Sterilization and disinfection
.Sterilization is the process by which surgical items are rendered free of viable microorganisms, including spores. The purpose of effective laparoscopic instruments sterilization is to provide the surgeon with a sterile product
HISTORY OF STERILIZATION
In Seventeenth century advancements in anatomy, physiology and medical instrumentation included the development of the microscope in 1683 by Anton van Leeuwenhoek which allowed bacteria to be studied.Lord Joseph Lister was the one who successfully identified the implications for surgical infections. Lister believed that infection could be prevented if he could prevent the airborne microbes from entering the wound. Further advances in aseptic techniques from 1881 to 1882 were possible when a German bacteriologist, Robert Koch, introduced methods of steam sterilization and developed the first non-pressure flowing steam sterilizer.
LEGISLATION IN STERILIZATION
.The sterilization of laparoscopic instruments must comply with safety standards. These vary depending upon legislation of the individual countries. In Germany legislation requires steam autoclaving at 134°C for 5 minutes. However, in France sterilization is practiced at this temperature for 18 minutes.
In USA, Food and Drug Administration (FDA) has established different sterilization criteria regarding sterilization of reusable instruments. General requirement for characterization of sterilizing agent and the development, validation and routine control of a sterilization process for laparoscopic instrument is provided by the manufacturer and sterilization should be performed strictly according to the manufacturer’s guidelines.
Cleaning
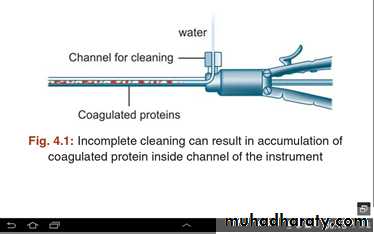
All used instruments, regardless of size, should be completely immersed in distilled water before leaving the operating room. The first step of the high level disinfection process is thorough cleaning . Cleaning removes debris, mucous, blood and tissue (bioburden) which would interfere with the action of the disinfectant.. Current recommendations specify disassembly of most laparoscopic equipment prior to sterilization. If the surgical assistants are unfamiliar with the proper assembly of laparoscopic instruments, it may cause patient injury from equipment malfunction. Because of the intricate internal parts of laparoscopic instruments, questions have been raised about the efficacy of cleaning and sterilization techniques.
bioburden can be removed by meticulous cleaning. Cleaning may be accomplished via manual or mechanical washing.
ULTRASONIC TECHNOLOGY FOR CLEANING
• Energy from high-frequency sound waves• Vigorous microscopic implosions of tiny vapor bubbles
• Millions of scrubbing bubbles do the job of cleaning
• Ultrasonic cleaners facilitate removal of organic material, decreasing the risk of contaminants .
The cleaning agent selected should be
: • Able to remove organic and inorganic soil
• Able to prevent water-borne deposits
• Low foaming
• Able to be rinsed completely
• Compatible with the materials being cleaned.
Following cleaning, items to be disinfected must be rinsed thoroughly to remove any residual detergent. After cleaning, instruments are subjected to sterilization.
Sterilization
The two methods of sterilization most commonly used for laparoscopic instruments.• Steam sterilization
• Chemical sterilization.
Autoclaving by means of steam was the oldest, safest and most cost-effective method of sterilization. When steam is placed under pressure and the temperature is raised, the moist heat produces changes within the cell protein, thereby rendering it harmless over a prescribed period of time.
The relationship between temperature, pressure and time of exposure is the critical factor in the destruction of microbes. Although steam sterilization in effective, an inexpensive. Most of the laparoscopic instrument can be safely autoclaved but some of the laparoscopic instruments cannot withstand the prolonged heat and moisture of the steam sterilization process.
Laparoscopic cameras, laparoscopes, light cables and flexible endoscopes are damaged by heat. Therefore, alternative methods of sterilization were needed to effectively sterilize moisture-sensitive and heatsensitive items that require rapid, frequent processing in the clinical setting.
One of the most common types of alternative of steam sterilization is chemical sterilization. Many chemicals are proven to have sterilizing property. Laparoscopic camera (CCD) is damaged by chemical sterilization with repeated exposure. In these expensive devices, a sterile plastic sleeve or sterile thick cloth sleeve should be used to avoid contamination.
Ethylene Oxide
One of the most common types of chemical sterilization uses ethylene oxide (EtO) gas, which is in use since the 1950 s. EtO is colorless at ordinary temperatures, has an odor similar to that of ether and is extremely toxic and flammable. Mixture of EtO with an inert gas such as carbon dioxide or a chlorofluorocarbon (CFC) was used to make it noninflammable. The most common combination was 12 percent EtO and 88 percent freon. A newer formulation uses EtO plus a hydrochlorofluorocarbonHCFC). EtO sterilization depends on four parameters:
1. Time2 . Temperature
3. Gas concentration
4. Relative humidity. All EtO sterilizers operate at low temperature, typically between 49° and 60 °C (130-140°F) and relative humidity of 40 to 60 percent. The humidity must be not less than 30 percent in order to hydrate the items during the sterilization process. These characteristics make EtO sterilization suitable for complex medical equipment.
Both temperature and humidity have a profound influence on the destruction of microorganisms because they affect penetration of the gas through bacterial cell walls, as well as through the wrapping and packaging materials. It typically takes between 3 and 6 hours for the sterilization portion of the cycle to be completed. Additionally, items sterilized by EtO must be aerated to make them safe for personnel handling and patient use. Therefore, the EtO sterilization and aeration processes can take up to 20 hours and should be used only when time is not a factor.
Hydrogen Peroxide Gas Plasma
Hydrogen peroxide is an oxidizing agent that affects sterilization by oxidation of key-cellular components. Plasma is a state of matter distinguishable from a solid, liquid, or gas. The cloud of plasma is composed of ions, electrons and neutral atomic particles that produce a visible glow. Hydrogen peroxide is bactericidal, virucidal, sporicidal and fungicidal, even at low concentration and temperature.A solution of hydrogen peroxide and water (59%is vaporized and allowed to surround and interact with the devices to be sterilized. Applying a strong electrical field then creates plasma. The plasma breaks down the peroxide into a “cloud” of highly energized species that recombine, turning the hydrogen peroxide into water and oxygen. No aeration time is required and the instruments may either be used immediately or placed on a shelf for later use.
A load of surgical instruments may be sterilized in less than one hour.,
Peracetic Acid
Liquid paroxyacetic, or peracetic acid, is a biocidal oxidizer that maintains its efficacy in the presence of high levels of organic debris. Peracetic acid is acetic acid plus an extra oxygen atom and reacts with most cellular components to cause cell death. The peracetic acid solution is heated to 50 to 56 º C (122-131º F) during the 20 to 30 minutes cycle. Peracetic acid must be used in combination with anticorrosive additives.Parameters for peracetic acid sterilizers include:
• Relatively short cycle times•Availability of the items for immediate use
• Sterilant can be discharged into the drainage system since it is not hazardos
• No aeration time is required for the sterilized items
• Items must be rinsed with copious amounts of sterile water after the sterilization process. Items processed by this method should be used immediately after processing, since the containers are wet and are not protected from the environment. This system must also be monitored for sterility with live spores
Glutaraldehyde
An activated 2 percent aqueous glutaraldehyde solution is recognized as an effective liquid chemical sterilant. Glutaraldehyde is most frequently used as a high-level disinfectant for lensed instruments because it is non-corrosive and has minimal harmful effect on the instrument . Sterilization can be achieved with an activated 2 percent glutaraldehyde solution after the item is completely immersed for 10 hours at 25 º C in especially designed tray
. Before immersion, the item must be thoroughly cleaned and dried. During immersion, all surfaces of the item must be in contact with the solution. After immersion, the item must be rinsed thoroughly with sterile water prior to use
Orthophthalaldehyde
For laparoscopic instruments, 0.55 percent orthophthalaldehyde is good option, it is non-glutaraldehyde solution for disinfection of delicate instruments. In fact, orthophthalaldehyde solution is one of the gentlest reprocessing options available, which means it can substantially reduce instrument damage and repair costs. It is good not only because of its speed and efficiency but also because of its environmental safety. It comes with the trade name of Cidex OPA. It has following advantage:No activation or mixing required
It can be used in both automated and manual reprocessingTwo years shelf life and 75 days open-bottle shelf life
Rapid 5 minutes immersion time at a minimum of 25 ºC in an automatic endoscope reprocessor
Efficient 12 minutes soak time at room temperature (20° C) for manual reprocessing
Effective against glutaraldehyde-resistant mycobacterium
It has following advantage:
Formaldehyde
Bactericidal properties and use of formaldehyde include: 37 percent aqueous solution (formalin) or 8 percent formaldehyde in 70 percent isopropyl alcohol kills microorganisms by coagulating intracellular protein. Solution is effective at room temperature.
Specially designed airtight formalin chambers are available, Eight to ten formalin tablets wrapped with moist gauge piece should be placed in the chamber and the door should be closed. The vapor of formalin acts for one week, after one week tablets should be changed. Although known to destroy spores, it is rarely used because it takes from 12 to 24 hours to be effective. Formalin chamber is used by
Formalin chamber is used by many surgeons to carry their sterilized instrument from one hospital to another. Pungent odor of formalin is quite objectionable and irritating to the eyes and nasal passages. The vapors can be toxic and ongoing controversy exists regarding its carcinogenic effects. However, lowtemperature steam with formaldehyde has been widely used in health care facilities in Northern Europe for the sterilization of reusable medical devices that cannot withstand steam sterilization
.Other Chemical Disinfectant
Recently, non-aldehyde instrument disinfectant is available for rapid decontamination of non-invasive and heat labile laparoscopic instruments. It contains halogenated tertiary amines, poly hexamethylene biguanide hydrochloride, ethyl alcohol B, dodecylamine and sulfamic acid. Contact time for bactericidal and fungicidal and virucidal protection is 10 minutes. For sporicidal protection, contact time is 30 minutes.CONCLUSION
Most of the laparoscopic instrument can be easily sterilized if the person knows how to dissemble, clean and use specificchemical for sterilization. Manufactures instruction is important to follow if desired effect has to be achieved. Expensive instruments should be handled carefully and all the insulated instruments should be checked thoroughly for any breach in insulation before sterilization.Apart from newer generation chemical disinfectant, low-temperature steam with formaldehyde has been widely used in health care facilities in Northern Europe for the sterilization of reusable medical devices that cannot withstand steam sterilization. Other key considerations in the sterilization process which should be taken care are:
• Packaging of the items after sterilization
• Monitoring the sterilization process
• Shelf life of the sterilized items
• Cost implications.