVEF, and increase in survival
Digoxin, which can lead to a small increase in cardiac output, improvement in heart failure symptoms, and decreased rate of heart failure hospitalizations
Anticoagulants to decrease the risk of thromboembolism
Inotropic agents to restore organ perfusion and reduce congestion
Acute heart failure treatment
Stabilize the patients’ clinical condition
Establish the diagnosis, etiology, and precipitating factors
Initiate therapies to rapidly provide symptom relief
Administration of oxygen, if oxygen saturation is less than 90%, and noninvasive positive pressure ventilation (NIPPV) provides patients with respiratory support to avoid intubation.
Medical therapy for heart failure patients, the majority who present with normal perfusion and evidence of congestion, focuses on the following goals:
Preload and afterload reduction for symptomatic relief using vasodilators (nitrates, hydralazine, nipride, nesiritide, ACEI/ARB) and diuretics
Inhibition of deleterious neurohormonal activation (renin-angiotensin-aldosterone system [RAAS] and sympathetic nervous system) using ACEI/ARB, beta-blockers, and aldosterone antagonists resulting in long-term survival benefit
Treatment of Heart Failure with Normal LVEF
• Low-sodium diet• Restricted fluid intake
• Daily measurement of weight
• Exercise
• Weight loss
• Diuretic therapy is recommended to reduce fluid retention. However, patients must be monitored carefully to avoid hypotension.
• ACEI/ARBs are used as indicated for patients with atherosclerotic disease, prior MI, diabetes mellitus, or hypertension.
• Beta-blockers are indicated for patients with prior MI or hypertension and for control of ventricular rate in those with atrial fibrillation
• Aldosterone receptor blockers are indicated for hypertension and to reduce myocardial fibrosis
Treatment of Right Ventricular Heart Failure
General measures should be applied, as follows:
Sodium and fluid restriction
Moderate physical activity, with avoidance of isometric exercises
Avoidance of pregnancy
Compliance with medications
Avoidance, or rapid treatment of, precipitating factors
Precipitating factors include the following:
Sleep apnea
Pulmonary embolism
Sepsis
Arrhythmia
Ischemia
High altitude
Anemia
Hypoxemia
Use of an ACEI/ARB is beneficial if RV failure is secondary to LV failure; the efficacy of these agents in isolated RV failure is not known.
The same recommendation applies for use of beta-blockers.
The role of nesiritide in RV failure is not well defined.
Use of digoxin in RV failure associated with chronic obstructive pulmonary disease (COPD) not associated with LV dysfunction appears not to improve exercise tolerance or RV ejection fraction.
Treatment of pulmonary-induced RV failure is to address the correction of a primary pulmonary etiology and a decrease in RV afterload via specific pulmonary artery vasodilatory therapies (please see Primary Pulmonary Hypertension for treatment).
In patients with severe, hemodynamically compromising RV failure, inotropic therapy is administered, using dobutamine (2-5 mcg/kg/min), dobutamine and inhaled nitric oxide, or dopamine alone. Milrinone is preferred if the patient is tachycardic or on beta-blockers.
Anticoagulation indications are standard for evidence of intracardiac thrombus, thromboembolic events, pulmonary arterial hypertension, paroxysmal or persistent atrial fibrillation/flutter, and mechanical right-sided valves.
Hypoxemia should be corrected, and positive pressure should be avoided when mechanical ventilation is needed.
Electrophysiologic Intervention
Devices for electrophysiologic intervention in heart failure include pacemakers, cardiac resynchronization therapy (CRT) devices, and implantable cardioverter-defibrillators (ICDs).Maintaining a normal chronotropic response and AV synchrony may be particularly significant for patients with heart failure.[5] Because RV pacing may worsen heart failure due to an increase in ventricular dysynchrony, the current 2010 HFSA Practice Guidelines recommend against placement of a dual-chamber pacemaker in heart failure patients in the absence of symptomatic bradycardia or high-degree AV block.
Implantable cardioverter-defibrillators
The AHA/ACC and ESC recommend ICD placement for the following categories of heart failure patients• Patients with LV dysfunction (LVEF ≤35%) from a previous MI who are at least 40 days post-Ml
• Patients with nonischemic cardiomyopathy; with an LVEF of 35% or less; in NYHA class II or III; receiving optimal medical therapy; and expected to survive longer than 1 year with good functional status [3, 5]
• Patients with ischemic cardiomyopathy who are at least 40 days post-MI; have an LVEF of 30% or less; are in NYHA functional class I; are on chronic optimal medical therapy; and are expected to survive longer than 1 year with good functional status
• Patients who have had ventricular fibrillation (VF)
• Patients with documented hemodynamically unstable ventricular tachycardia (VT) and/or VT with syncope; with an LVEF less than 40%; on optimal medical therapy; and expected to survive longer than 1 year with good functional statusRevascularization Procedures
Percutaneous intervention (PCI)Coronary artery bypass graft (CABG)
Valvular Surgery
• Aortic valve replacement
• Mitral valve repair
• Mitral valve replacement
Ventricular Assist Devices
Ventricular assist devices (VADs) are invaluable tools in the treatment of heart failure. A number of these devices are available to support the acutely or chronically decompensated heart (ie, ACC/AHA stage D). Depending on the particular device used, the right ventricle and left ventricle can be assisted with a left ventricular assist device (LVAD), a right VAD (RVAD), or a biventricular assist device (BiVAD). An alternative term for a VAD is a ventricular assist system (VAS).LVADs, RVADs, and BiVADs are similar. Blood is removed from the failing ventricle and diverted into a pump that delivers blood to either the aorta (in the case of an LVAD) or the pulmonary artery (in the case of an RVAD). An exception is the Impella (Abiomed, Inc). This device is inserted percutaneously into the left ventricle; it draws blood from the left ventricle and expels it into the ascending aorta.
Heart Transplantation: indications
• Refractory cardiogenic shock• Dependence on IV inotropic support for adequacy of organ perfusion
• Peak oxygen consumption per unit time (VO 2) less than 10 mL/kg/min
• Severe ischemic symptoms with consistent limitations of routine activity that are not amenable to revascularization procedures (CABG, percutaneous coronary intervention)
• Recurrent symptomatic ventricular arrhythmias despite all therapeutic interventions