
Body Fluid physiology
1
إعداد
.د
هافع عاوي الفياض
إخصائي جراح العي ن

حقيق يث ت ا العلم
2

Body Fluid Compartments
Why do you need to understand body fluid
compartments?
Many of you will be applying IV care for
patients.
3
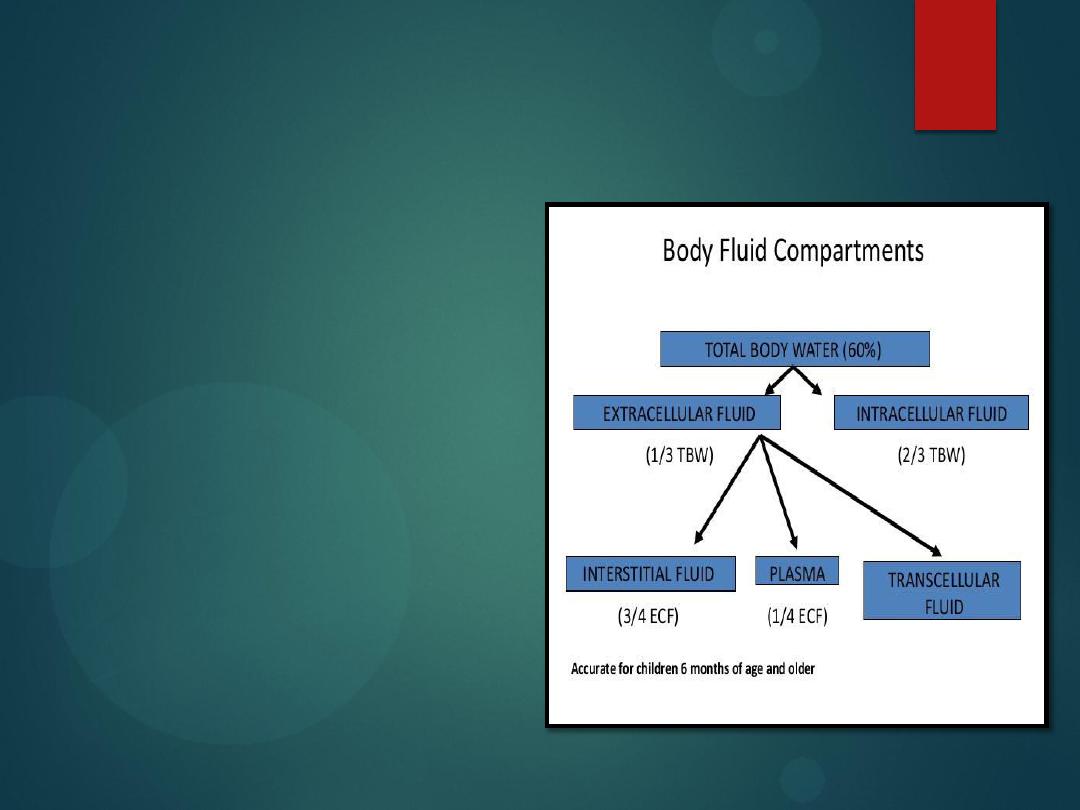
Water
Water makes up 60% of
our body weight
Divide this into two
compartments
Intracellular water
Extracellular
water
4
Diffusion
If the plasma becomes diluted to 260
mOsm, and the cells next to a blood
vessel are still at 300 mOsm, the cells
now have more particles. By a law of
physics, all particles want to move from
an area of high concentration to an
area of low concentration.
5

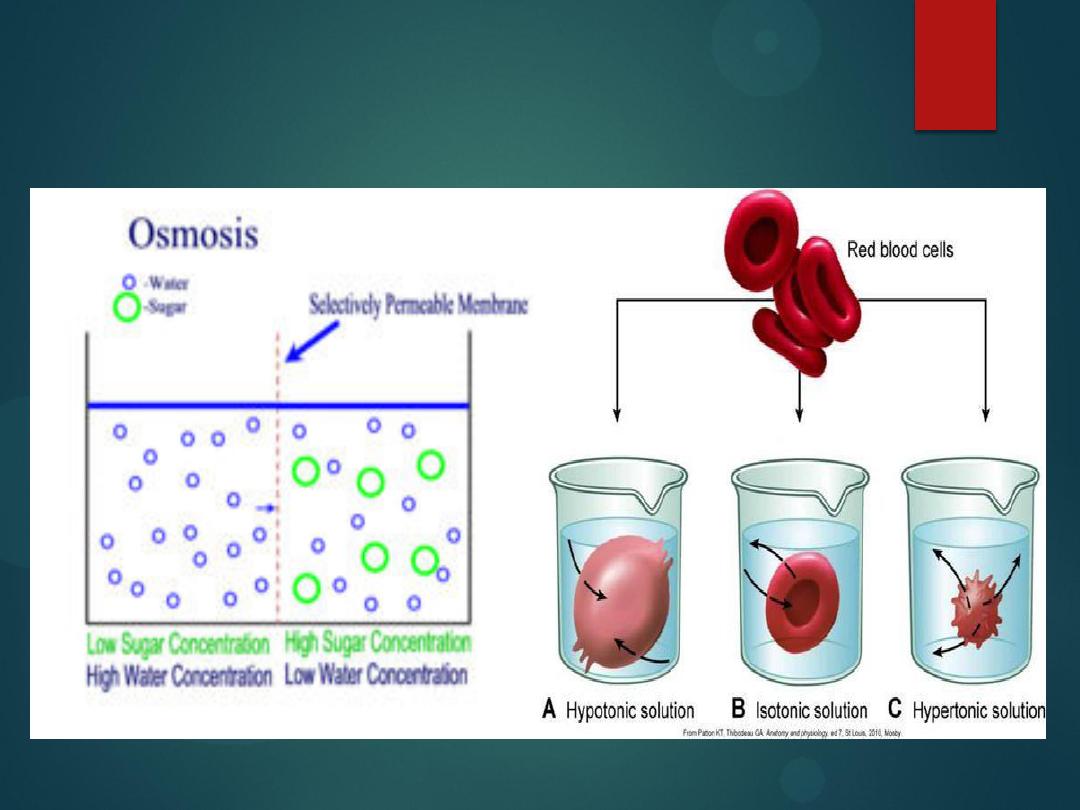
Diffusion vs osmosis
Diffusion:
The process of which molecules move from an area of
high
concentration to an area of
low
concentration.
Osmosis:
The process by which molecules of a solvent tend to pass
through a semipermiable membrane from a
less concentrated
solution into
a
more concentrated
one, thus equalizing the concentrations on each side
of the membrane.
particles suck water.
6

Diffusion
What will move, in order to dilute the cells?
Water. Why?
Because
particles suck
!
The compartment with more particles (the cells) will suck
the water in. Therefore, water will move from the plasma
to the adjacent cells.
What will that do to the cells? They will lyse (rupture).
7
Diffusion
8

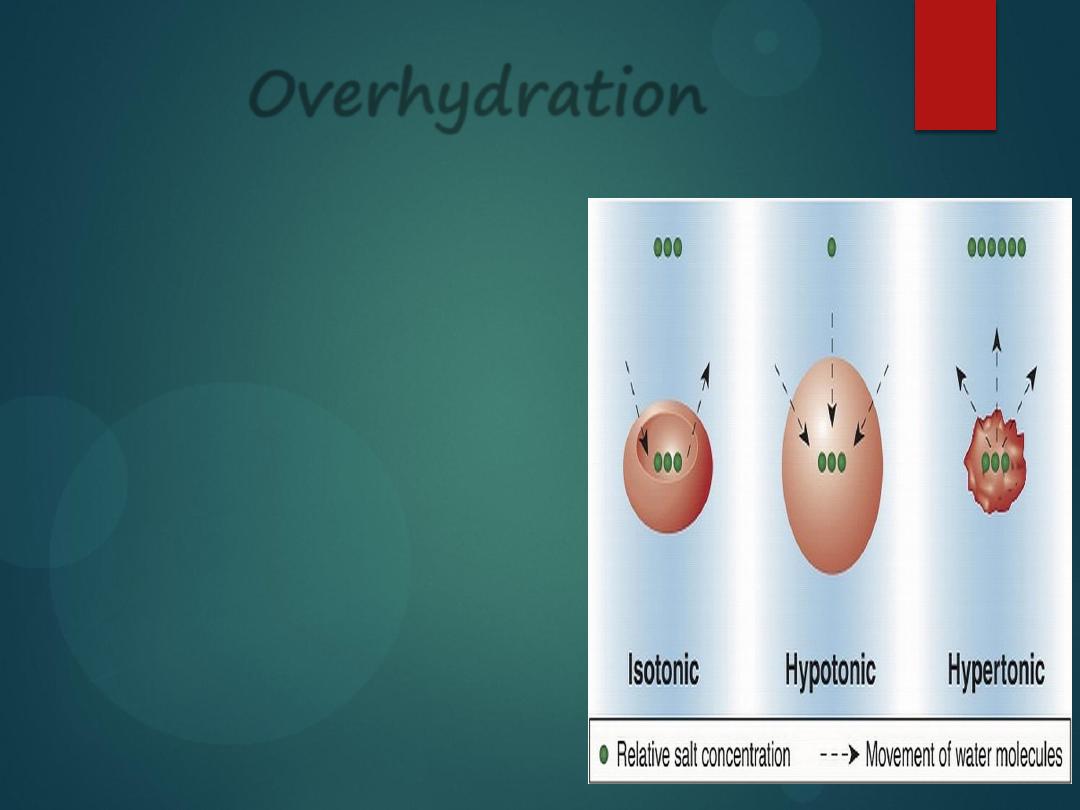
Overhydration
Drinking too much water changes the extracellular osmolality.
When the plasma is too dilute (too much water, too few solutes),
water will leave the bloodstream to enter the tissues, where there are
more solutes (
solutes SUCK
!).
Water will enter the tissues (intracellular body fluid compartment),
including the brain.
The excess water will cause the brain to swell.
9
Overhydration
Thus, we learn that if a person
is over-hydrated, the plasma
will be diluted below 300
mOsm, but the cells still have
300 mOsm in particles.
So, the cells will draw in more
water from the plasma and the
cells will
enlarge and rupture
.
10

Overhydration
Thus, we learn that if a person is over-hydrated, the plasma will
be diluted below 300 mOsm, but the cells still have 300 mOsm in
particles.
So, the cells will draw in more water from the plasma and the
cells will
enlarge and rupture
.
11

Dehydration
if a patient is mildly dehydrated, you will give an IV that was
hypotonic
(less than 300 mOsm, be careful of the drip rate) to
balance out the number of particles per liter within the plasma
and within the adjacent cells.
12

Dehydration
If a doctor accidentally tells you to give a
mildly dehydrated
patient an IV solution that
is hypertonic
(greater than
300mOsm), the plasma will have more particles than the cells,
and the cells will have the water sucked out of them, which also
causes
death
.
Hypertonic solutions
are only okay for an
overhydrated person
,
or a dehydrated person who has lost particles, such as from
blood or electrolyte loss after surgery
.
Understanding body fluid compartments is important!
13
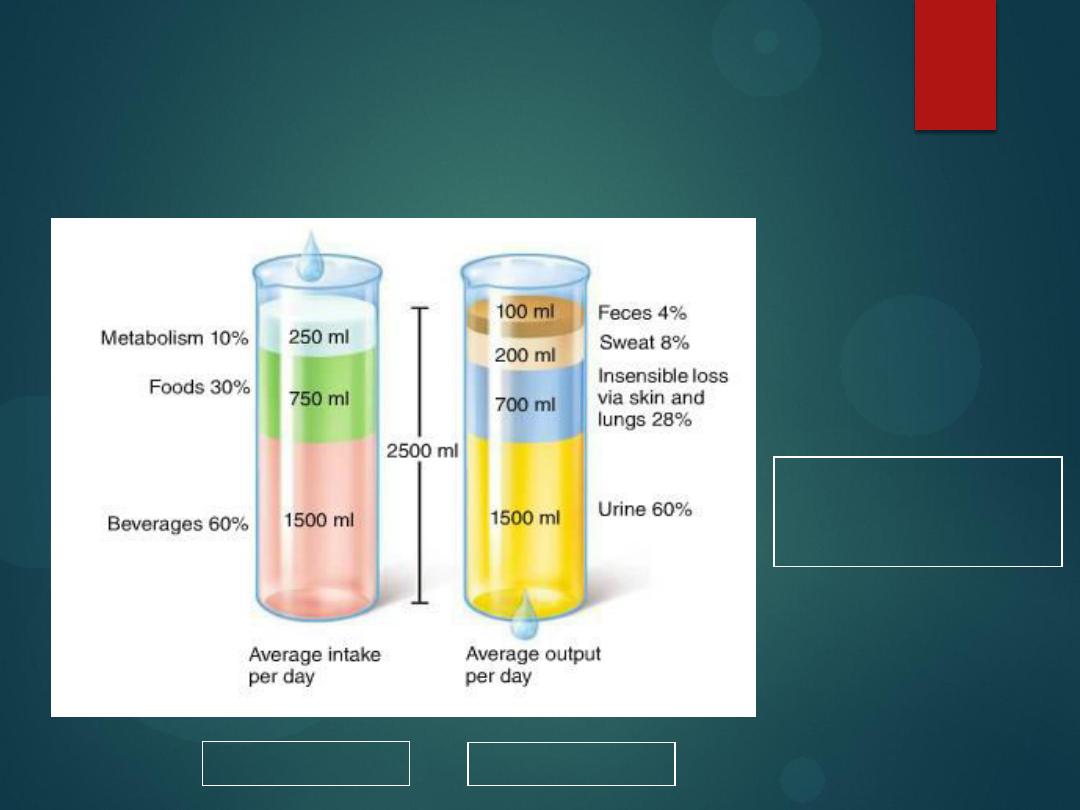
Sources of water
intake/output
14
Water intake
must equal
water output
2500 ml
2500 ml
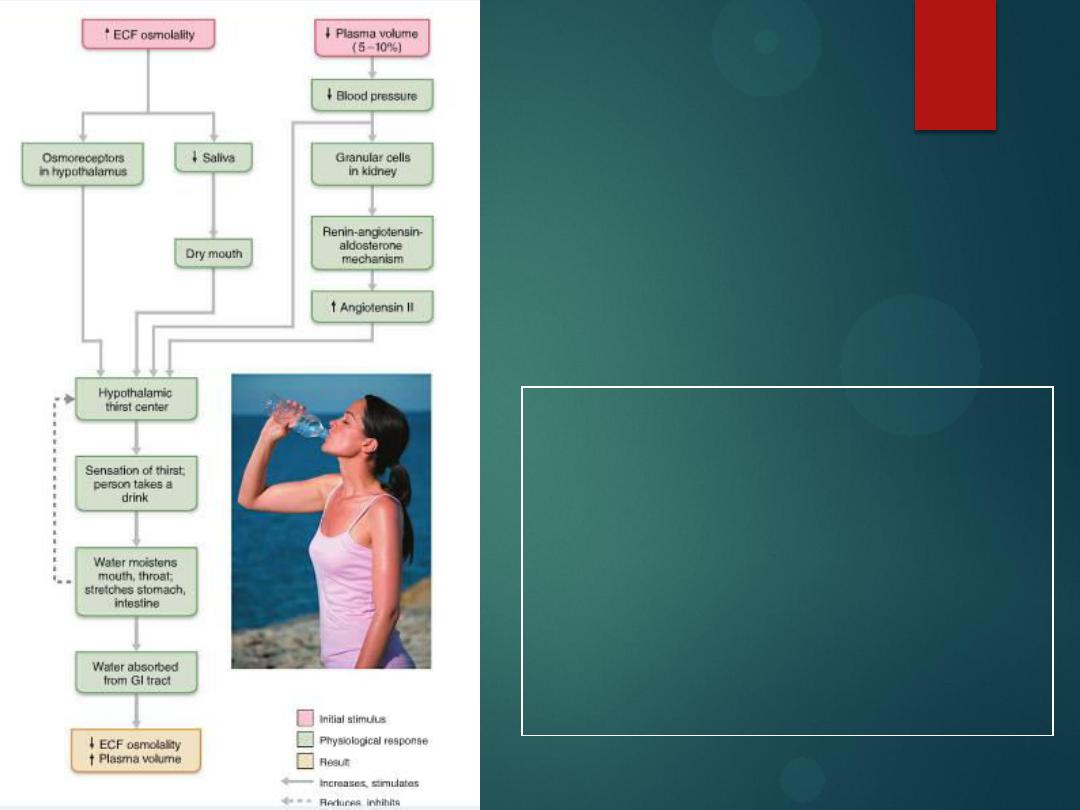
Thirst mechanism
for regulating
water intake
15
Na+ acts
as a powerful
water magnet
.
Drinking water satisfies thirst before the
water is absorbed because the mouth,
throat, and stomach sensors provide
feedback signals that inhibit the thirst
center in the brain.

Types of Dehydration
Isotonic Dehydration
Hypotonic Dehydration
Hypertonic Dehydration
16

Isotonic Dehydration
Type of Loss
: solute and water loss proportional, no change in plasma
volume, serum sodium level is decreased to 125-150 mEq/L.
The cause of the fluid loss
is GIT fluid loss (vomiting or diarrhea), large
urine loss and decreased oral intake.
Fluid Replacement Guidelines
: Initially, a bolus of 0.9% sodium chloride
or Ringer's lactate is given followed by 5% Dextrose in water and 0.45%
sodium chloride. Half of the deficit should be replaced in the first 8
hours and the remaining half over the next 16 hours
17

Hypotonic fluid deficit
Type of Loss
:
More solute is lost than water. Plasma volume moves
from the ECF to the ICF. Serum sodium levels are decreased below
125 mEq/L.
The cause of the fluid loss
is often a GI fluid loss with hypotonic oral
intake.
Fluid Replacement Guidelines
:
Initially a bolus of 0.9% sodium
chloride or Ringer's Lactate followed by 5% Dextrose in water and
0.9% sodium chloride.
18

Hypertonic fluid deficit
Type of Loss
: There is greater water loss than solute loss. Volume moves
from the ICF to the ECF. Sodium levels are maintained at over 150 mEq/L.
The cause
is GI fluid loss with hypertonic oral intake, diabetes insipidus,
fever and hyperventilation.
Fluid Replacement Guidelines
: 5% Dextrose in water and 0.45% sodium
chloride. If the patient is hypertensive 0.9% sodium chloride or Ringer's
lactate should be given.
19
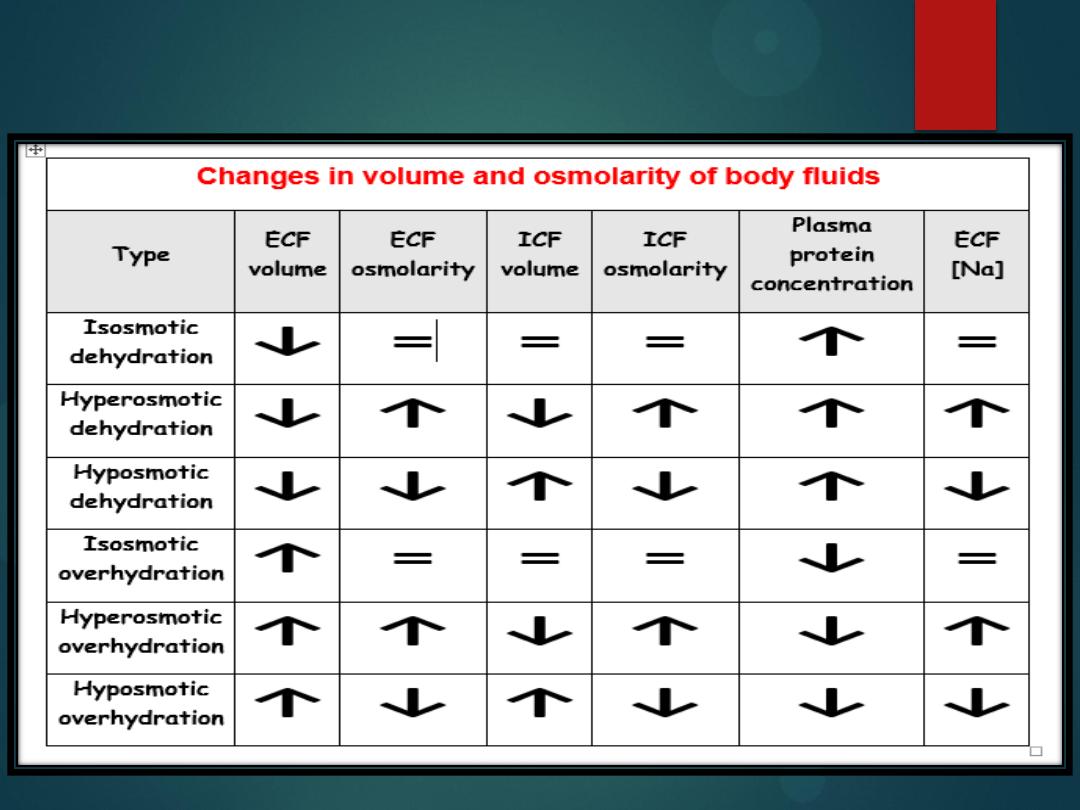
Changes in the volume and osmolarity
20

Body Fluid Compartments
Intracellular Fluid (60%)
Extracellular Fluid
Interstitial fluid
(
the water immediately outside cells,
between and around cells
)
(
30%
)
Plasma fluid
(
the water inside blood vessels, but not in
blood cells
)
(
9%
)
Transcellular fluid
(
the water enclosed in chambers lined
by epithelial membranes , including the GI tract and synovial
joints
)
(
1%
)
21

Composition of Compartments
All compartments are not the same size.
Which is the biggest?
What’s the smallest?
The
inside of cell
is
low
Na
and
Ca
, and
high
in
K
and
proteins
Outside of cells
(in the plasma) are
high
in
Na
and
Ca
,
low
in
K
and
proteins
.
Sodium has the highest extracellular fluid to
intracellular fluid concentration ratio for most
mammalian cells.
22
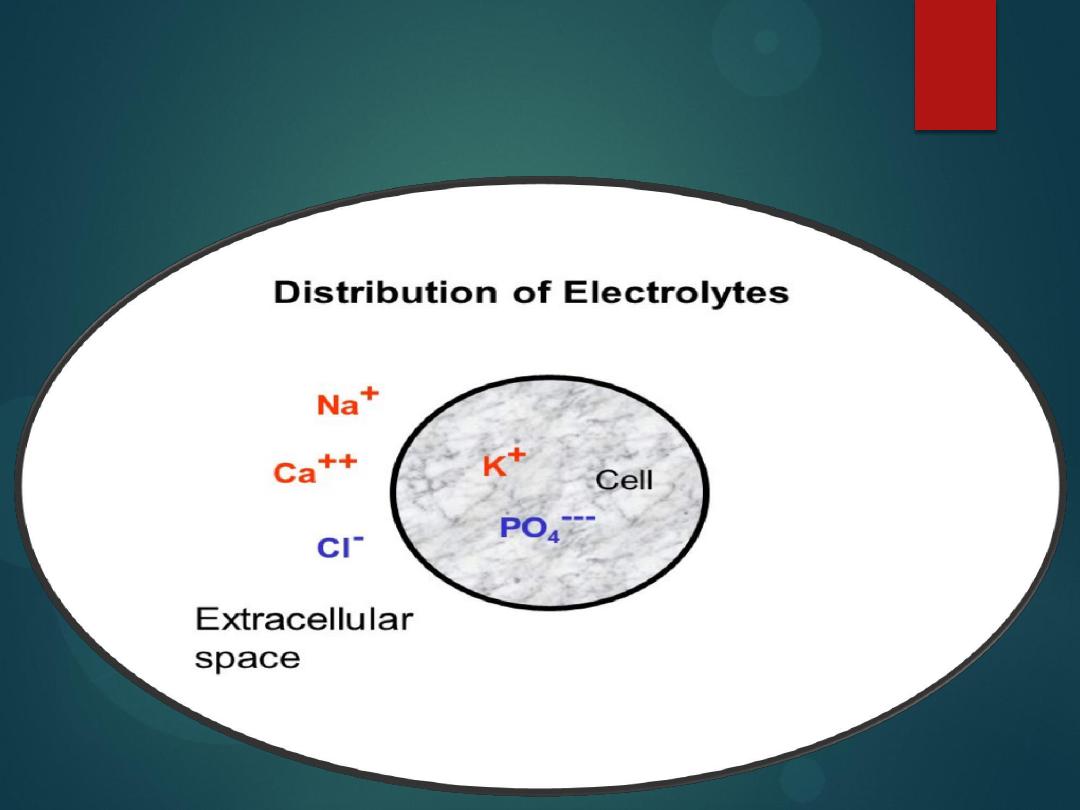
Electrolytes distribution
23
Electrolytes distribution
24

Cell membranes are semi-permeable
the cell membranes are designed to be semi-
permeable (they will only let certain
substances come into and go out of the cell).
25

26
What particles can cross the cell membrane?
Gases (O2, CO2)
Lipids and lipid-loving (hydrophobic or lipophilic)
substances, such as alcohol

Functions of Membrane
Selectively permeable- allows some substances to pass
between intracellular and extracellular fluids
Only small uncharged molecules or fat soluble molecules can
pass through membrane without help. They get through by
one of two types of ways:
Passive transport
means that energy (
ATP
) is
not
needed to get
a particular substance across the cell membrane.
Active transport
means that
ATP is used
.
27
Membrane Function
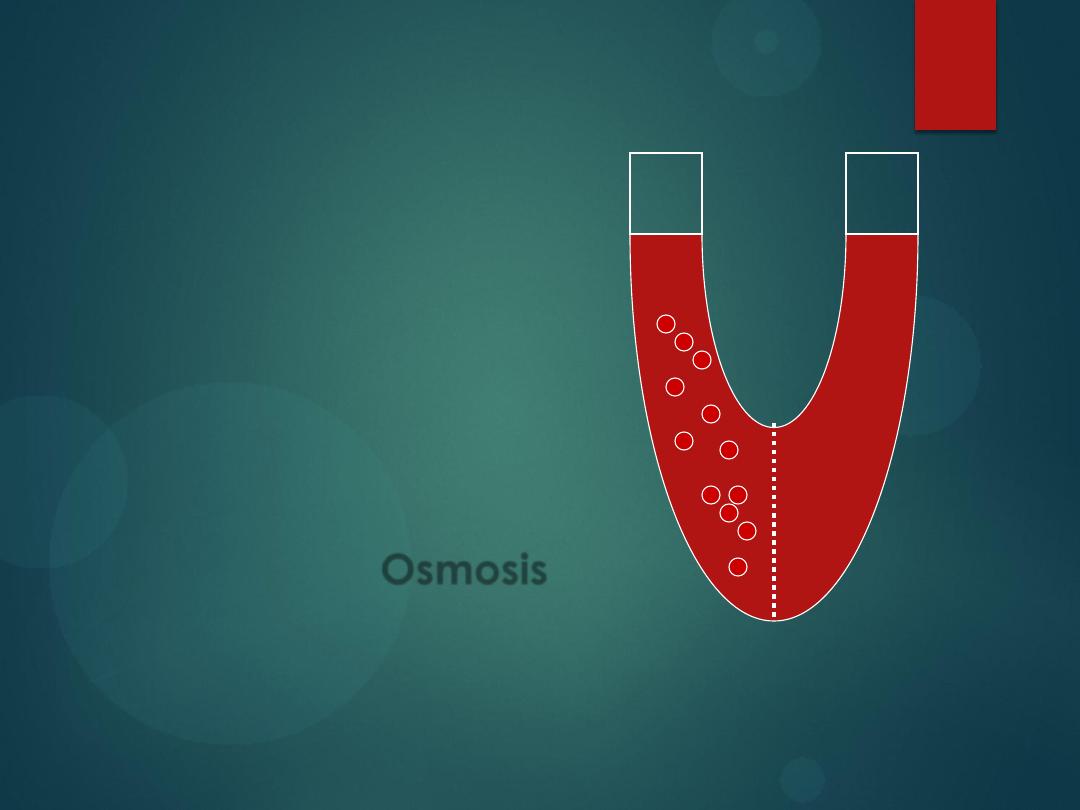
There are 3 types of Passive
Transport:
1)
Simple Diffusion
2)
Facilitated Diffusion
3)
Osmosis
All of these involve particles
crossing from high to low
concentration.
Osmosis
is
diffusion of water across a
cell membrane from low to
high concentration.
28

Facilitated diffusion
Facilitated diffusion
is when an ion wants to travel
down its concentration gradient, but there is a
channel
in the cell membrane that opens and closes
by a protein which enlarges or shrinks to open or
block the channel (remember, this is still
passive
transport
, so it
does not need ATP
).
29
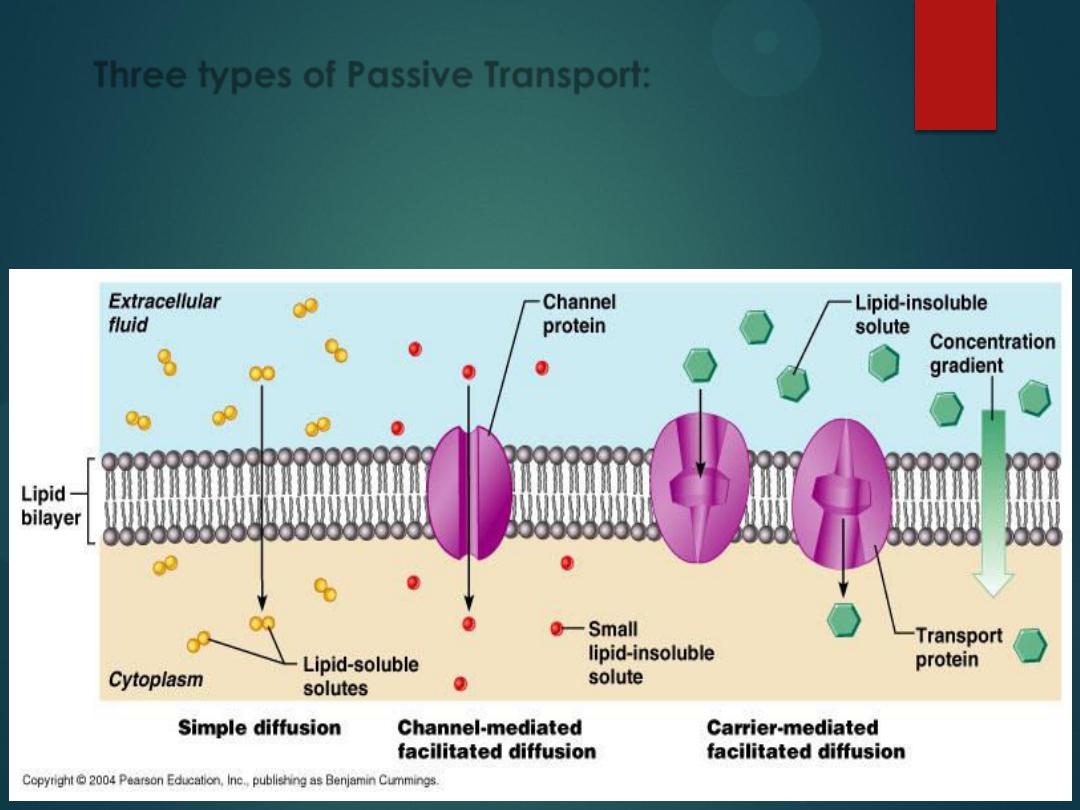
Three types of Passive Transport:
Simple Diffusion
Facilitated Diffusion
Osmosis
30
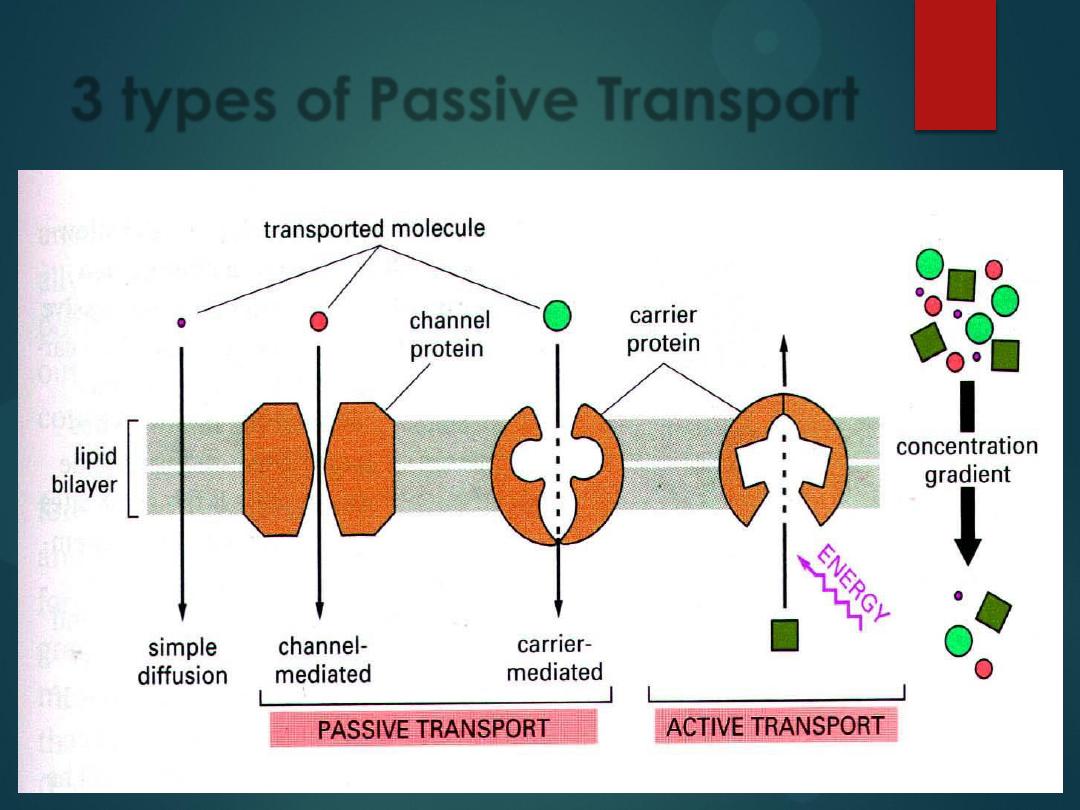
3 types of Passive Transport
31

osmosis
32

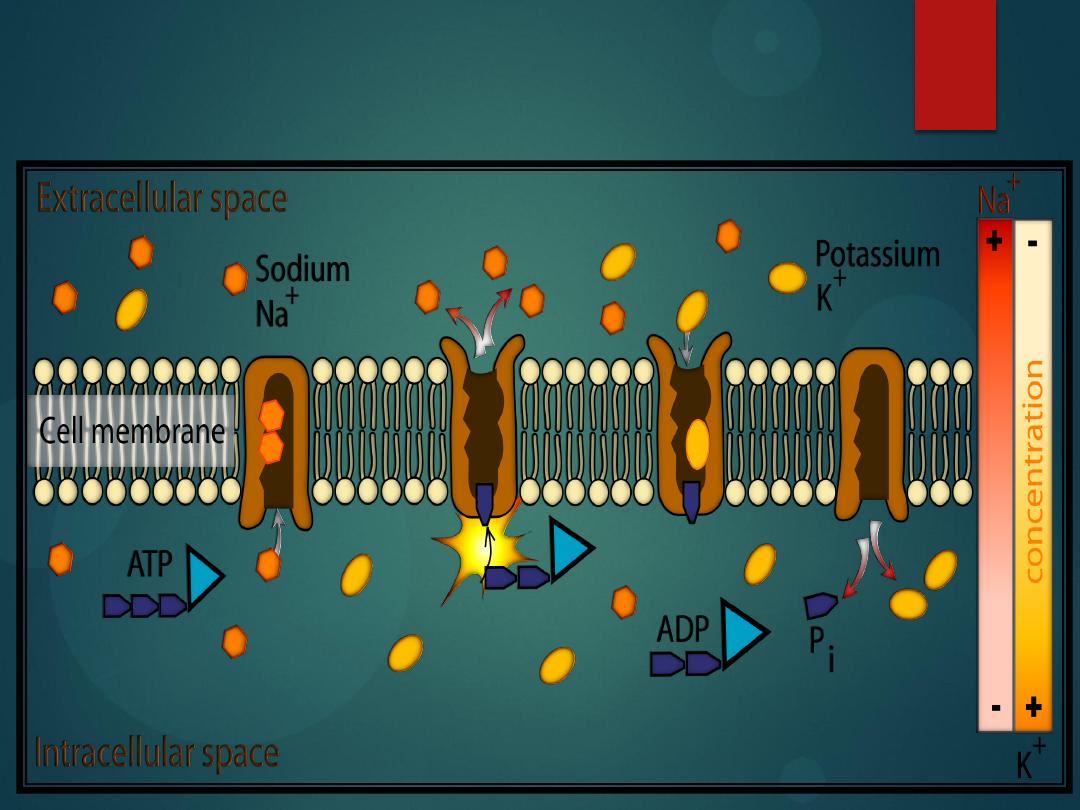
Active Transport
is when a substance needs to move against its
concentration gradient (it is moved from an
area of
low concentration
on one side of the
cell
membrane
to
an
area
of
high
concentration
on the other side of the cell
membrane).
It accomplishes this because a protein
embedded in the cell membrane grabs onto
the substance and drags it across the cell
membrane (this
requires ATP
).
33

Active Transport
There are three main types of active transport:
Ion Pumps
Cotransport
Endocytosis
these are active transport mechanisms,
ATP is used
.
34

Passive vs. Active Transport
35

Transport of Water
There are two ways that water can move:
Osmosis
Hydrostatic pressure
36
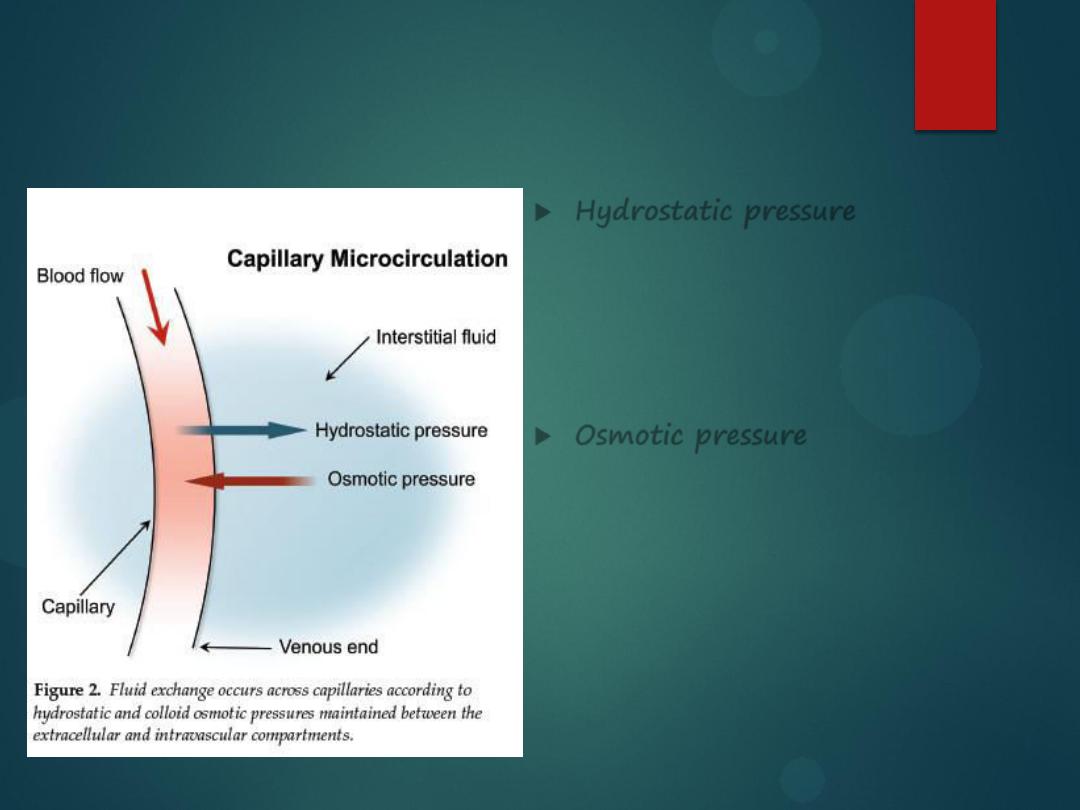
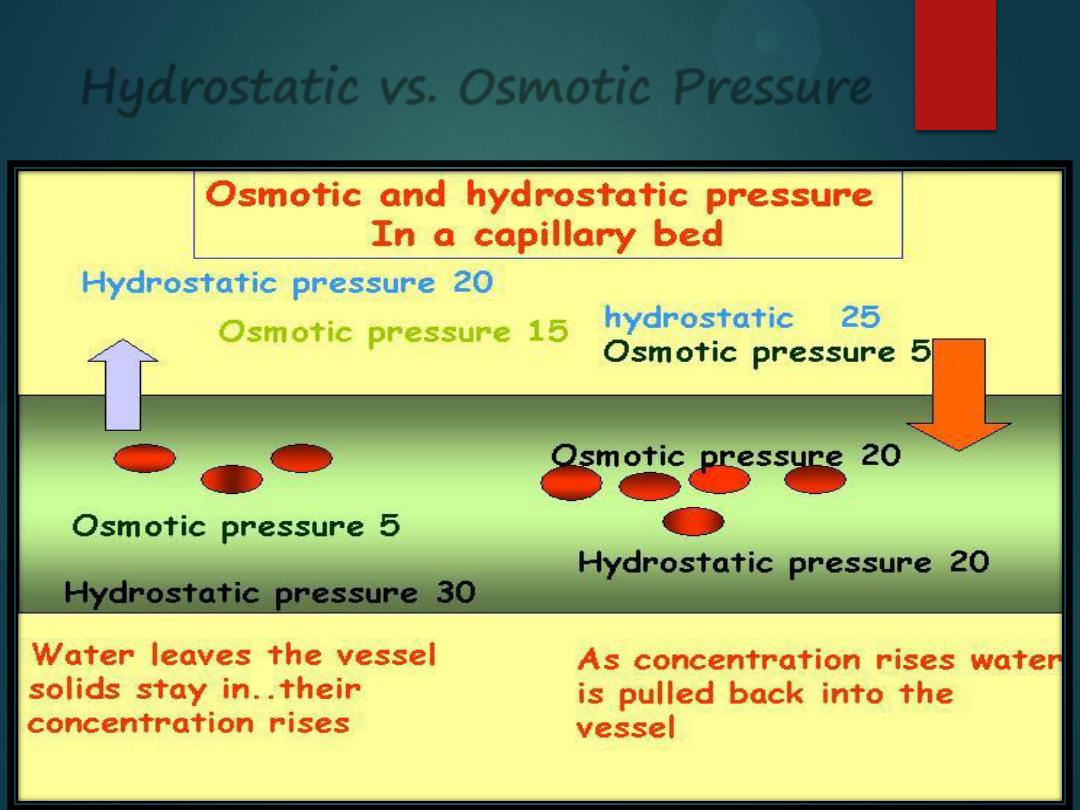
Hydrostatic vs. Osmotic Pressure
Hydrostatic pressure
is water
being pushed out by some force. If
there is a lot of water in the blood
vessel, it will get pushed out,
causing edema in the tissues.
Osmotic pressure
is water moving
from its area of
high
concentration
to its area of
low
concentration. If
there are too many particles in the
plasma, water will be sucked into
the blood vessel, causing the
blood pressure to elevate.
37
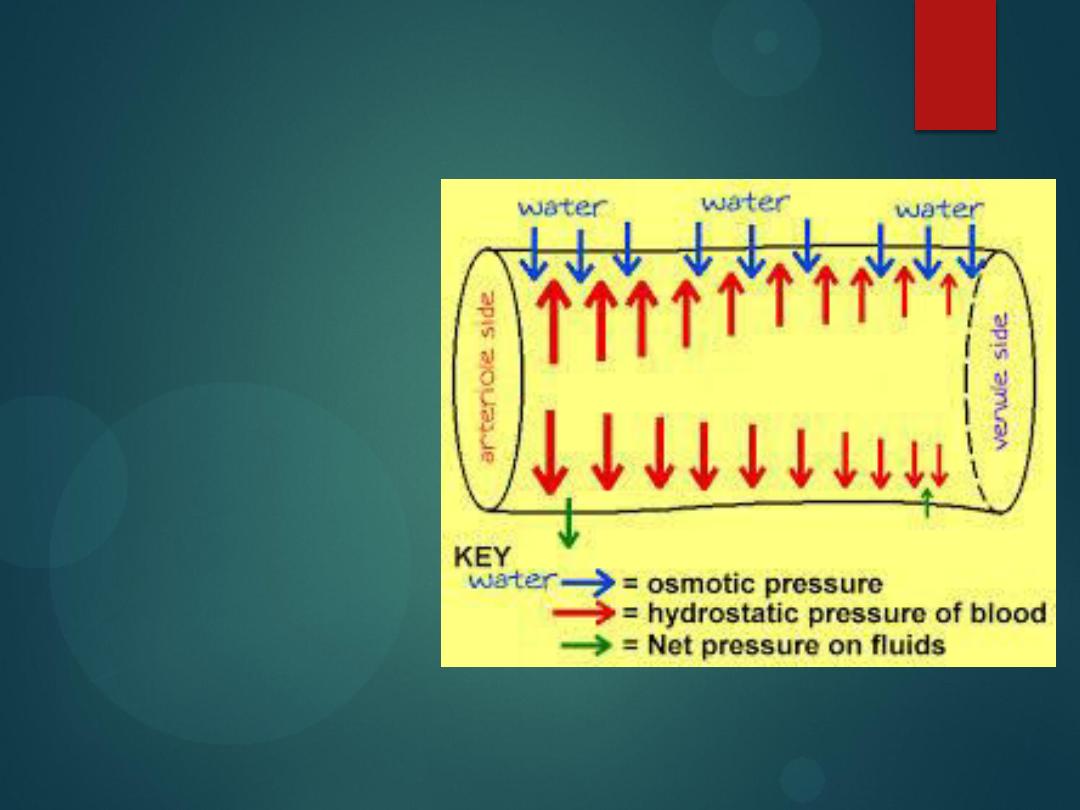
Hydrostatic Pressure
Fluid is forced out
of systemic
capillaries at the
arteriolar end
because the
hydrostatic
pressure of the
blood is greater
than the osmotic
pressure.
38
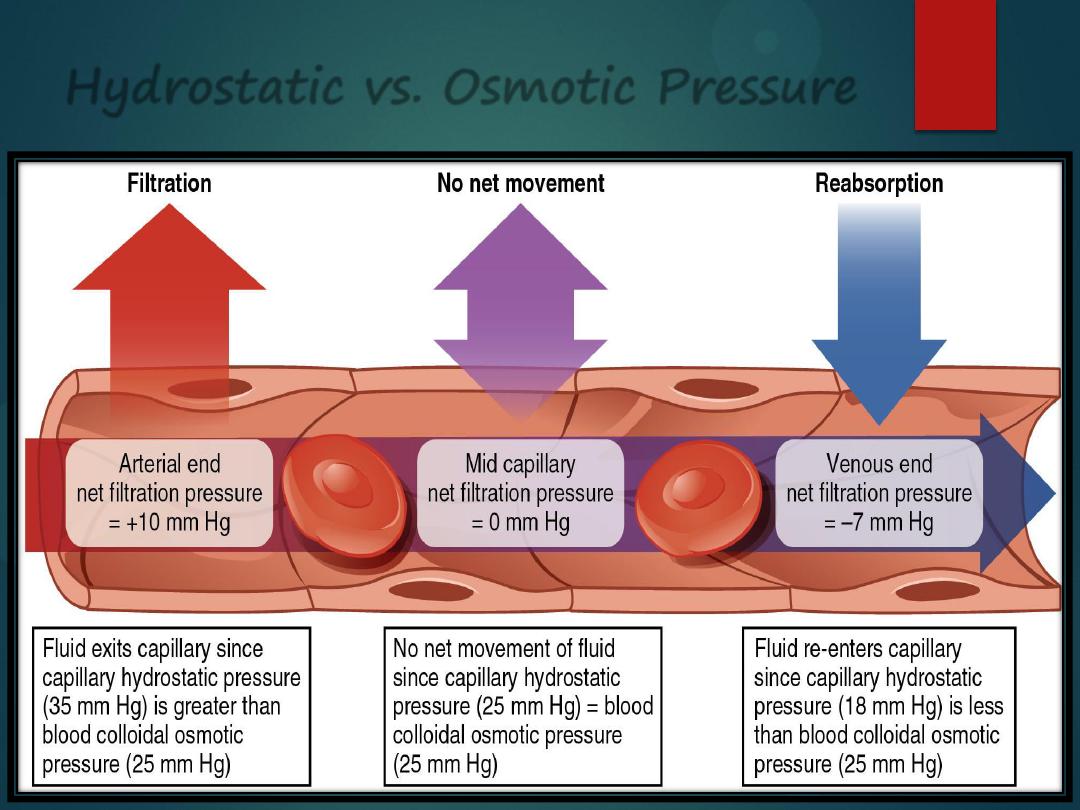
Hydrostatic vs. Osmotic Pressure
39
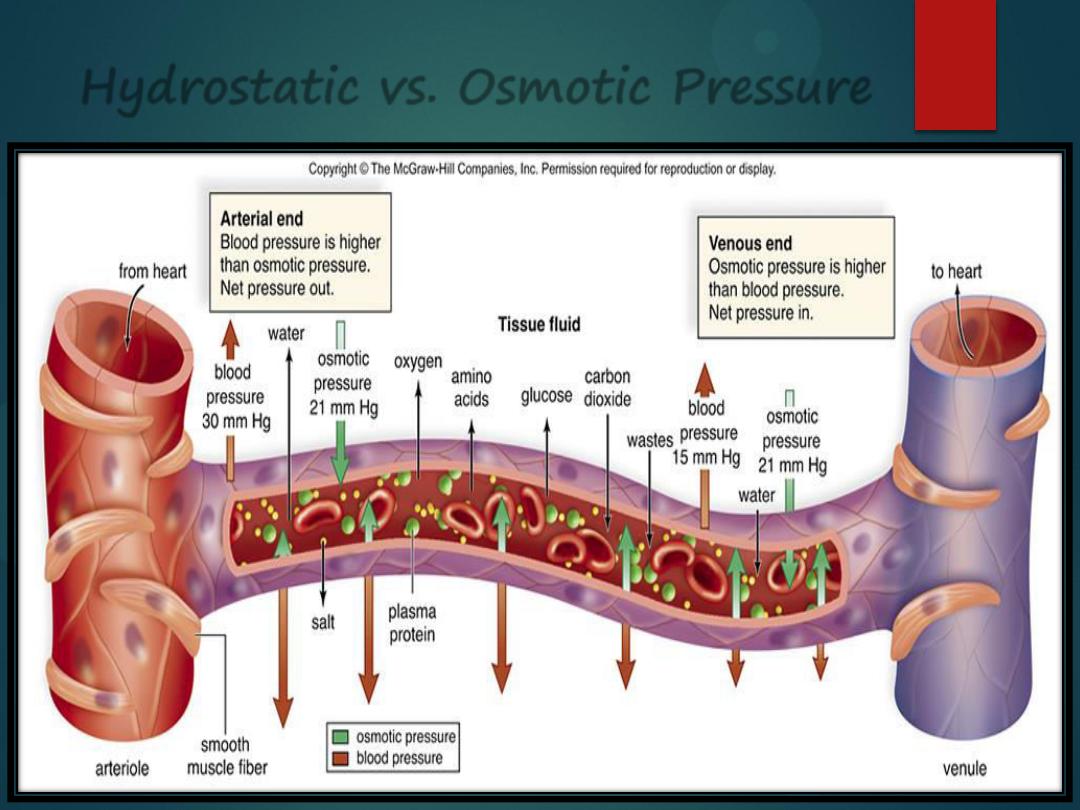
Hydrostatic vs. Osmotic Pressure
40
Hydrostatic vs. Osmotic Pressure
41

42
