- 1 -
Orientation in disubstituted benzenes
The presence of two substituents on a ring makes the problem of orientation
more complicated, but even here we can frequently make very definite
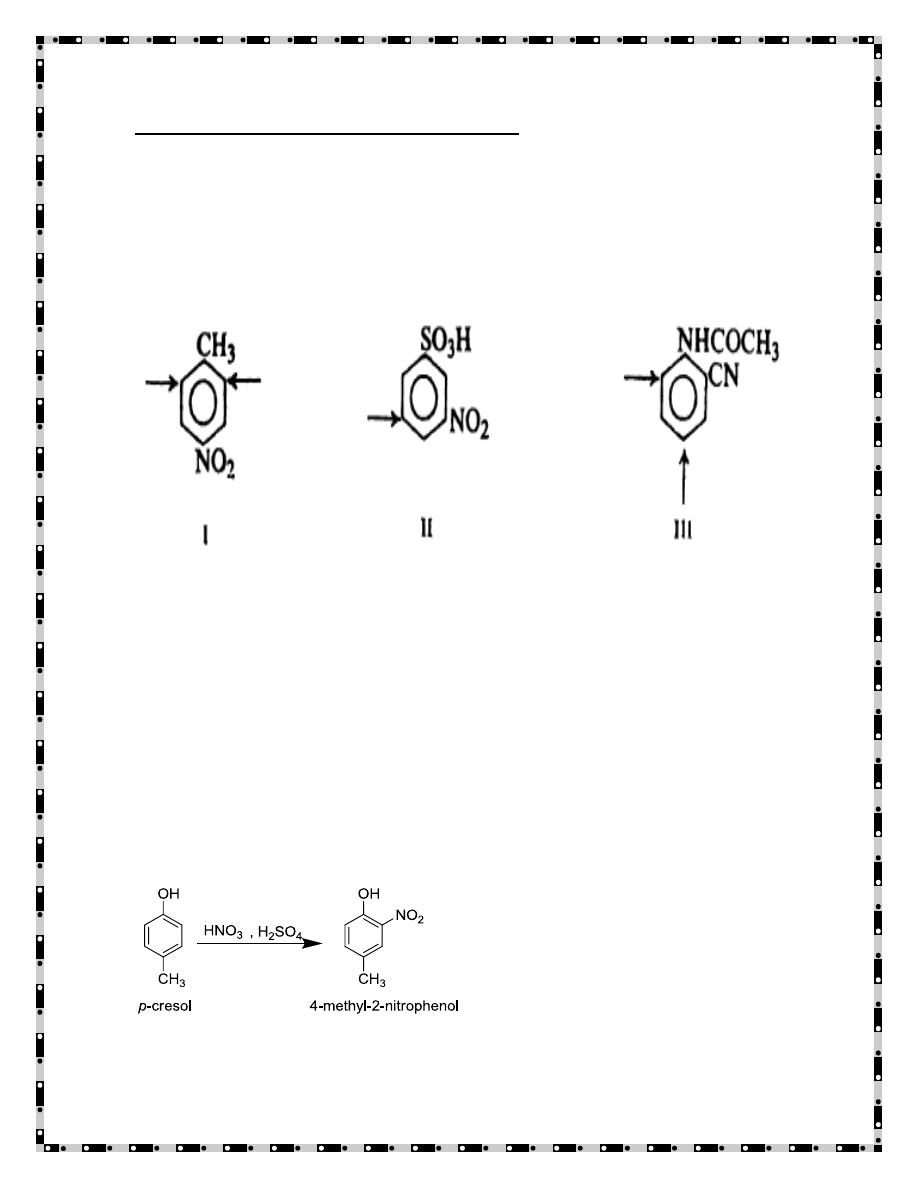
predictions. First of all, the two substituents may be located so that the
directive influence of one reinforces that of the other for example, in I, II,
and III the orientation clearly must be that indicated by the arrows.
On the other hand, when the directive effect of one group opposes that of the
other, it may be difficult to predict the major product; in such cases
complicated mixtures of several products are often obtained.Even where
there are opposing effects, however, it is still possible in certain cases to
make predictions in accordance with the following generalizations.
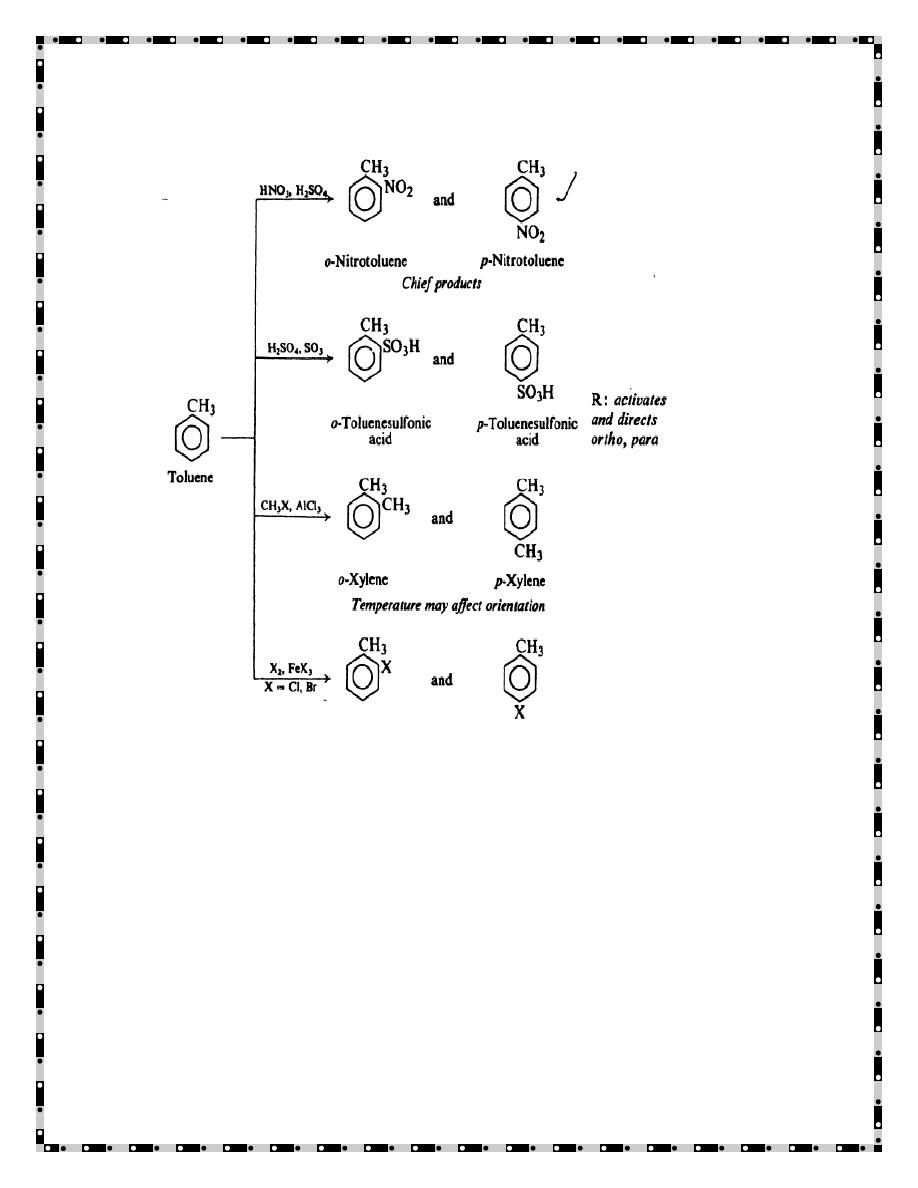
(a) Strongly activating groups generally win out over deactivating or weakly
activating groups. The differences in directive power in the sequence
-NH
2
, -OH > -OCH
3,
-NHCOCH
3
> -C
6
H
5
, -CH
3
> Meta directors
For example:
- 2 -
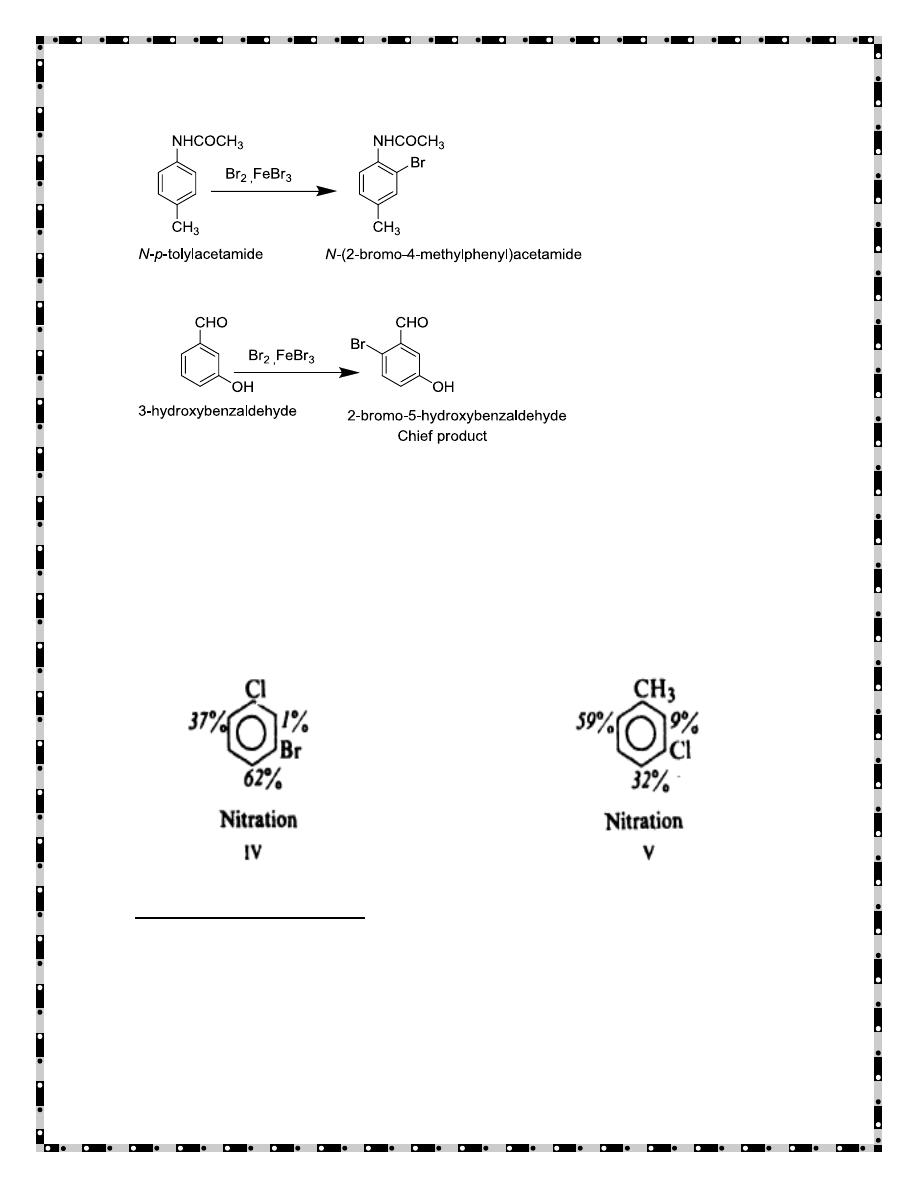
(b) There is often little substitution between two groups that are Meta to
each other. In many cases it seems as though there just is not enough room
between two groups located Meta to each other for appreciable substitution
to occur there, as illustrated by IV and V.
Orientation and synthesis
A laboratory synthesis is generally aimed at obtaining a single, pure
compound. Whenever possible we should avoid use of a reaction that
produces a mixture, since this lowers the yield of the compound we want
and causes difficult problems of purification. With this in mind, let us see
some of the ways in which we can apply our knowledge of orientation to the
- 3 -
synthesis of pure aromatic compounds. First of all, we must consider the
order in which we introduce these various substituents into the ring. In the
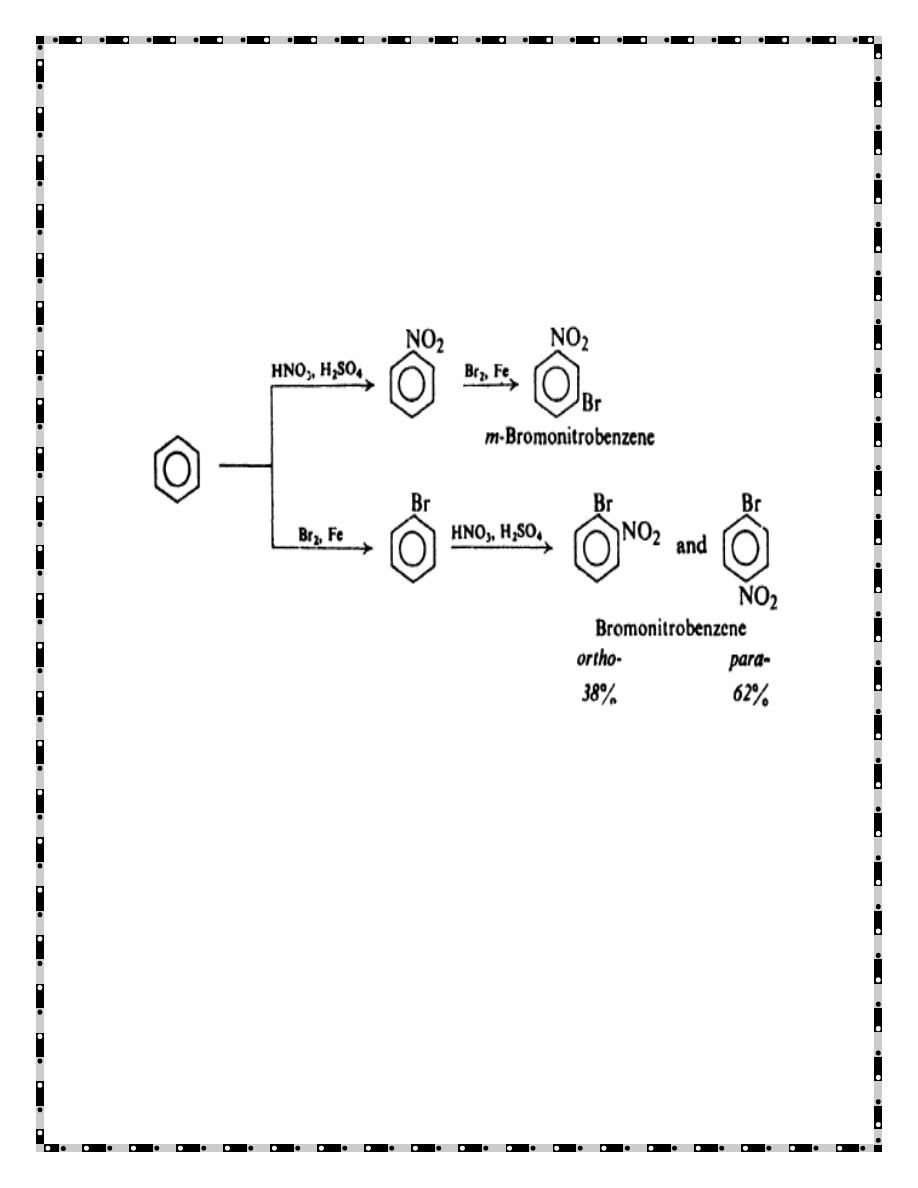
preparation of the bromonitrobenzenes, for example, it is obvious that if we
nitrate first and then brominate, we will obtain the m-isomer; whereas if we
brominate first and then nitrate, we will obtain a mixture of the o- and p-
isomers. The order in which we decide to carry out the two steps, then,
depends upon which isomer we want
Next, if our synthesis involves conversion of one group into another, we
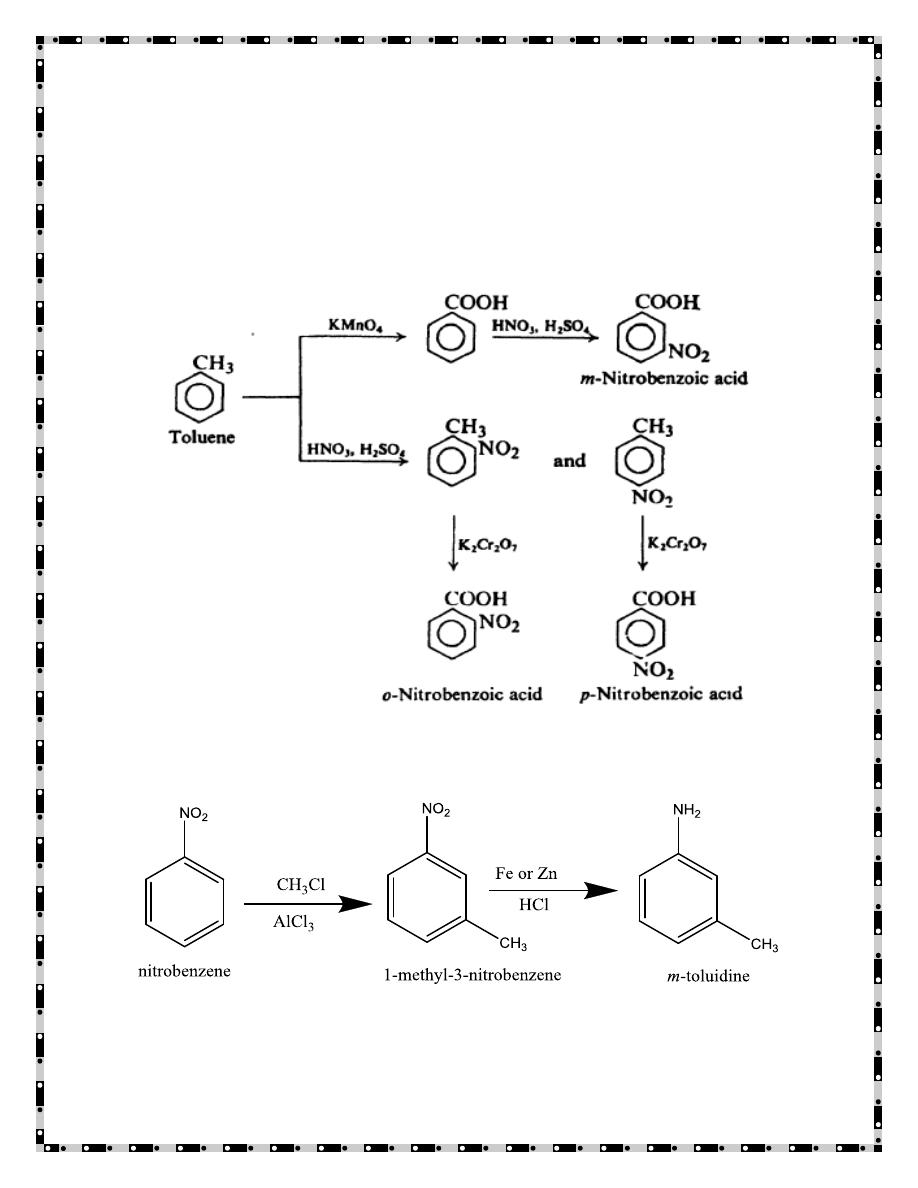
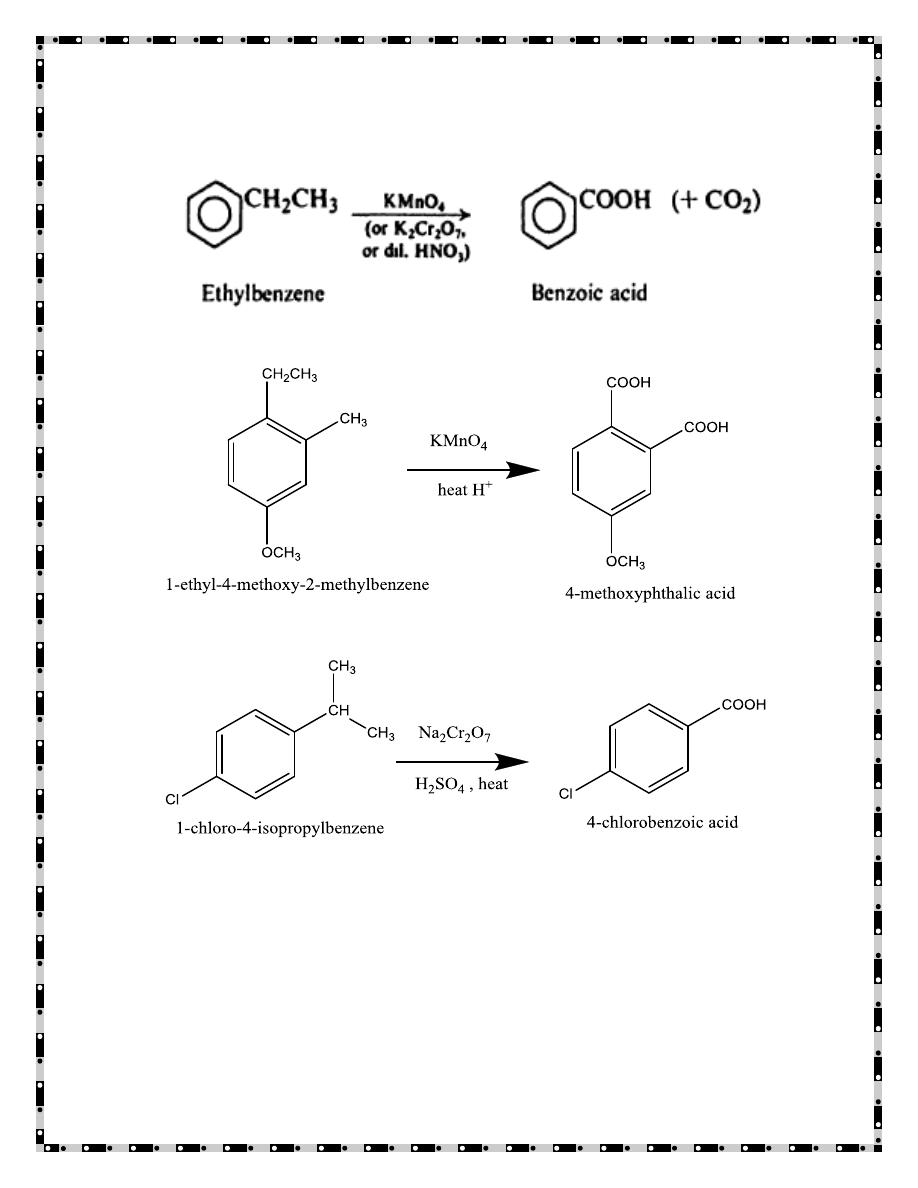
must consider the proper time for this conversion. For example, oxidation of
a methyl group yields a carboxyl group. In the preparation of nitrobenzoic
acids from toluene, the particular product obtained depends upon whether
oxidation or nitration is carried out first.
Substitution controlled by an activating group yields a mixture of ortho and
para isomers; nevertheless, we must often make use of such reactions, as in
the examples just shown. It is usually possible to obtain the pure para
isomer from the mixture by fractional crystallization. As the more
symmetrical isomer, it is the less soluble, and crystallizes while the solvent
still retains the soluble ortho isomer. Some para isomer, of course, remains
- 4 -
in solution to contaminate the ortho isomer, which is therefore difficult to
purify. As we shall see, special approaches are often used to prepare ortho
isomers
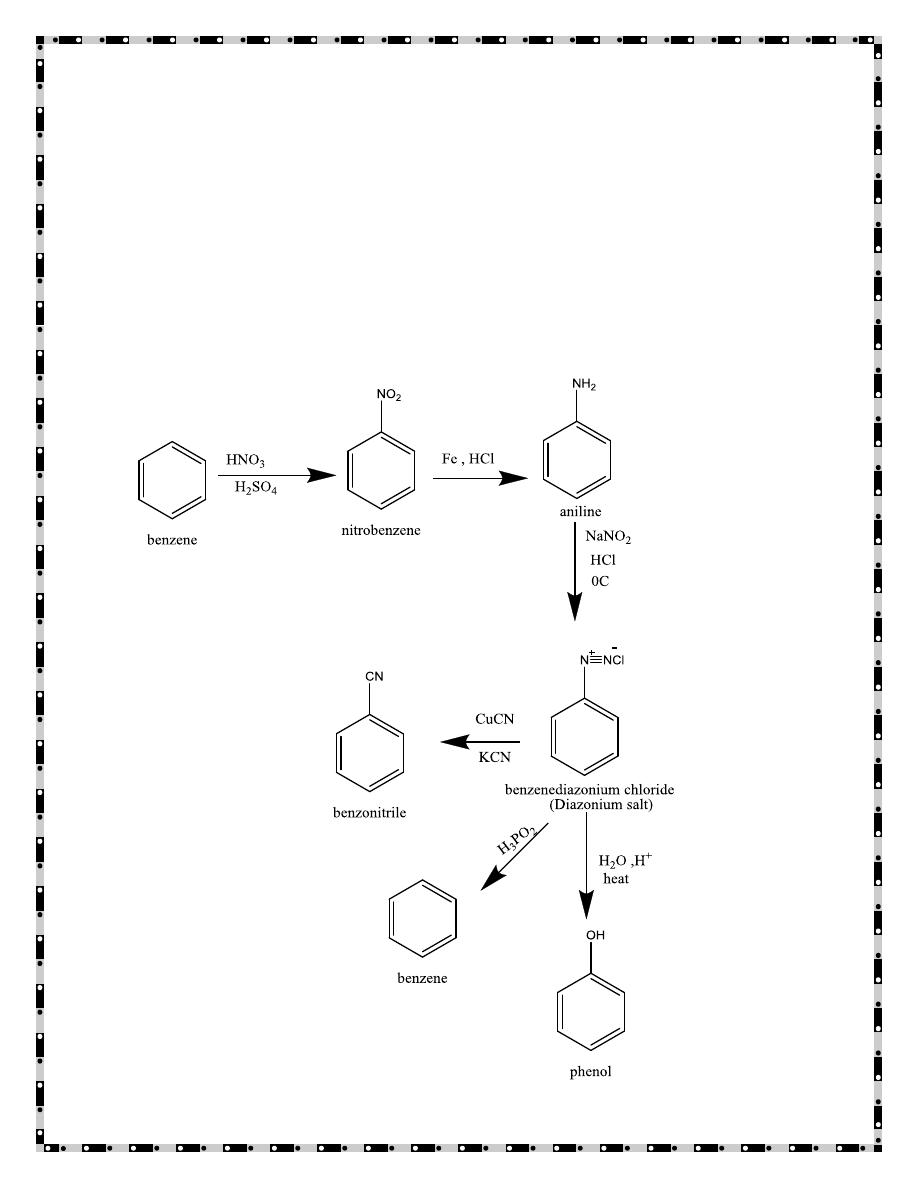
- 5 -
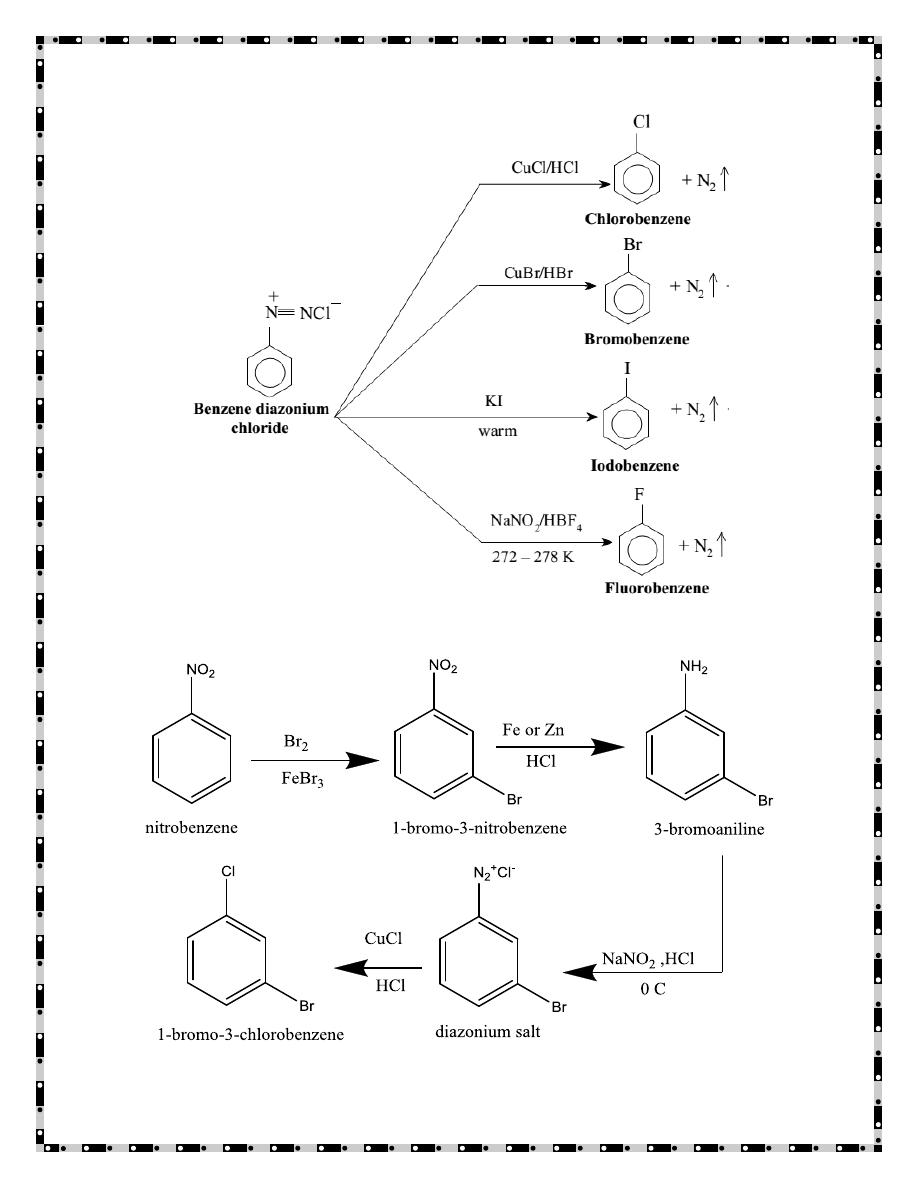
- 6 -

- 7 -
Aliphatic-aromatic hydrocarbons
Many important compounds are not just aliphatic or just aromatic, however,
but contain both aliphatic and aromatic units; hydrocarbons of this kind are
known collectively as arenes. Ethylbenzene, for example, contains a
benzene ring and an aliphatic side chain
.
The ring of ethylbenzene undergoes the electrophilic substitution
characteristic of benzene, and the side chain undergoes the free radical
substitution characteristic of ethane. Second, the properties of each portion
of the molecule should be modified by the presence of the other portion. The
ethyl group should modify the aromatic properties of the ring, and the ring
should modify the aliphatic properties of the side chain .Treatment of
ethylbenzene with nitric acid and sulfuric acid, for instance, introduces a
nitro group into the ring; treatment with bromine in the presence of light
introduces a bromine atom into the side chain. But because of the ethyl
group, nitration takes place more readily than with benzene itself, and occurs
chiefly at the positions ortho and para to the ethyl group; and because of the
ring, bromination takes place more readily than with ethane, and occurs
exclusively on the carbon nearer the ring. Thus each portion of the molecule
Affects the reactivity of the other portion and determines the orientation of
attack
The simplest of the alkyl benzenes, methylbenzene, is given the special
name of toluene. Compounds containing longer side chains are named by
prefixing the name of the alkyl group to the word -benzene, as, for example,
in ethylbenzene, n-propyl benzene, and isobutyl benzene.
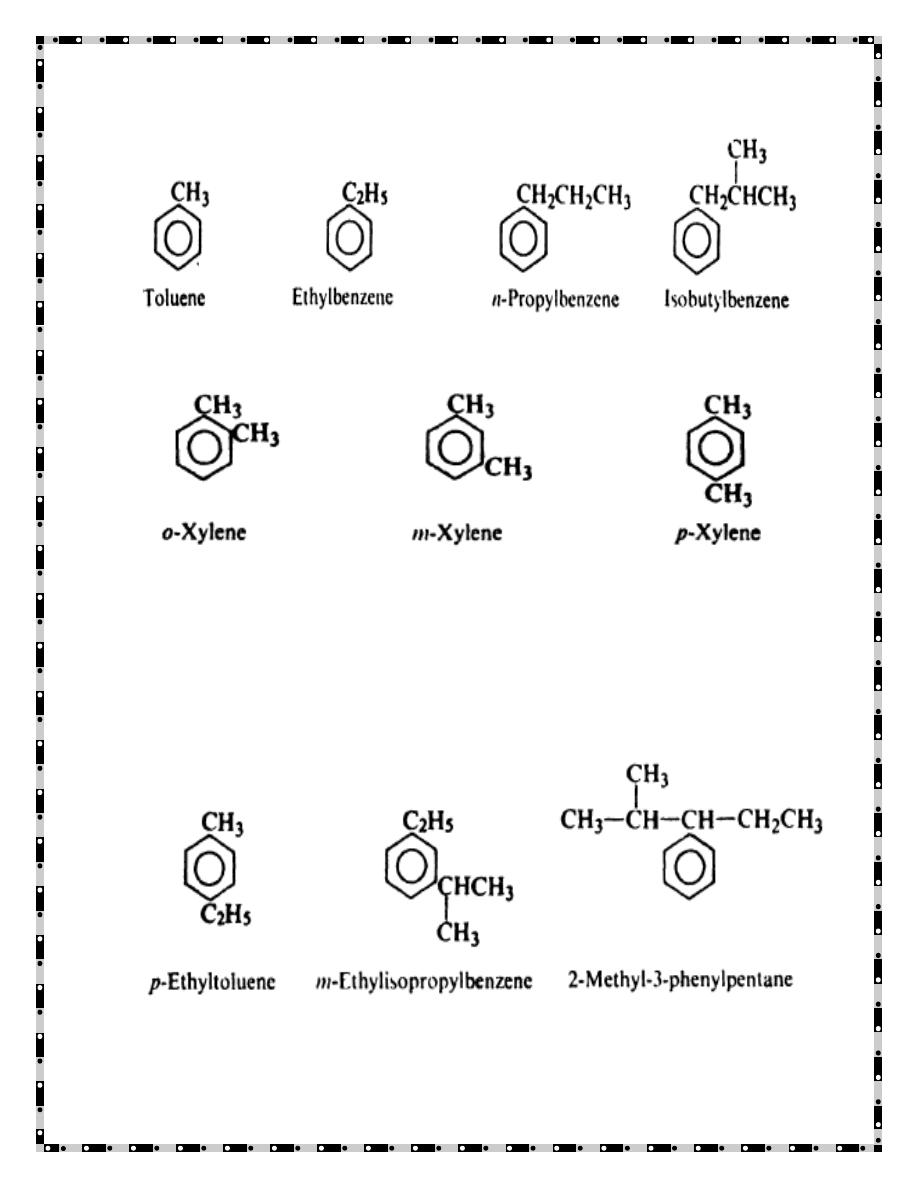
- 8 -
A compound containing a very complicated side chain might be named as a
phenyl alkane (C
6
H
5
= phenyl). Compounds containing more than one
benzene ring are nearly always named as derivatives of alkanes.
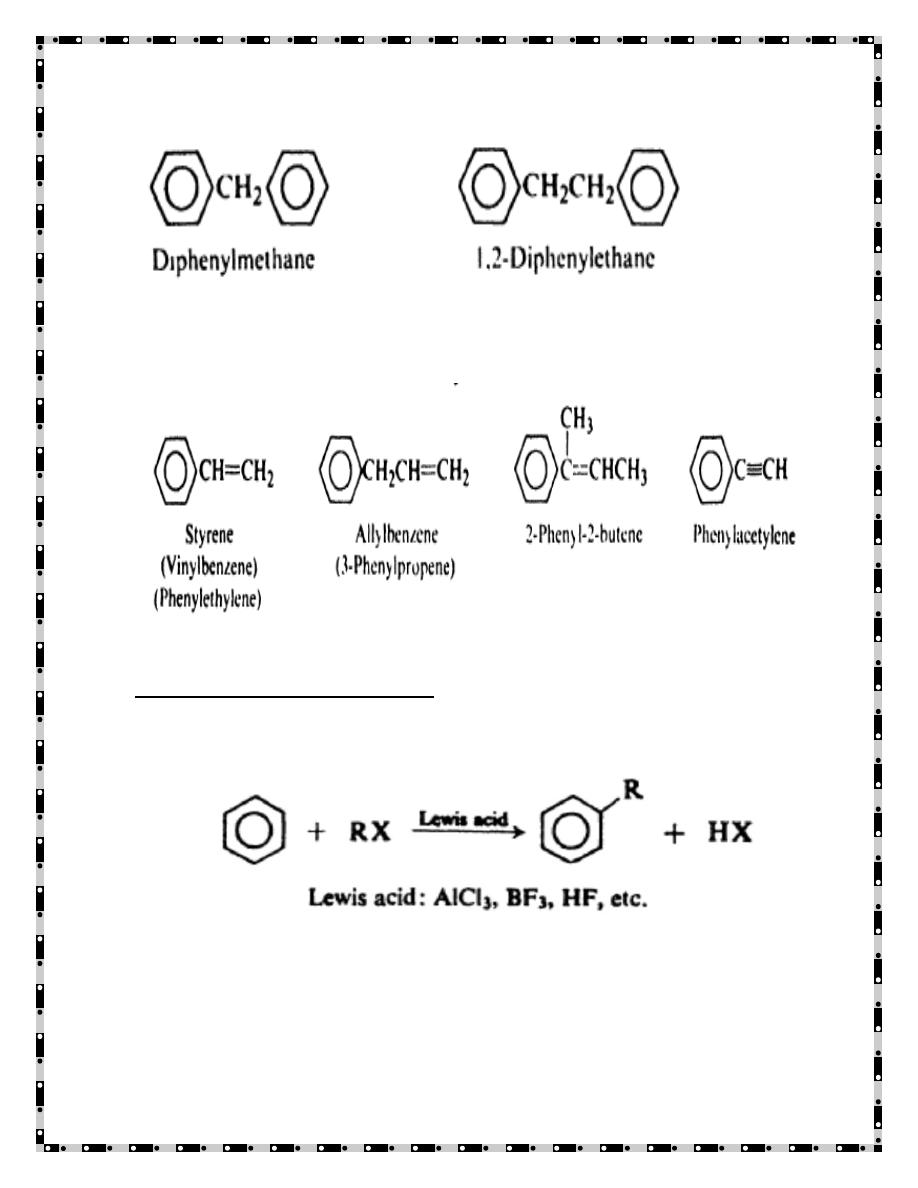
- 9 -
Preparation of alkylbenzenes
1. Friedel-Crafts alkylation
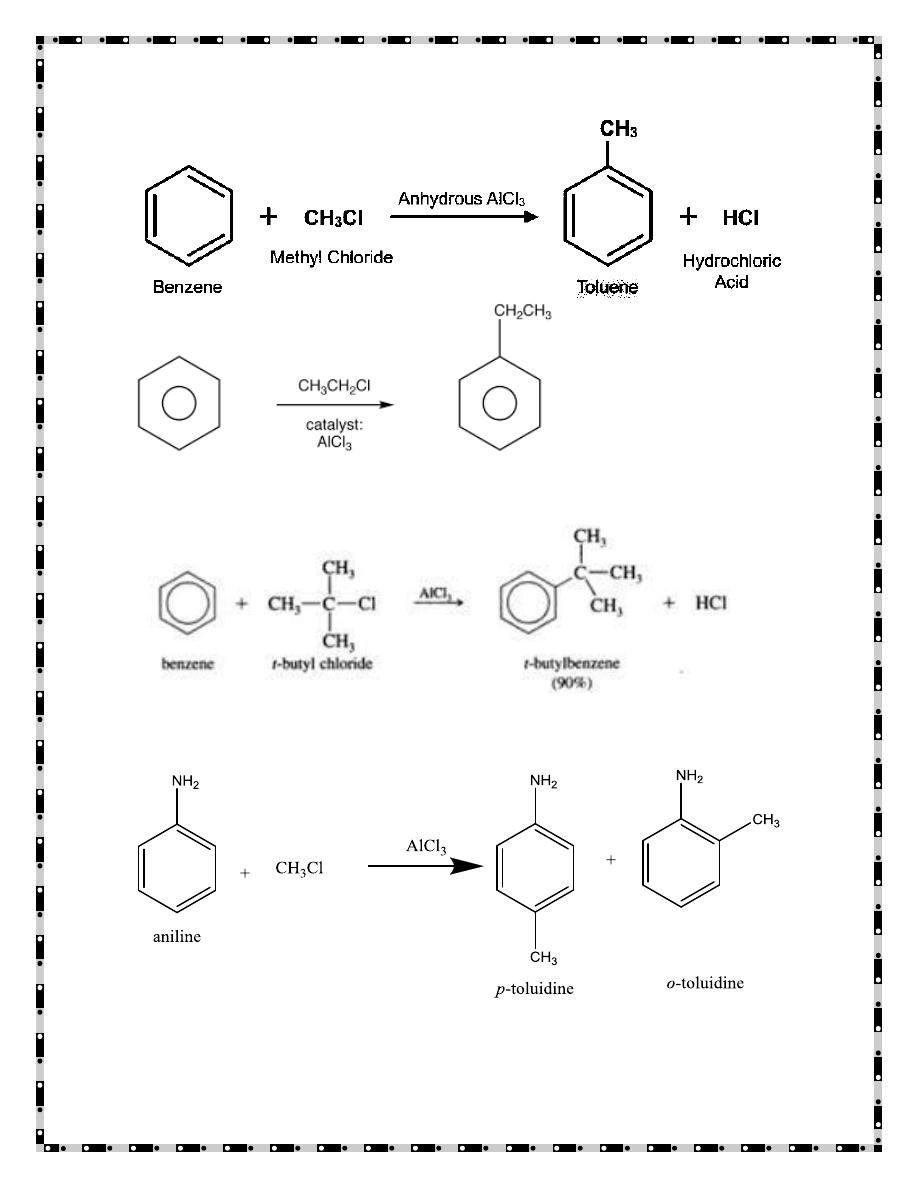
- 10 -
- 11 -
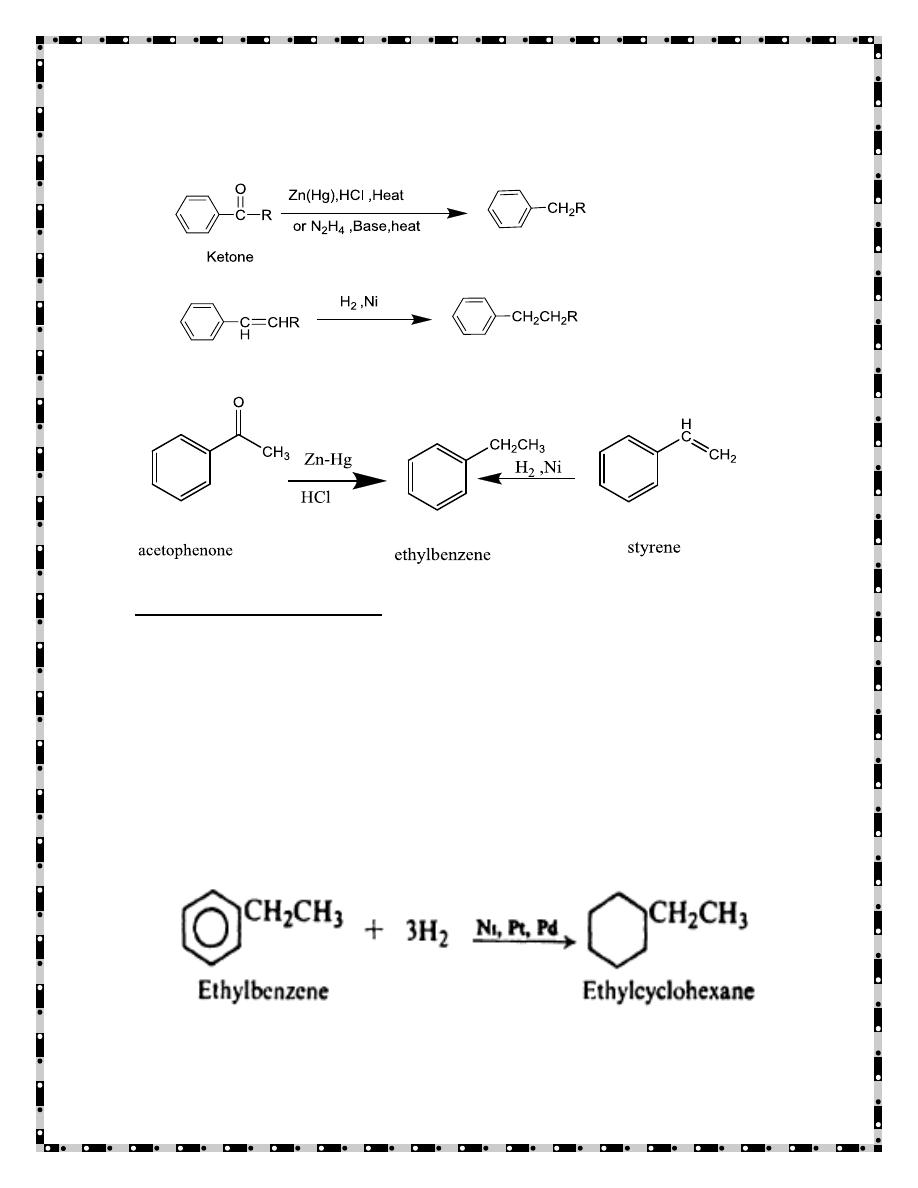
2. Conversion of side chain
Reactions of alkylbenzenes:
The most important reactions of the alkyl benzenes are outlined below, with
toluene and ethylbenzene as specific examples; essentially the same
behavior is shown by compounds bearing other side chains. Except for
hydrogenation and oxidation, these reactions involve either electrophilic
substitution in the aromatic ring or free-radical substitution in the
aliphatic side chain.
1. Hydrogenation:
- 12 -
2. Oxidation
- 13 -
2) Electrophilic aromatic substitution
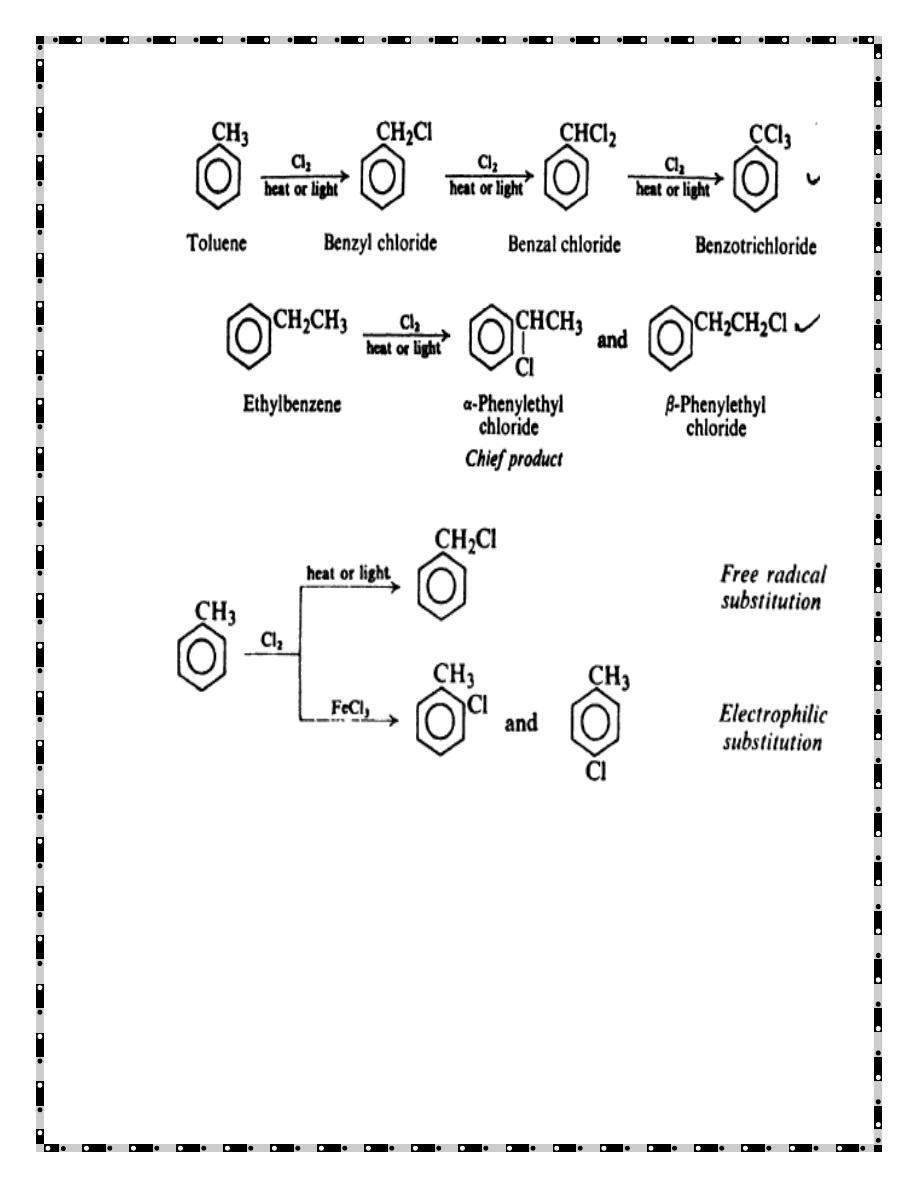
3) Side-chain halogenation (Free-radical halogenations) of
alkylbenzenes
An alkyl benzene with a side chain more complicated than methyl offers
more than one position for attack, and so we must consider the likelihood of
obtaining a mixture of isomers. Bromination of ethylbenzene, for example,
could theoretically yield two products: 1-bromo-l-phenylethane and 2-
bromo-l-phenylethane.
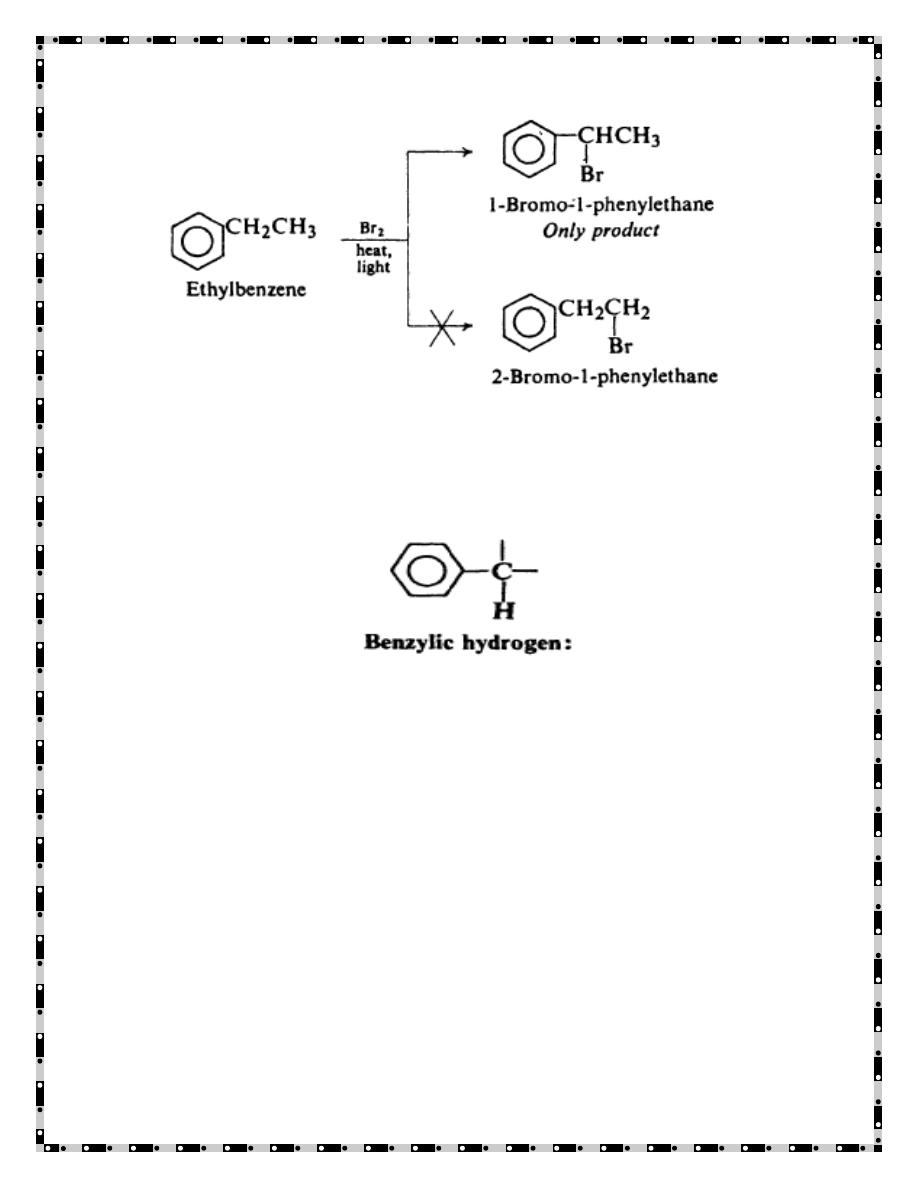
- 14 -
Practically the 1-Bromo product is the only product. Abstraction of the
hydrogen’s attacked to the carbon next to the aromatic ring is greatly
preferred .Hydrogen atoms attached to carbon joined directly to an aromatic
ring are called benzylic hydrogens.
- 15 -
