1
Organic chemistry II
Amines
Amines may be regarded as the derivatives of ammonia in which one
or more or all the hydrogen atoms are replaced by organic groups,
such as alkyl or aryl
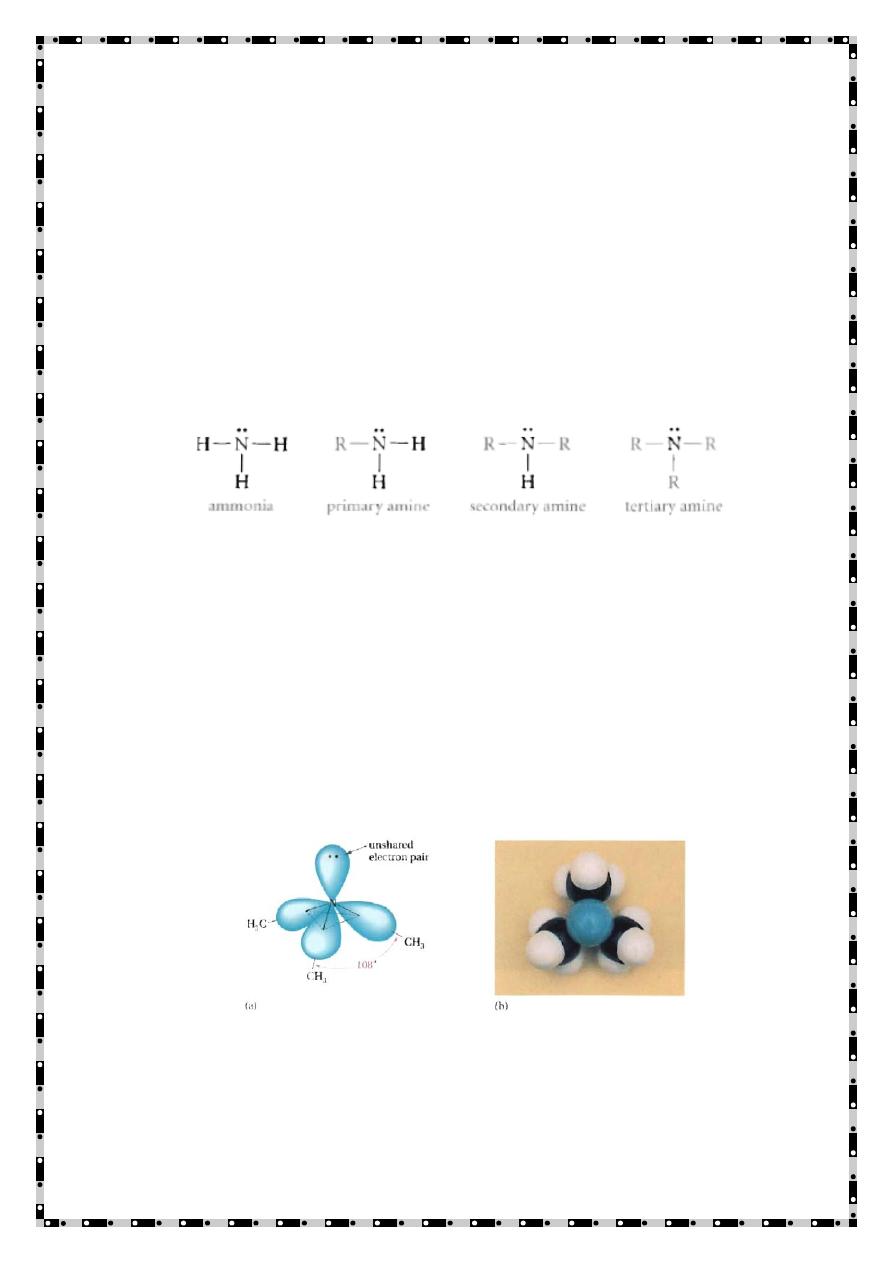
Structure The relation between ammonia and amines is as
follows:
1º Amines have one organic group attached to the N atom
2º Amines have two organic groups attached to the N atom
3º Amines have three organic groups attached to the N atom
In some 2º and 3º amines the N may be part of a ring
The N atom in amines is trivalent and it carries an unshared
electron pair
The N orbitals are sp
3
-hybridized, and the overall geometry is
pyramidal (almost tetrahedral)
2
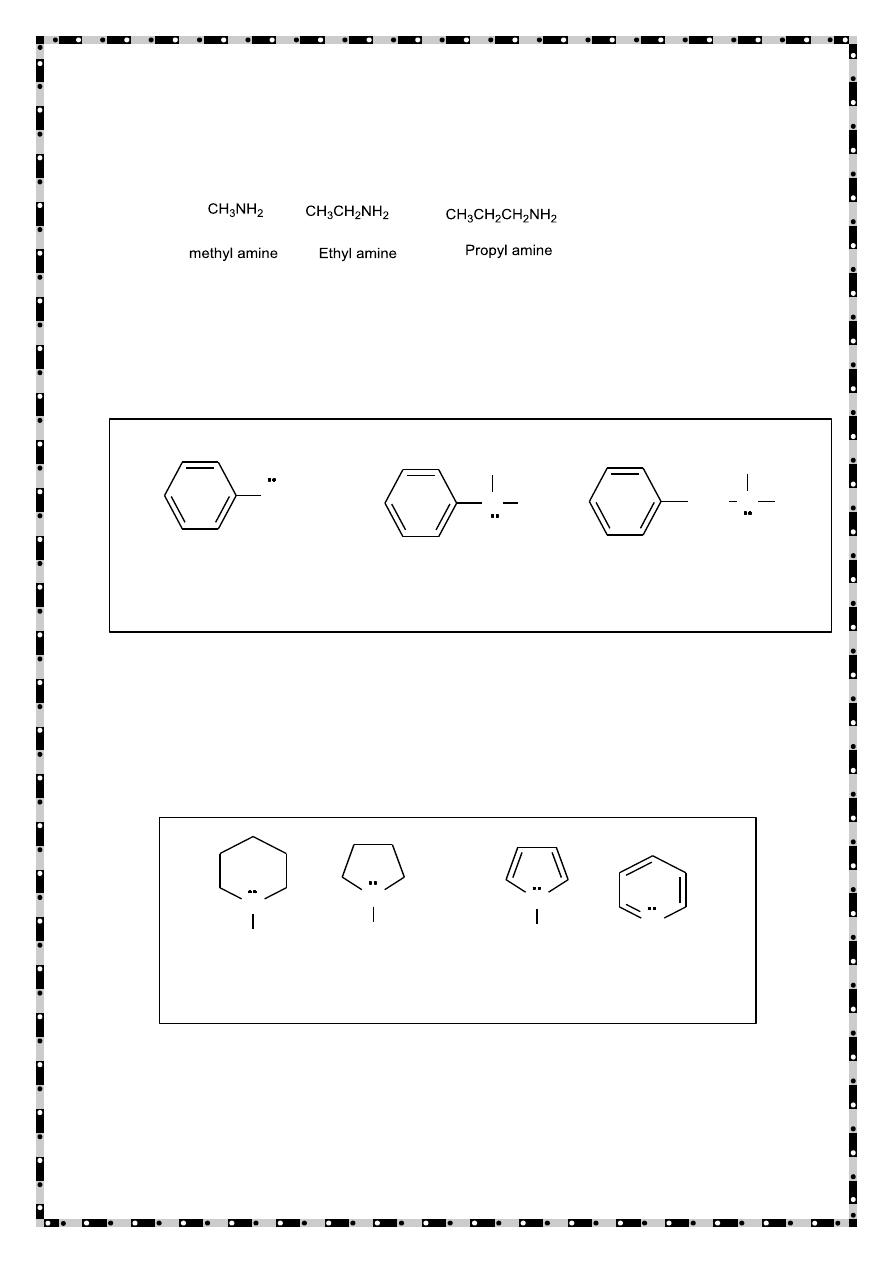
Classification of amines
a) Primary (1°) Amines: - An amine in which one hydrogen of
ammonia has been replaced by an alkyl or aryl group.
b) Secondary (2°) Amines: - An amine in which two hydrogen of
ammonia have been replaced by alkyl or aryl groups.
c) Tertiary (3°) Amines: - An amine in which all three hydrogens of
ammonia have been replaced by alkyl or aryl groups.
d) Quaternary (4°) ammonium ion:- An ion in which nitrogen is
bonded to four carbons and bears a positive charge.
NH
3
CH
3
NH
2
CH
3
NH
CH
3
CH
3
N
CH
3
CH
3
Ammonia Methylamine Dimethylamine Trimethylamine
(1° amine) (2° amine) (3° amine)
(CH
3
)
4
N
+
Cl
-
NCH
2
(CH
2
)
12
CH
3
Cl
-
CH
2
N(CH
3
)
3
OH
-
Tetramethylammonium Tetradecylpyridinium chloride Benzyltrimethylammonium
chloride (Cetylpyridinium chloride) hydroxide
3
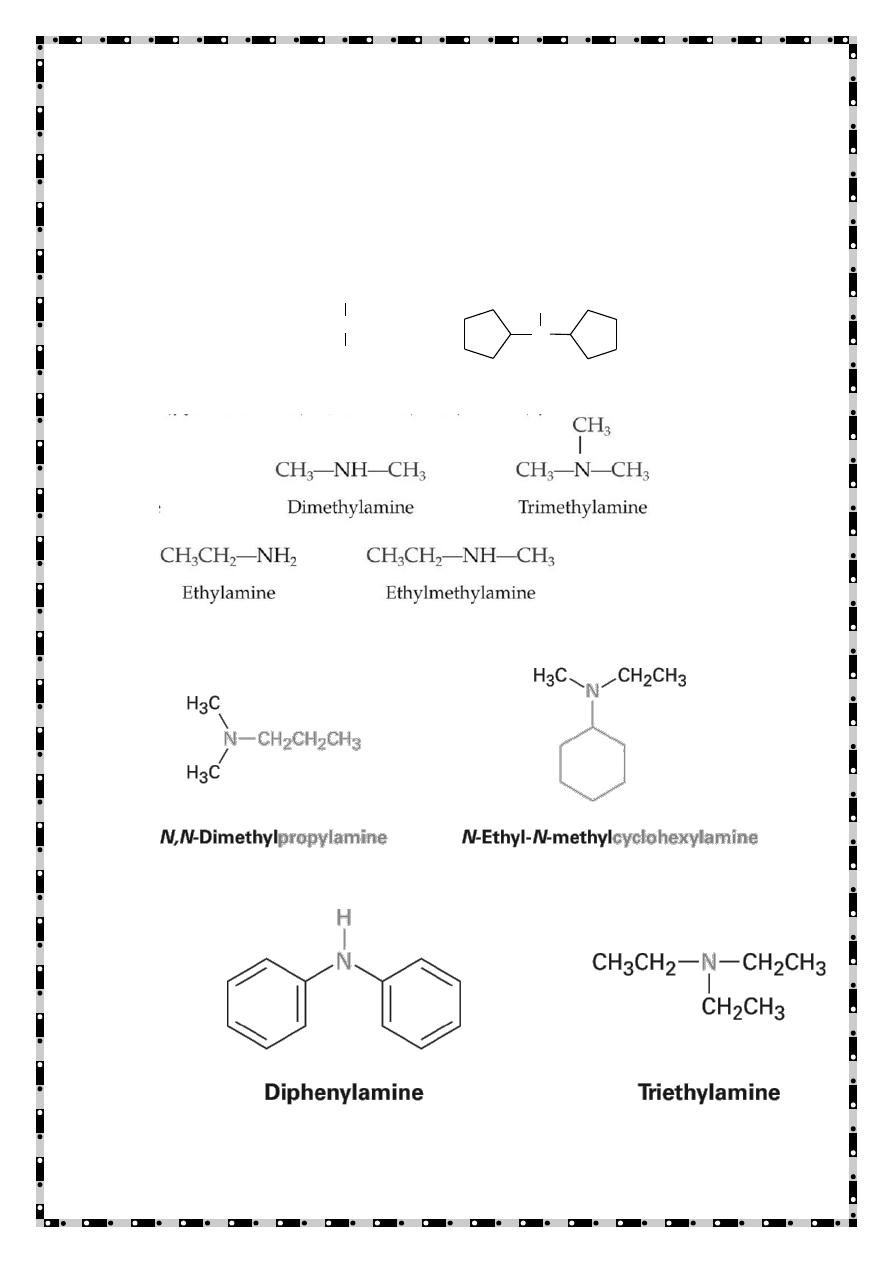
e) Aliphatic amines: - An amine in which nitrogen is bonded only
to alkyl groups.
f) Aromatic amine: - An amine in which nitrogen is bonded to one or
more aryl groups.
g) Heterocyclic amines:-An amine in which nitrogen is one of the atoms
or a ring.
NH
2
N H
CH
3
CH
2
N
CH
3
CH
3
Aniline N-Methylaniline Benzyldimethylamine
(1° aromatic amine) (2° aromatic amine) (3° aliphatic amine)
N
H
N
H
N
N
H
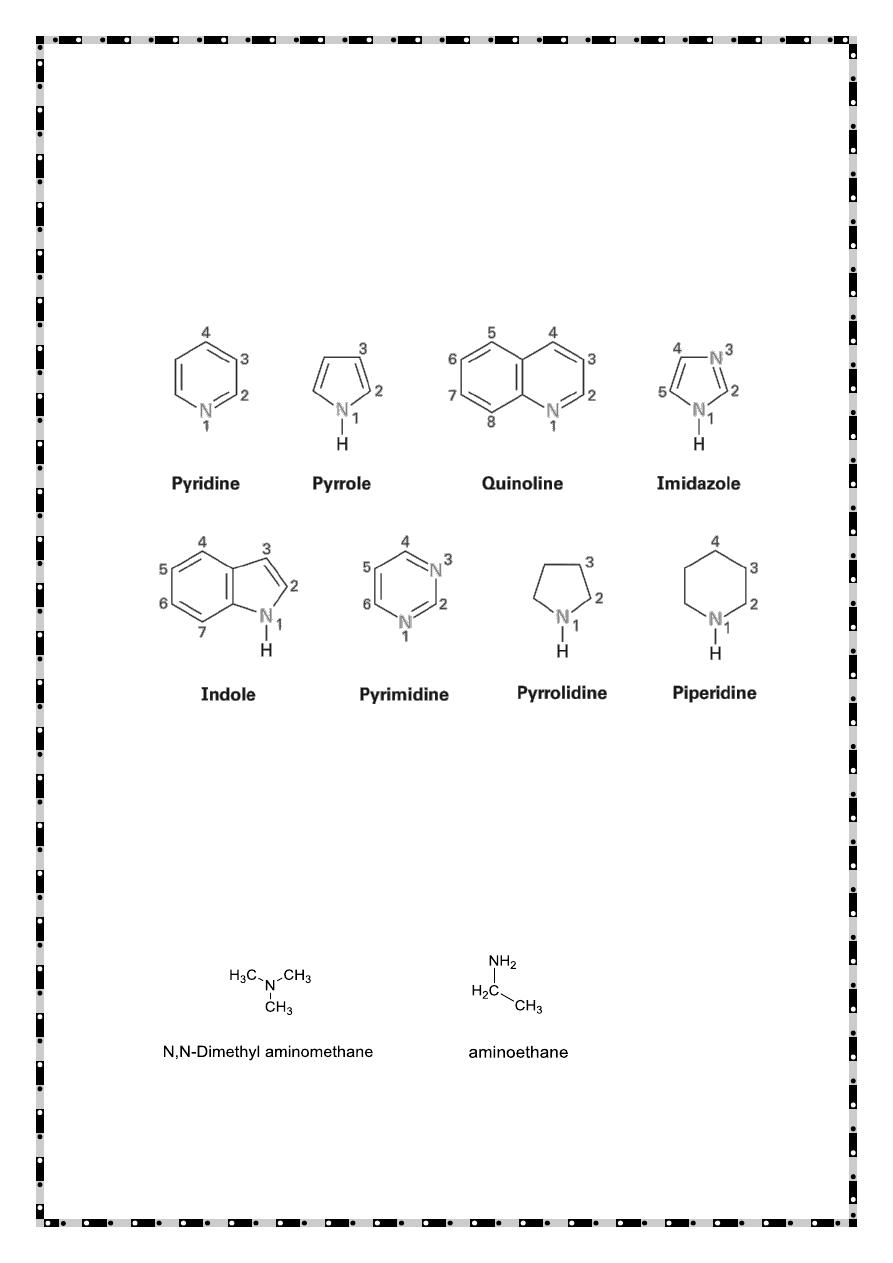
Piperidine Pyrrolidine Pyrrole Pyridine
(heterocyclic aliphatic amines) (heterocyclic aromatic amines)
4
Nomenclature of Amines
(Common Names)
Naming Simple Amines
• Are named as alkylamines.
• List the names of the alkyl groups bonded to the N atom in
alphabetical order in front of amine.
CH
3
NH
2
CH
3
CNH
2
CH
3
CH
3
N
H
CH
3
CH
2
NCH
2
CH
3
CH
2
CH
3
Methylamine tert-Butylamine Dicyclopentylamine Triethylamine
5
If the nitrogen atom occurs as part of a ring, the compound
is designated as being heterocyclic
Each ring system has its own parent name
IUPAC system
- The prefix amino and a number designates the position of the
amino group on an alkane parent chain
- A substituent on the N uses the –N prefix as with the
systematic naming
6
When other functional groups are present, the amino group is named
as a substituent:
Aromatic amine
IUPAC nomenclature retains the common name aniline for C
6
H
5
NH
2
,
the simplest aromatic amine. Its simple derivatives are named using
the prefixes o-, m-, p-, or numbers to locate substituents. Several
derivatives of aniline have common names that are still widely used.
Among those are toluidine for a methyl-substituted aniline and
anisidine for a methoxy-substituted aniline.
H
2
NCH
2
CH
2
OH
C
CH
2
OH
NH
2
H
(CH
3
)
2
CH
CO
2
H
H
2
N
2-Aminoethanol ( S)-2-Amino-3-methyl 4-Aminobenzoic acid
1-butanol
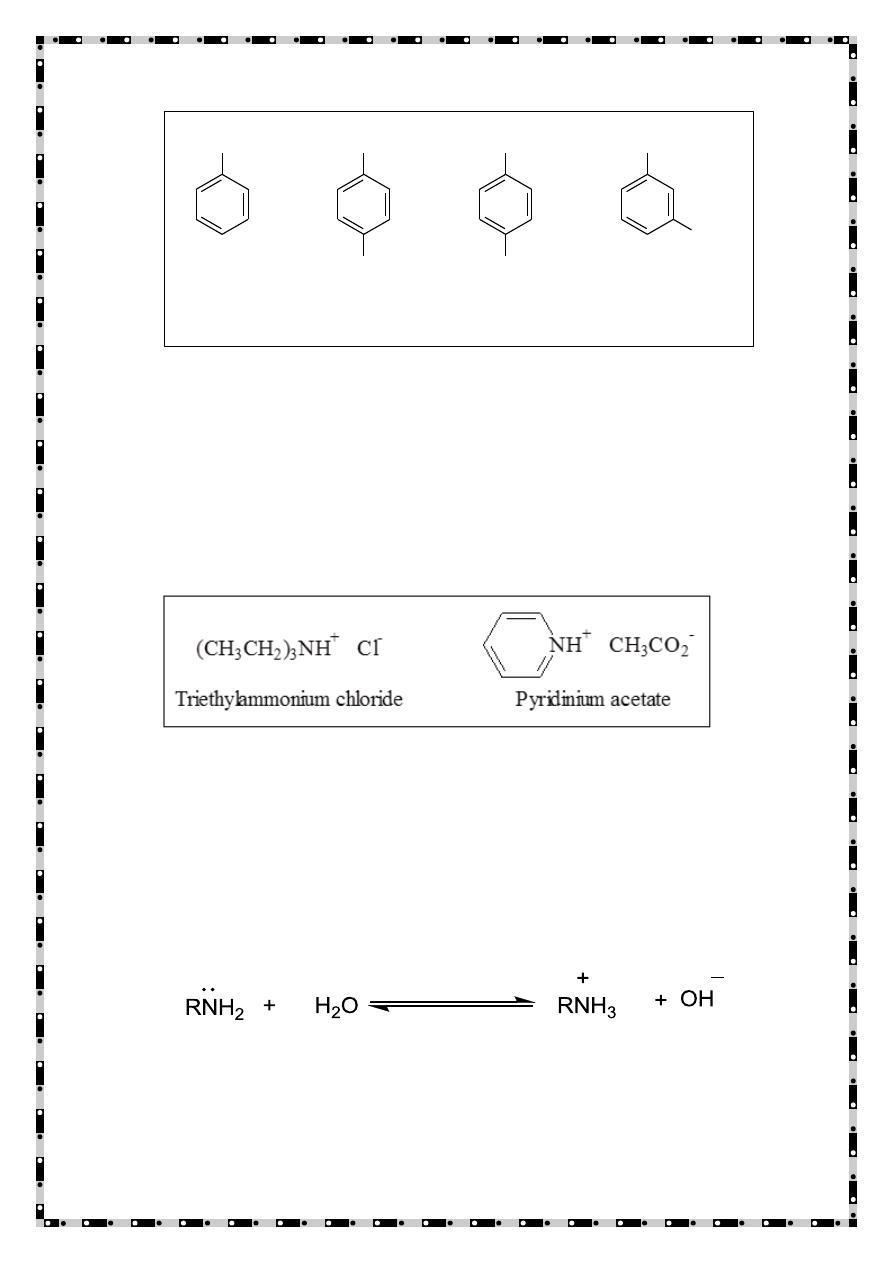
7
When four atoms or groups of atoms are bonded to a nitrogen atom,
the compound is named as a salt of the corresponding amine. The
ending -amine (or -aniline, or pyridine, and so on) is replaced by
ammonium (or anilinium, or pyridinium, etc.) and the name of the
anion (chloride, acetate, etc.) is added.
Basicity of amines
Amines are the organic derivatives of ammonia, it is highly
reasonable to assume that amines may be basic in character. In fact,
amines, like ammonia, give an alkaline reaction in aqueous solution
and form salts with acids. In aqueous solution, amine exists in the
following equilibrium
According to this modem concept the strength of any base
depends upon the simultaneous of two conditions:
NH
2
NH
2
NO
2
NH
2
OCH
3
NH
2
CH
3
Aniline 4-Nitroaniline 4-Methylaniline 3-Methoxyaniline
(p-Toluidine) (m-Anisidine)
8
(1) Availability of electrons
(2) Stability of conjugate acid formed in solution.
Any factor that can increase electron density of the species under
consideration increases the basicity. Similarly increase in the stability
of conjugate acid increases the basic strength of the compound, but
both these factors are to be considered simultaneously. Based on the
above concept regarding the strength of the bases the observations
noted in the table may be properly rationalized It has been observed
that aliphatic secondary amine is most basic among ammonia, primary,
secondary and tertiary amines, i.e., the order is
NH
3
< RNH
2
<R
2
NH>R
3
N>NH
3
The greater basicity of all the three types of amines than ammonia is
attributable to the electron release by alkyl groups which increases the
electron density on nitrogen and at the same time the conjugate acids
formed are moderately stable, as a result of which the average effect of
Both the factors are greater in these amines than in ammonia, and
amines are more basic than ammonia. But among the three types of
amines, average effect of these two factors is not the same.
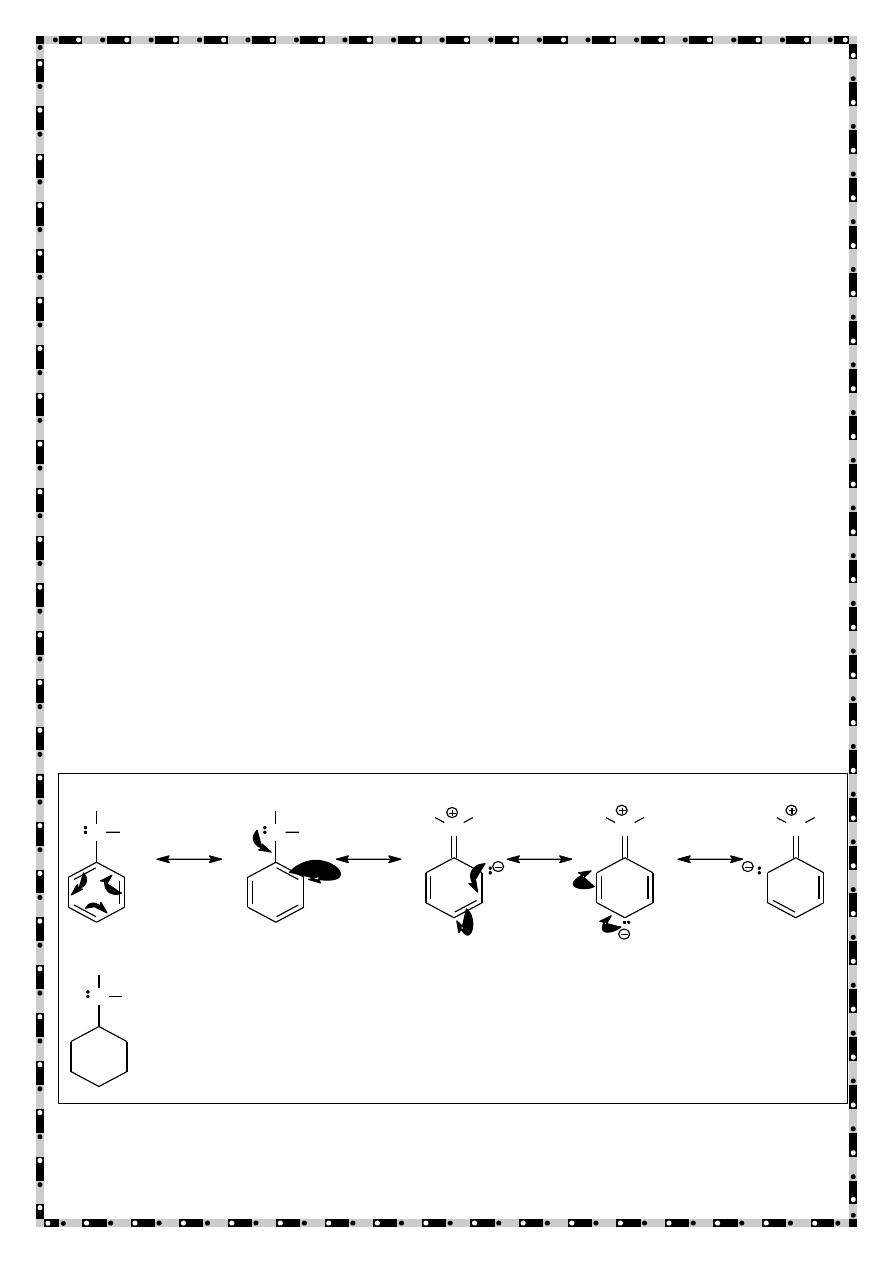
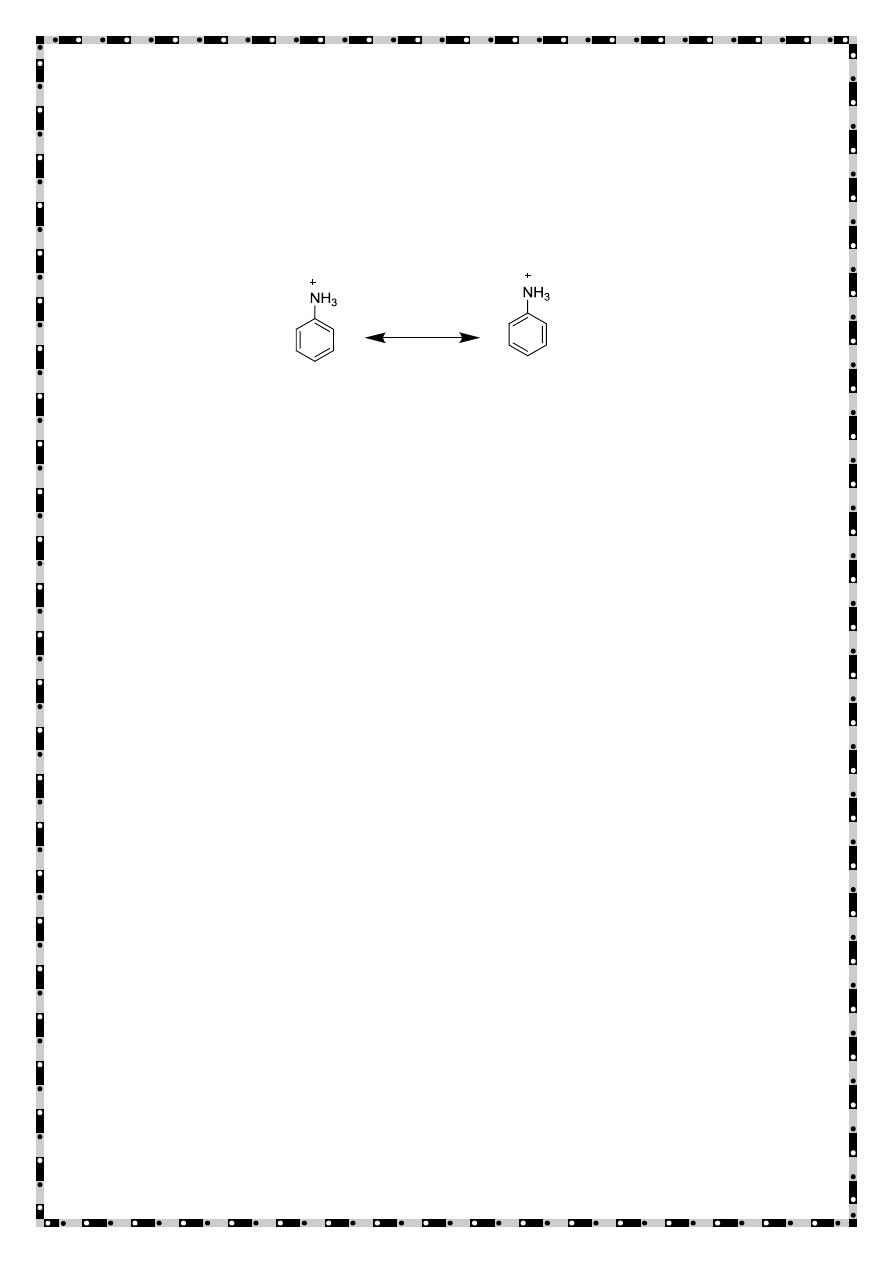
Weak basicity of aromatic amines is due to the resonance stabilization
as indicated below
N
H
H
No resonance possible
with alkylamines
N
H
H
N
H
H
N
H
H
N
H
H
N
H
H
Interaction of the electron
pair on nitrogen with the pi
system of the aromatic ring.
9
Owing to the above resonance, the lone pair of electrons on nitrogen is
less available for coordinating with a proton and at the same time, the
small charge on nitrogen atom would tend to repel a proton. Further,
the conjugate acid, the anilinum ion is less stable than parent amine
due to lesser number of resonating structures (only two)
Electron releasing groups (e.g., methyl, ethyl, and other
alkyl groups) increase the basicity of aromatic amines, whereas
electron-withdrawing groups (halogen, nitro, and carbonyl)
decrease their basicity. The decrease in basicity on halogen
substitution is due to the electron withdrawing inductive effect
of the electronegative halogen.
Physical properties
The lower members of the amine family are gases and soluble in
water, amine containing three To eleven carbon atoms are solid.
Amines are polar compounds, and both primary and secondary
amines form intermolecular hydrogen bonds. An N-H---N hydrogen
bond is not as strong as an O-H---O hydrogen bond because the
difference in electronegativity between nitrogen and hydrogen (0.9) is
not a great as that between oxygen and hydrogen (1.4). The effect of
intermolecular hydrogen bonding can be illustrated by comparing the
boiling points of methylamine and methanol. Both compounds have
polar molecules that interact in the pure liquid by hydrogen bonding.
Hydrogen bonding is stronger in methanol than in methylamine, and,
therefore, methanol has the higher boiling point. All classes of amines
form hydrogen bonds with water and are more soluble in water than
hydrocarbons of comparable molecular weight. Most low-molecular-
weight amines are completely soluble in water. Higher molecular-
weight amines are only moderately soluble or insoluble.
10
Preparation of amines
Alkylation of ammonia
Ammonia reacts as a nucleophile with alkyl halides to give primary
amines in a nucleophilic substitution reaction. A yield are often
poor as the product, a primary amine, RNH
2
, is itself a
nucleophile and can react with more alkyl halide. The result is
mixtures containing primary amines, secondary amines, tertiary
amines and quaternary ammonium salts.This can be avoided if a
large excess of ammonia is used.
Aryl halides do not undergo simple nucleophilic substitution,
they cannot be prepared using this method.
N
H
R
R
H
R
R
N
d+
d-
d-
d+
hydrogen bonding
11
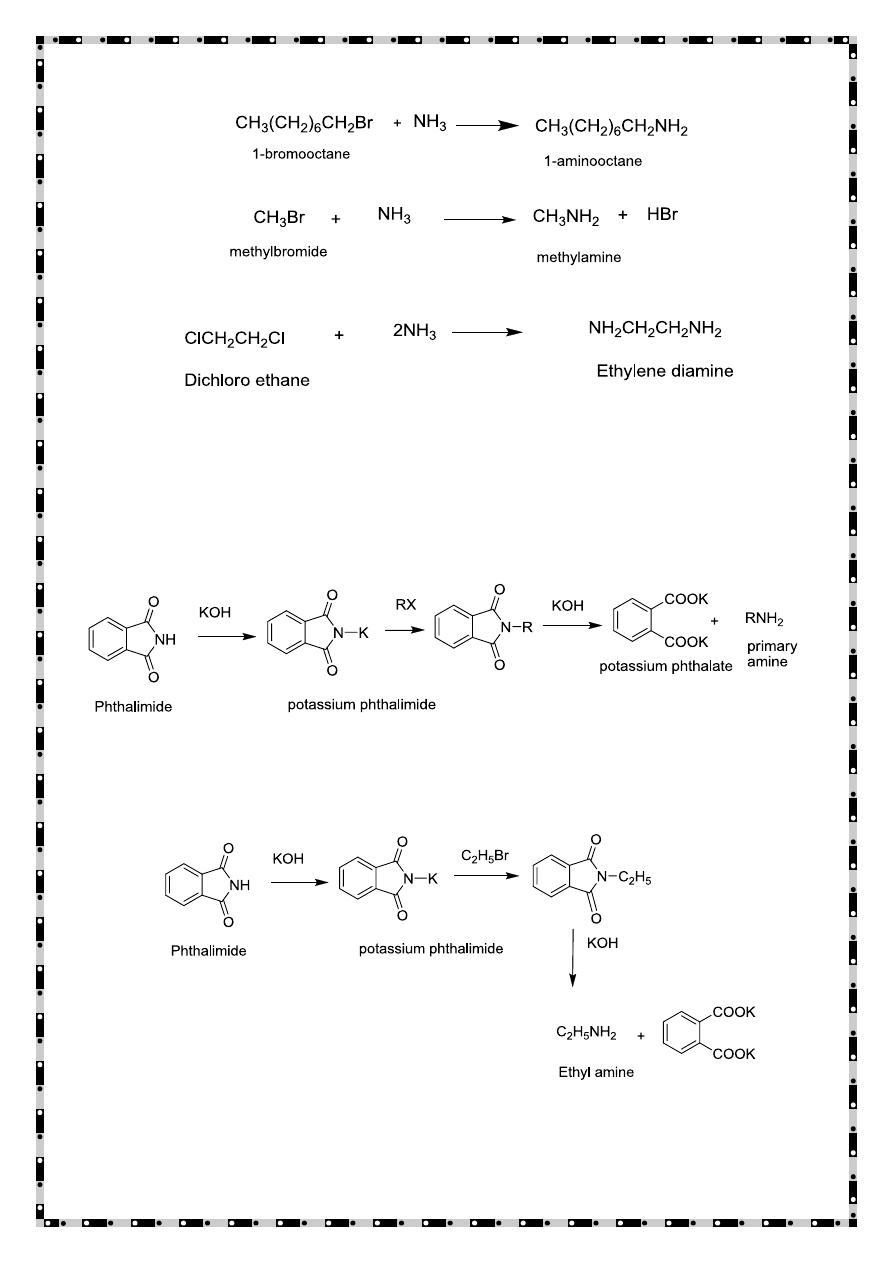
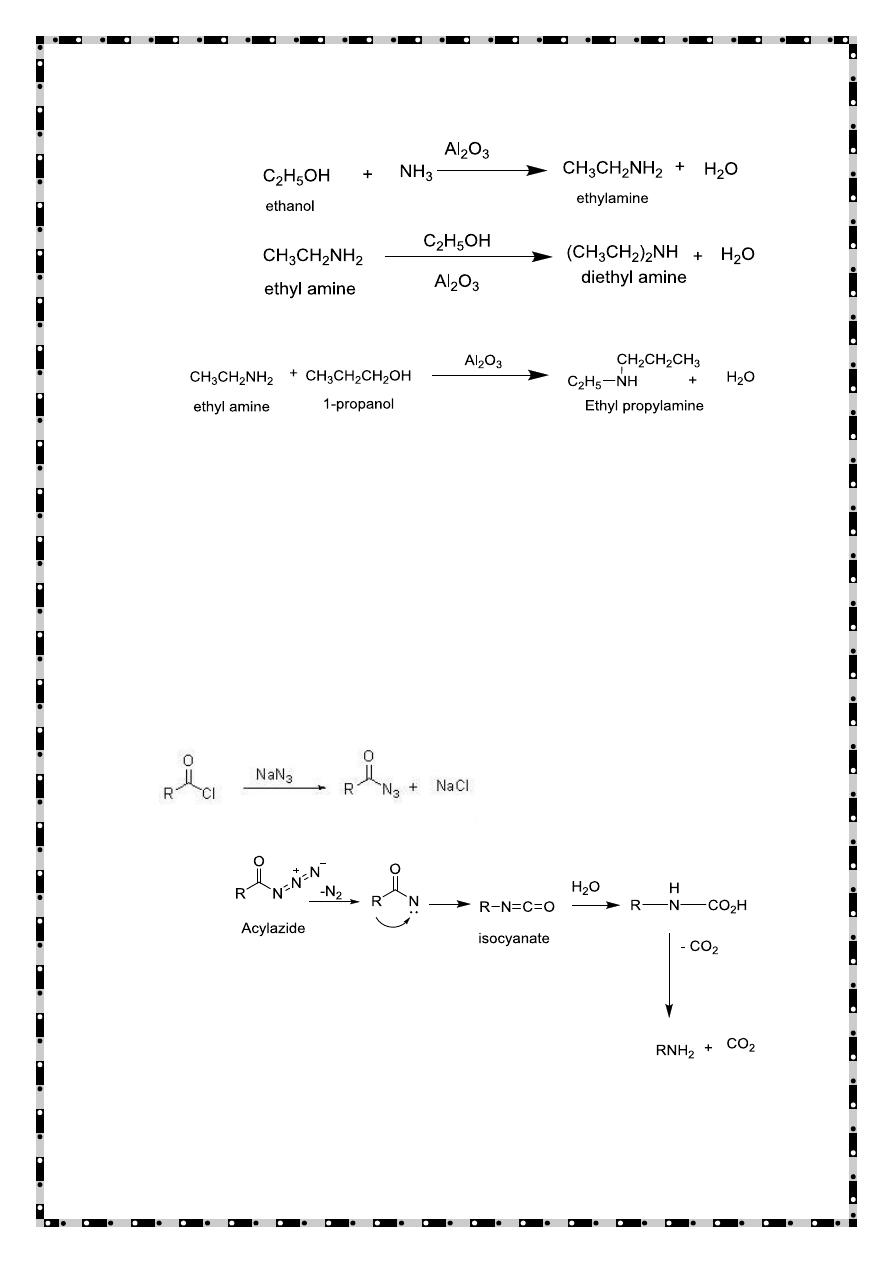
Gabriel Synthesis
Pure primary amines can be prepared by this method by using
phthalimide with alkyl halid in presence of potassium hydroxide.
12
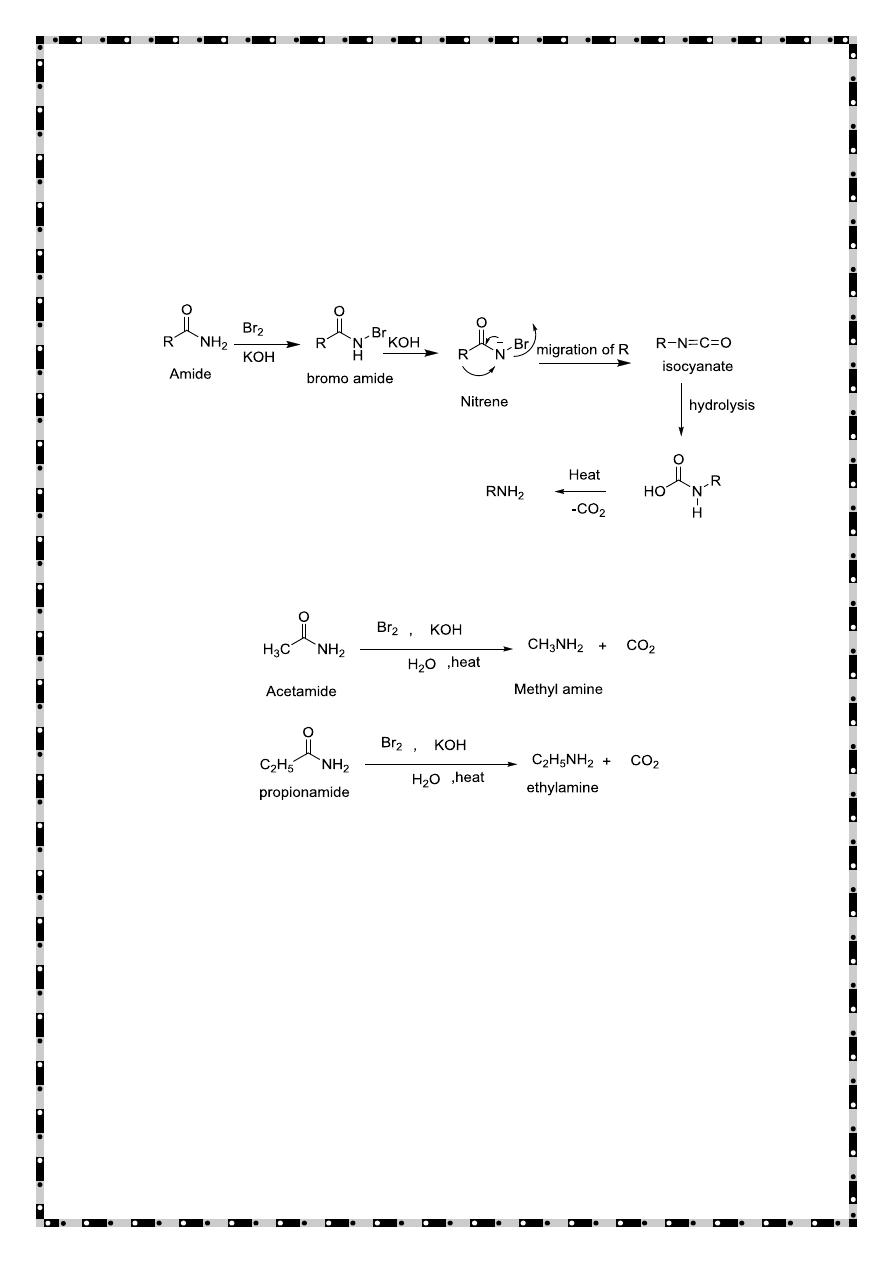
Hofmann rearrangement
Hofmann rearrangement, also known as Hofmann degradation is the
reaction of a primary amide with a halogen (chlorine or bromine) in
strongly basic (sodium or potassium hydroxide) aqueous medium, in
this reaction the amide converts to a primary amine with one
fewer carbon atoms.
Reduction of reducible compounds
Easily reducible compounds like nitriles, oxime, amides, and nitro
compounds on reduction yield amines
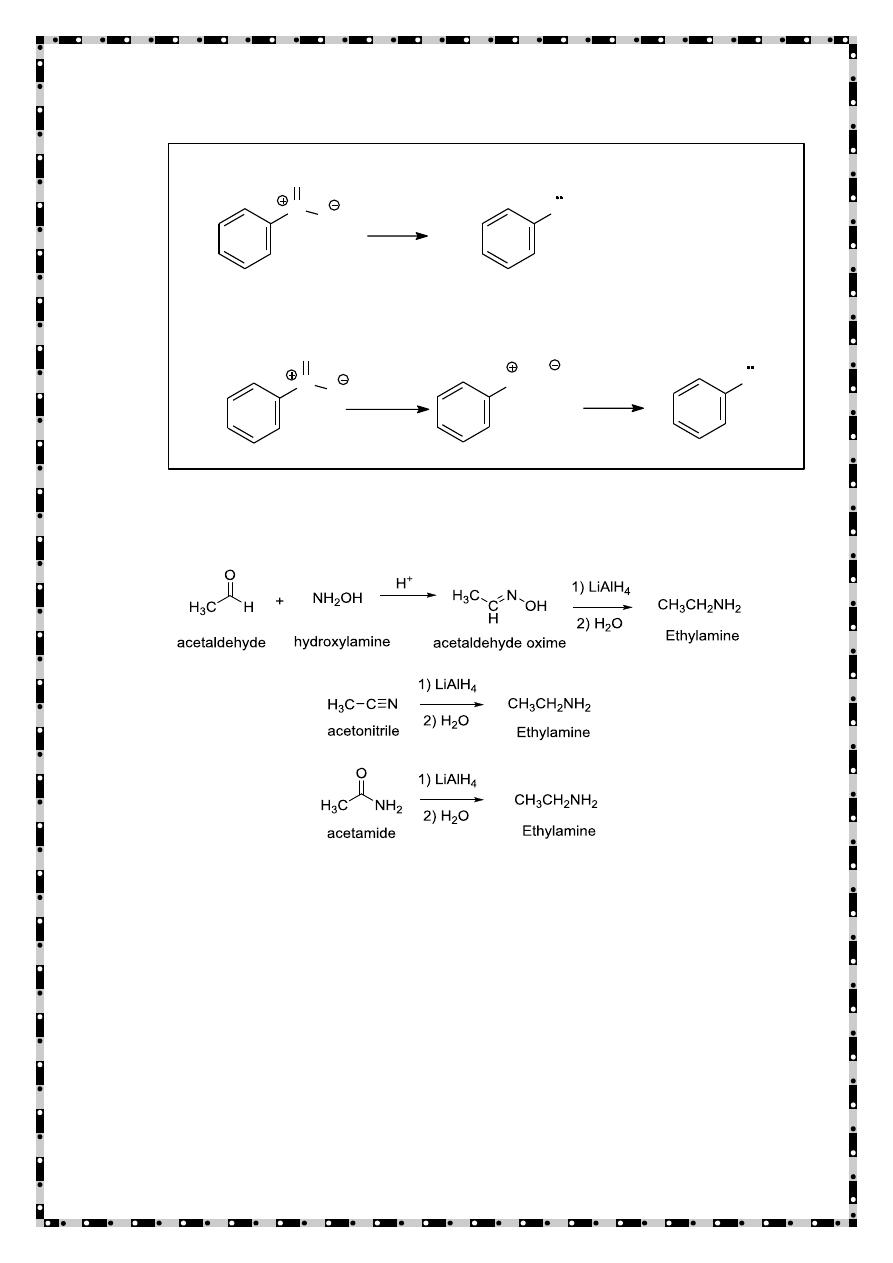
13
a) Reduction of Nitro Groups
b) Reduction of Oximes, Nitriles, and Amides by Lithium
Aluminum Hydride
Action of ammonia on alcohols
When a mixture of alcohol and ammonia is passed over heated
alumina all the three types of amines are obtained.
Ni
3 H
2
N
O
O
N
O
O
NH
3
Cl
NH
2
NH
2
Fe / HCl
-
OH
Catalytic
Chemical
14
Curtius rearrangement
The Curtius rearrangement is an organic reaction used to convert an
acyl azide to an isocyanate under thermal conditions. The mechanism
consists of an alkyl shift of the R group from the carbonyl carbon to
the closest nitrogen with the release of nitrogen gas. The release of gas
drives the reaction forward and results in the formation of the
isocyanate product which can potentially react further in the presence
of nucleophiles in solution.
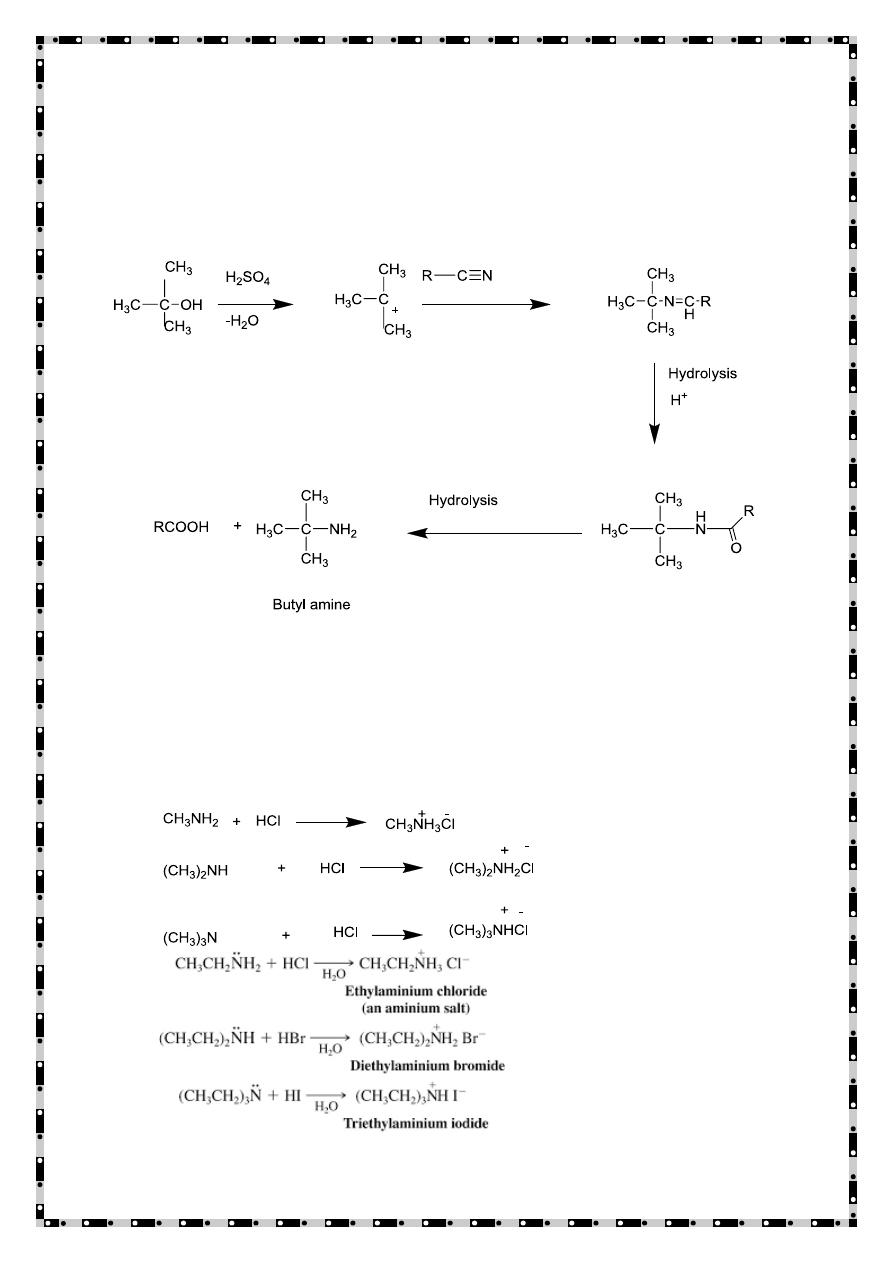
15
Ritter method
This method involves the reaction of tertiary alcohol with alkyl
cyanide in presence of strong sulphuric acid.
Chemical properties
a) Reactions with acids
Amines react with mineral acids to form salt .Salt formation with
mineral acids is analogous to ammonium salt and is formed both in
aqueous and anhydrous condition.

16
Reaction with nitrous acid
to form
)
2
(HNO
acid
reacts with nitrous
aliphatic amine
Primary
-
1
initially an unstable diazonium compound which decomposes to
evolve nitrogen. Nitrous acid is unstable and it is prepare d in situ by
reaction of sodium nitrite with hydrochloric
The carbocation formed combines with water in aqueous solution to
form alcohol
Thus primary aliphatic amines normally yield primary alcohols on
reaction with nitrous acid.
17
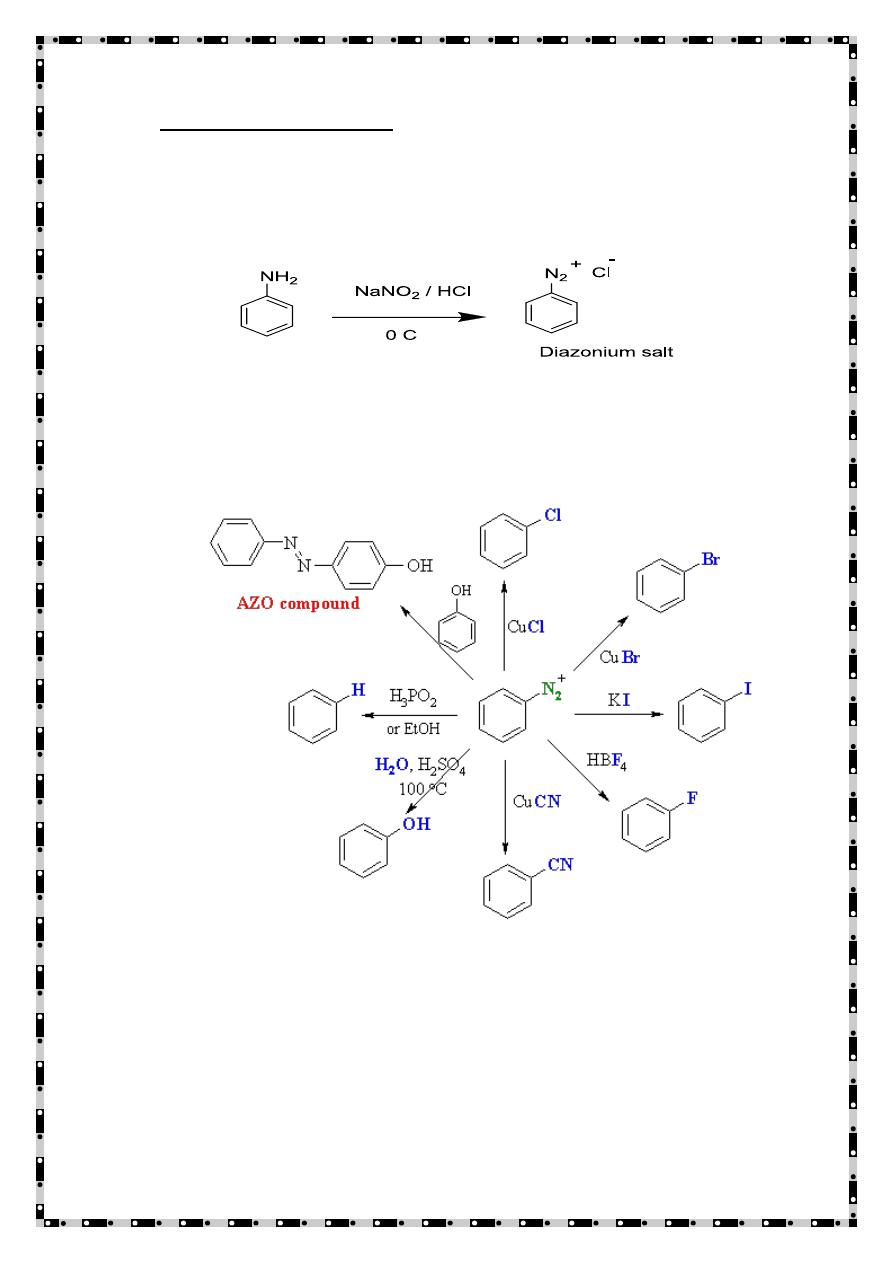
Primary aromatic amines
-
2
The nitrosation of primary aromatic amines with nitrous acid
(generated in situ from sodium nitrite and a strong acid, such as
hydrochloric acid, sulfuric acid) leads to diazonium salts.
Diazonium salts are important intermediates for the preparation of
halides (Sandmeyer Reaction), and azo compounds as the following.
18
3
)
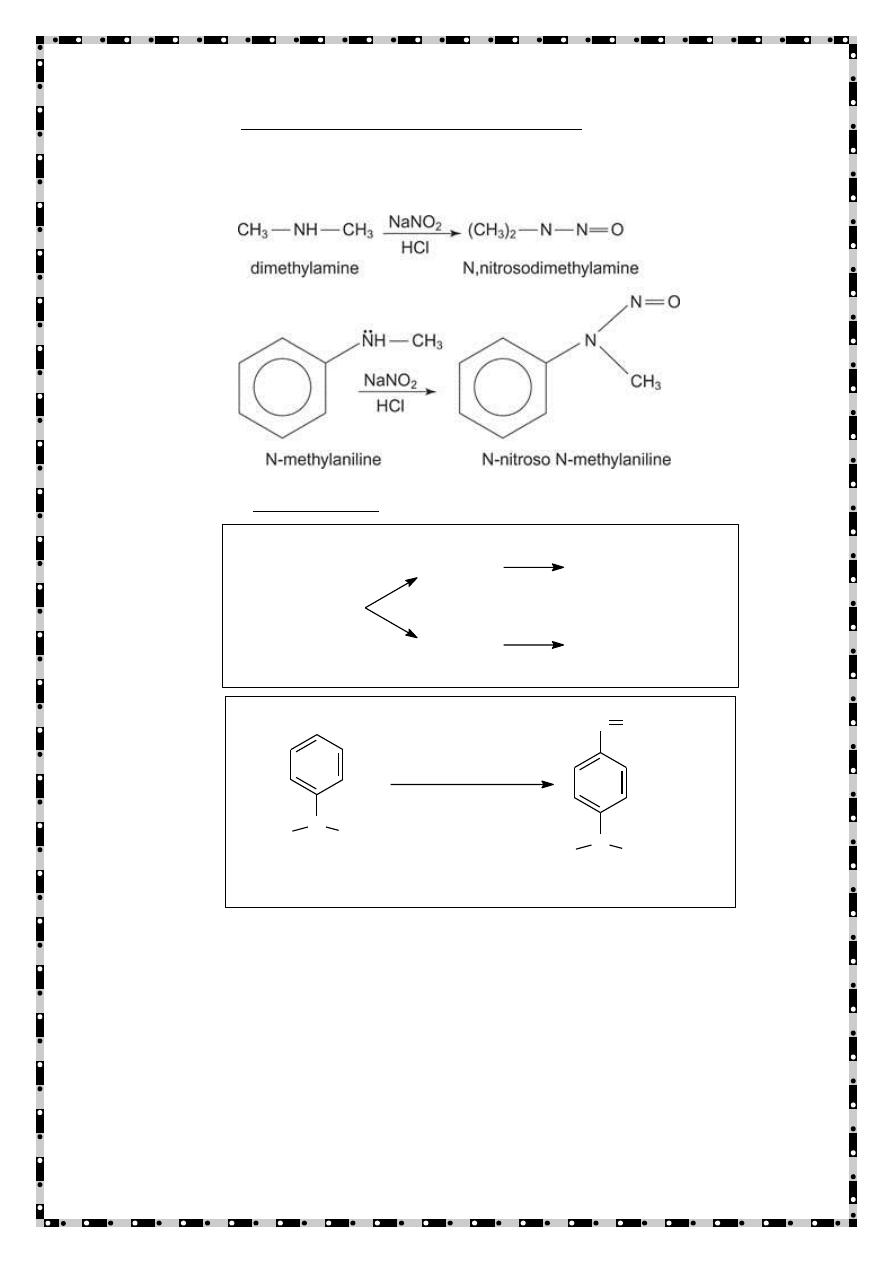
Secondary aliphatic and aromatic amines form N- nitrosoamine
with nitrous acid.
4) Tertiary Amines
Tertiary Amines
Aliphatic
Aromatic
No Reaction
Nitrosoamines
N
CH
3
CH
3
N
O
N
CH
3
CH
3
NaNO
2
/ HCl / O°
NaOH / H
2
O
1.
2.
N,N-Dimethylaniline
N,N-Dimethyl-4-nitrosoaniline
19
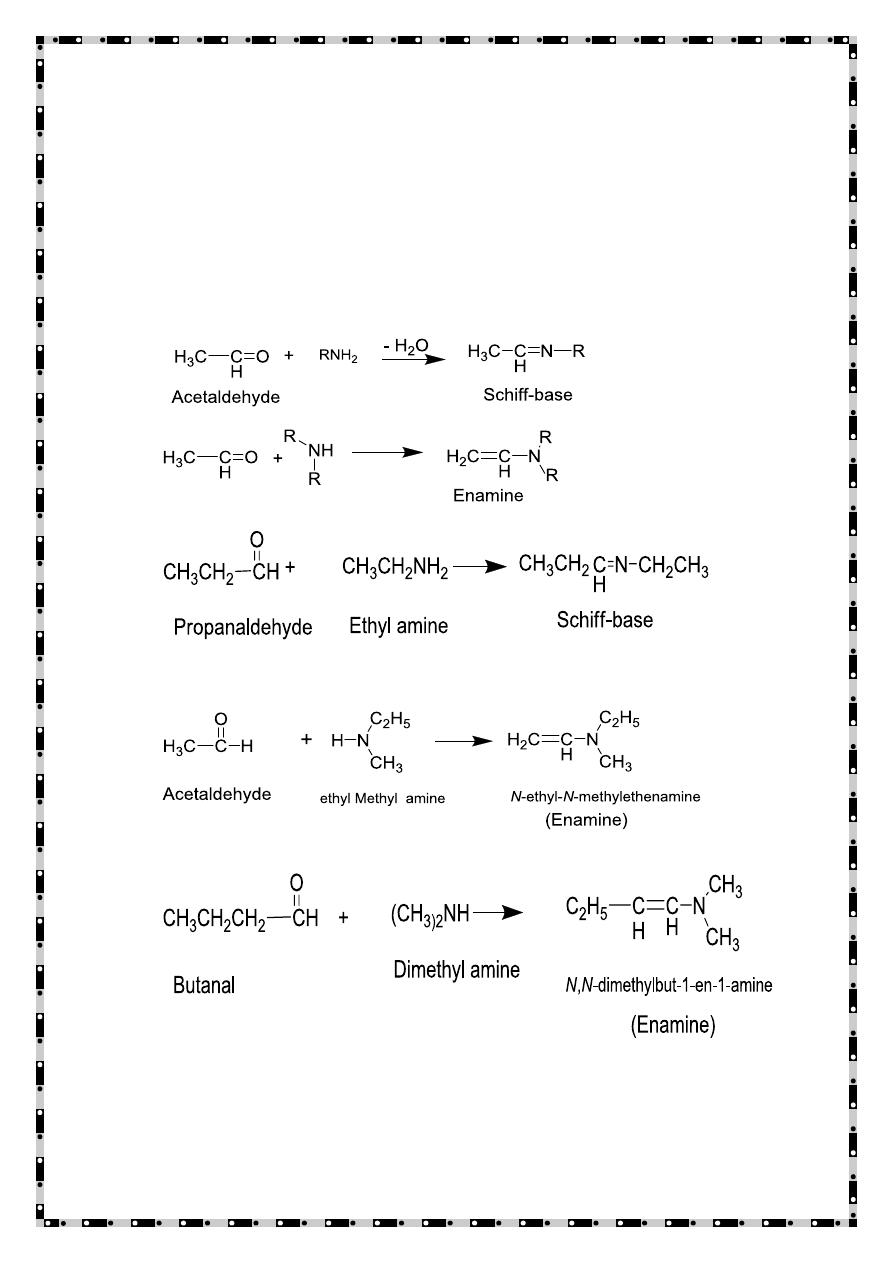
Reactions of Amines with compounds containing a
carbonyl group
Amines react with compounds having carbonyl function as
electron donor species(nucleophile), therefore the reaction aldehyde
and primary amine produce Schiff-base ,while Secondary amines
reacts with aldehydes having α-hydrogen atom produce enamines
.Tertiary amines do not react with aldehyde
20
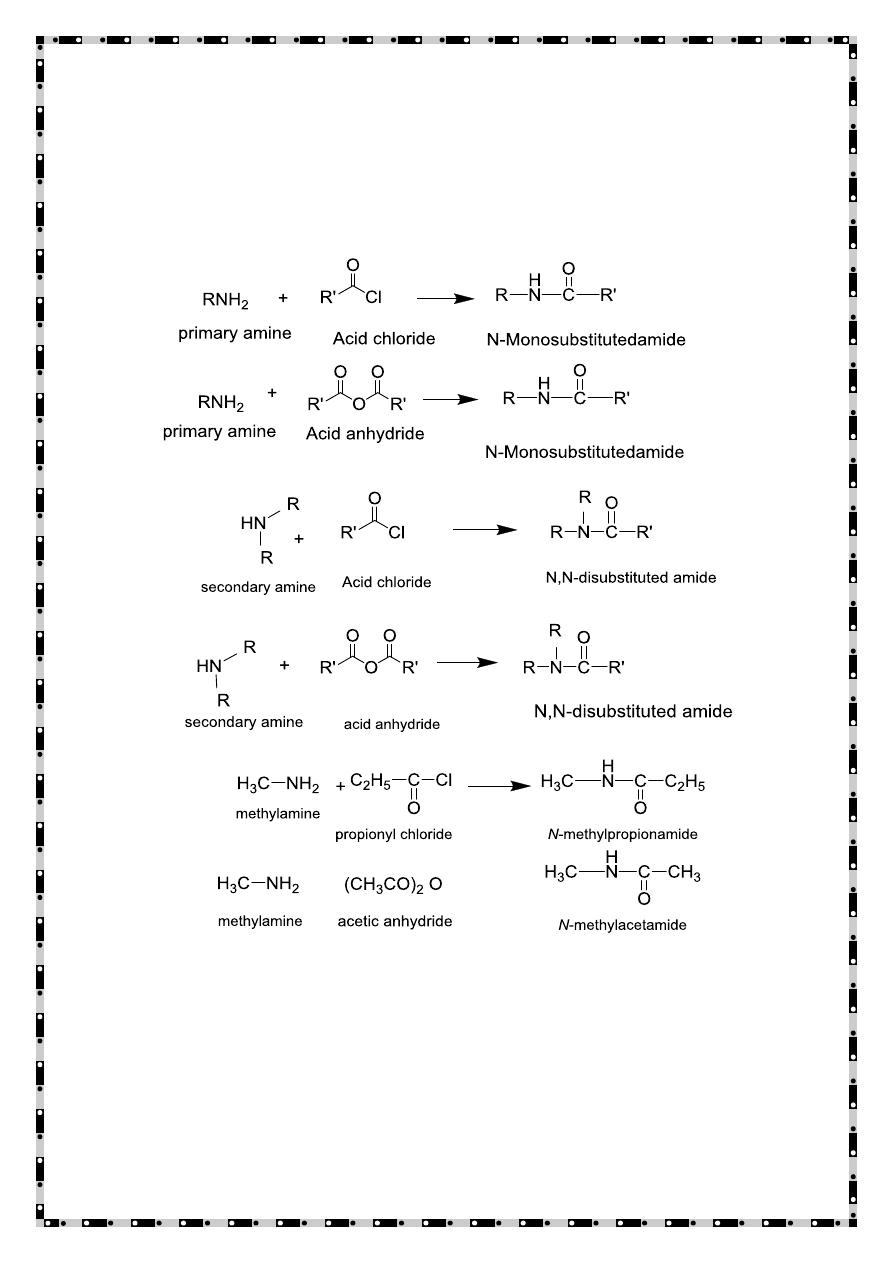
Reaction with acid-chloride and acid-anhydride
Primary and secondary amines on reaction with acid-chloride
and anhydride form acyl derivatives. But tertiary amines do not form
acyl derivative as they have no replaceable hydrogen atom.
Reaction with carbon disulphide
When primary amine is warmed with carbon disulphide in presence of
mercuric chloride as catalyst, isothiocyanate is formed.
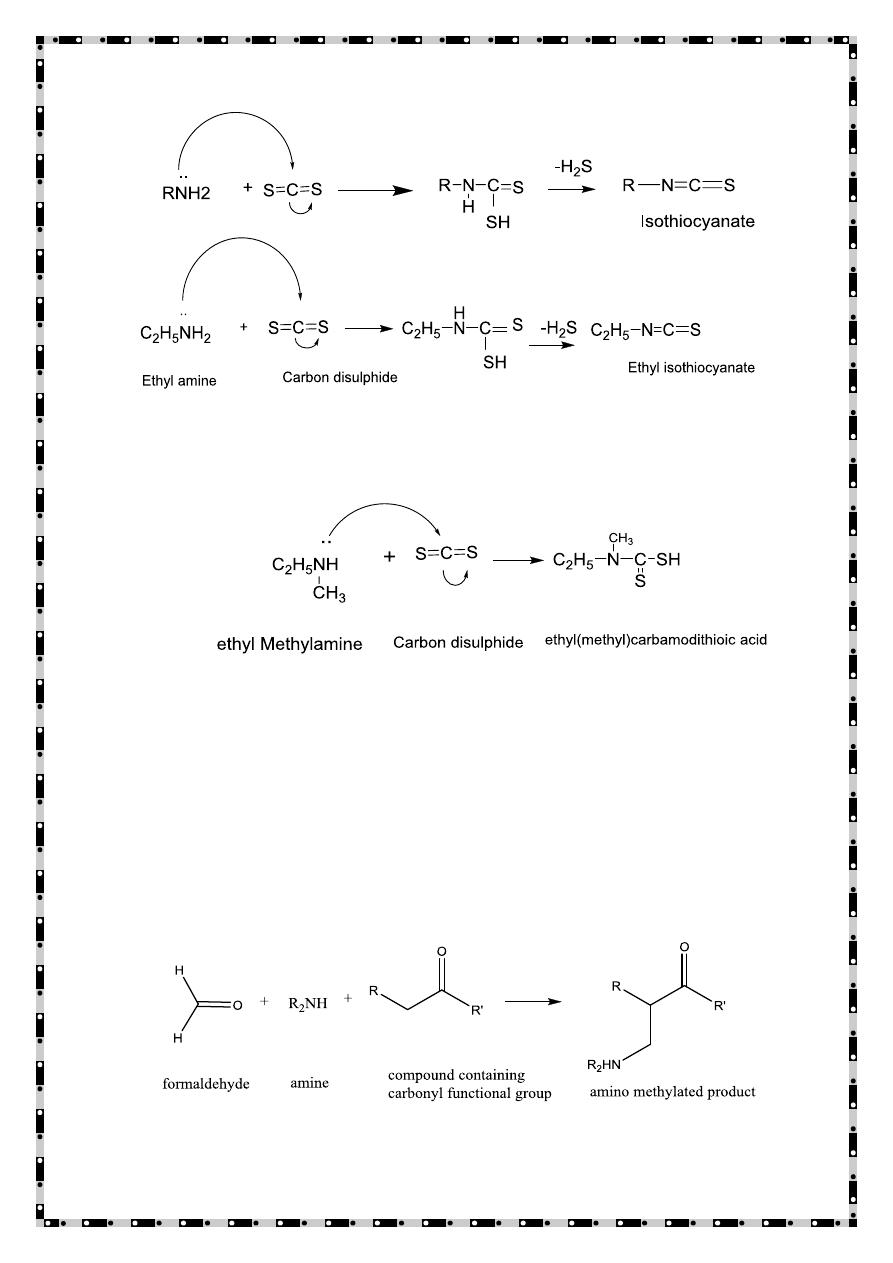
21
Secondary amines react with carbon disulphide to form dithiocarbamic
acid
Tertiary amines don't react with carbondisulphide.
Mannich condensation reaction
Mannich condensation is a reaction of a compound containing at least
one active hydrogen atom with formaldehyde and ammonia or
ammonia derivatives. Only primary and secondary amines can
participate in Mannich condensation reaction in this reaction active
hydrogen atom is replaced by amino methyl group.
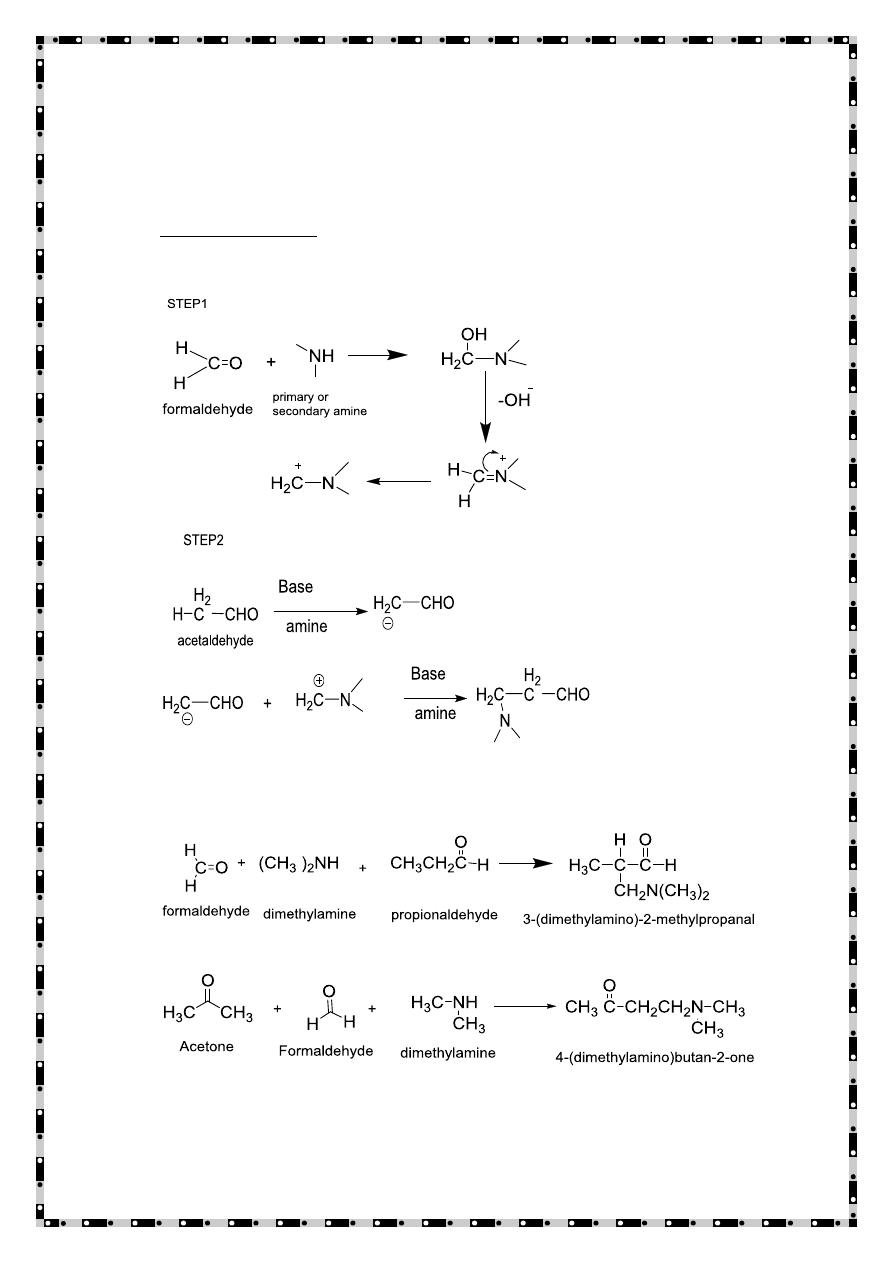
22
Mechanism of reaction
23
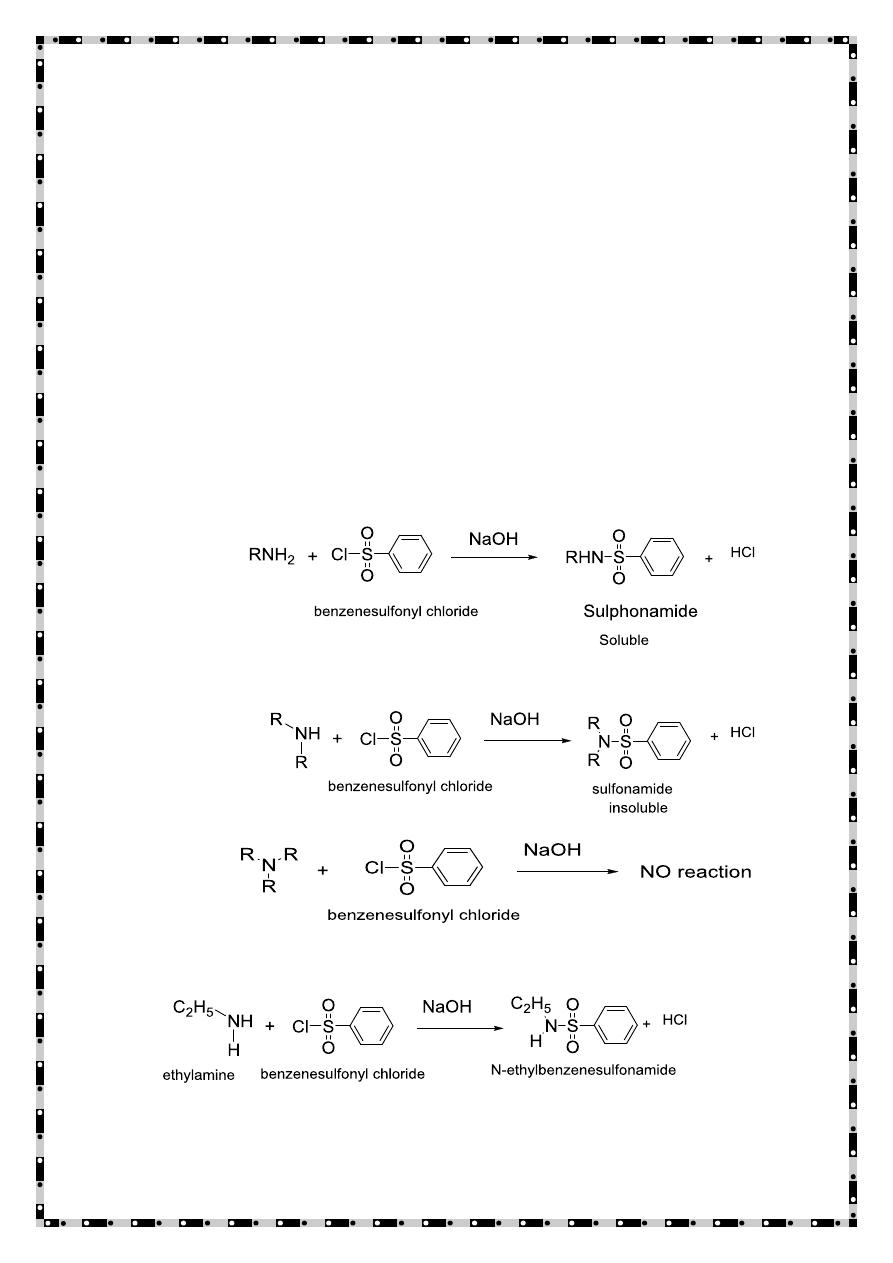
Detection of amines(
Hinsberg reaction
)
for the detection
a
is
Hinsberg reaction
The
. It is an excellent test for distinguishing primary, secondary
of
y amines. In this test, the amine is shaken well with
and tertiar
Hinsberg reagent in the presence of aqueous alkali (either KOH or
solution
). A reagent containing an aqueous
NaOH
is added to a substrate. A
and
after
salt which
will form a soluble
. A secondary amine in the same
addition of diluted
reaction will directly form an insoluble sulfonamide. A tertiary amine
In this way the reaction
.
yl chloride
sulfon
benzen
ct with
will not rea
.
can distinguish between the three types of amines

24
