Metabolic response to injury
The classical neuroendocrine pathways of the stress response consist of afferent neurones, the spinal cord, thalamus, hypothalamus and pituitary. Corticotrophin releasing factor (CRF) released from the hypothalamus increases adrenocorticotrophic hormone (ACTH)which is released from the anterior pituitary. ACTH then acts on the adrenal to increase the secretion of cortisol.Hypothalamic activation of the sympathetic nervous system causes release of adrenalin and also stimulates release of glucagon.
There are, however, many other players,include
1 .alterations in insulin release and sensitivity.2 . hypersecretion of prolactin
3. inactivation of peripheral thyroid hormones and gonadal function. Of note, GH has direct lipolytic, insulin-antagonising and proinflammatory properties
Proinflammatory cytokines including (interleukin-1 (IL-1, tumour necrosis factor alpha (TNF), IL-6 and IL-8 in the first 24 hours and act directly,on
1. the hypothalamus to cause pyrexia.
2. skeletal muscle to induce proteolysis
3. inducing acute phase protein production in the liver.
4. the development of peripheral insulin resistance.
Within hours of the upregulation of proinflammatory cytokines, antagonists enter the circulation
1- interleukin-1 receptor antagonist (IL-1Ra) and
2- TNFsoluble receptors (TNF-sR-55 and 75.
3- development of counterinflammatory response by IL-4 -5 -9 and -13, and results in immunosuppression and an increased susceptibility to opportunistic (nosocomial) infection
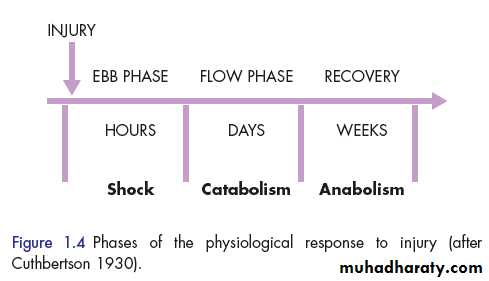
THE ‘EBB AND FLOW’ MODEL In the natural world, if an animal is injured, it displays a characteristic response, which includes immobility, anorexia and catabolism .
In 1930, Sir David Cuthbertson divided the metabolic response to injury in humans into ‘ebb’ and ‘flow’ phases
The ebb phase
begins at the time of injury and lasts for approximately 24-48 hours. It may be attenuated by proper resuscitation, but not completely abolished.
The ebb phase is characterised by
1. hypovolaemia2. decreased basal metabolic rate,
3. reduced cardiac output,
4. hypothermia and
5. lactic acidosis.
The predominant hormones regulating the ebb phase
these are
1. catecholamines
2. cortisol and
3. aldosterone (following activation of the renin–angiotensin system).
The main physiological role of the ebb phase is to conserve both circulating volume and energy stores for recovery and repair.
flow phase, which corresponds to SIRS. This involves the mobilisation of body energy stores for recovery and repair, and the subsequent replacement of lost or damaged tissue. It is characterised by
1 . tissue oedema (from vasodilatation and increased capillary leakage)
2. increased basal metabolic rate (hypermetabolism)
3. increased cardiac output,
4 .raised body temperature,
5 .leukocytosis,
6 increased oxygen consumption and
7. Increased gluconeogenesis.
The flow phase may be subdivided into
1. acatabolic phase 3-10 days, followed by2. an anabolic phase, which may last for weeks .
During the catabolic phase
1. the increased production of hormones ,(catecholamines, cortisol, insulin and glucagon)2. weight loss and increased urinary nitrogen excretion.
3. insulin resistance and, therefore, injured patients often exhibit poor glycaemic control. The combination of prolonged catabolism with insulin resistance places patients at increased risk of complications, particularly infectious.
KEY CATABOLIC ELEMENTS OF THE FLOW PHASE
Hypermetabolism
1- The majority of trauma patients demonstrate energy expenditures approximately 15-25 per cent above predicted healthy resting values,2- abnormalities in wound circulation (hyperaemic areas cause an increase in cardiac output)
Alterations in skeletal muscle protein metabolism
Muscle protein is synthesised and broken down with a turnover rate in humans of 1-2 per cent per day.Under normal circumstances, synthesis equals breakdown and muscle bulk remains constant. Physiological stimuli that promote net muscle protein accretion include feeding (especially extracellular amino acid concentration)and exercise. Paradoxically, during exercise, skeletal muscle protein synthesis is depressed, but it increases again during rest and feeding.
During the catabolic phase of the stress response,
muscle wasting occurs as a result of an increase in muscle protein degradation (via enzymatic pathways), coupled with a decrease in muscle protein synthesis.(In major sepsis), urinary nitrogen losses can reach 14-20 g/day
equivalent to the loss of 500 g of skeletal muscle per day. It is that muscle catabolism cannot be inhibited fully by providing artificial nutritional support as long as the stress response continues.in critical care, it is now recognised that ‘hyperalimentation’ represents a metabolic stress in itself, and that nutritional support should be at a modest level to attenuate rather than replace energy and protein losses.
Clinically
a patient with skeletal muscle wasting will experience1. asthenia
2. fatigue
3. reduced functional ability
4.decreased quality of life and
5.an increased risk of morbidity and mortality
1. Albumin is the major protein produced by the liver and is renewed at the rate of 10 per cent per day
2. transcapillary escape rate (TER) of Albumin may increased three-fold following major injury/sepsis
due to increased vascular permeability.
Alterations in hepatic protein metabolism:
The liver and skeletal muscle together account for > 50 per cent of daily body protein turnover.
Skeletal muscle has a large mass but a low turnover rate (1-2 per cent per day), whereas the liver has a relatively small mass (1.5 kg) but a much higher protein turnover rate 10-20 per cent per day.
fall in albumin following injury, reflect increased transcapillary escape, secondary to an increase in microvascular permeability.
Insulin resistance
Following surgery or trauma, postoperative hyperglycaemia develops as a result of increased glucose production combined with decreased glucose uptake in peripheral tissues. Decreased glucose uptake is a result of insulin resistance which is transiently induced within the stressed patient.The mainstay of management of insulin resistance is intravenous insulin infusion. in either
1. an intensive approach (i.e. sliding scales are manipulated to normalise the blood glucose level) or
2.a conservative approach (i.e. insulin is administered when the blood glucose level exceeds a defined limit and discontinued when the level falls).
intensive insulin therapy is superior to conservative insulin approaches in reducing morbidity rates .
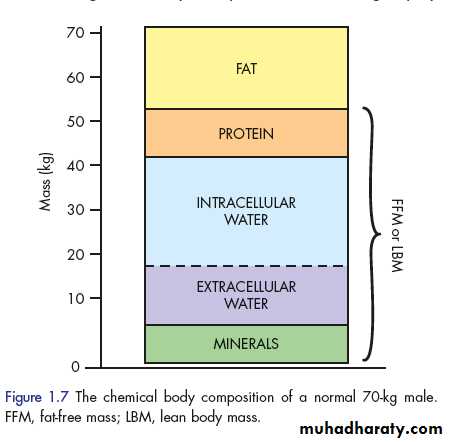
CHANGES IN BODY COMPOSITION FOLLOWING INJURY The average 70-kg male can be consist of fat (13kg) and fat-free mass (or lean body mass: 57kg). In such an individual, the lean tissue is composed primarily of protein (12kg), water (42kg) and minerals (3 kg) . The protein mass can be considered as two basic compartments, skeletal muscle (4 kg) and non-skeletal muscle (8 kg), which includes the visceral protein mass. The water mass (42litres) is divided into intercellular
(28 litres) and extracellular (14litres) spaces. Most of the mineral mass is contained in the bony skeleton.
1. each 1 g of nitrogen is contained within 6.25 g of protein, which is contained in 36 g of wet weight tissue.
2.the loss of 1 g of nitrogen in urine is equivalent to the breakdown of 36 g of wet weight lean tissue
3. A normal human ingests about 70-100 g protein per day, which is metabolised and excreted in urine as ammonia and urea (i.e. approximately 14g N/day).
During total starvation, urinary loss of nitrogen is rapidly attenuated by a series of adaptive changes. thus accounting for the survival of hunger strikers for a period of 50-60 days.
Following major injury, this adaptive change fails to occur, and there is a state of ‘autocannibalism’, resulting in continuing urinary nitrogen losses of 10-20 g N/day (equivalent to 500 g of wet weight lean tissue per day), once loss of body protein mass has reached 30-40 per cent of the total, survival is unlikely.
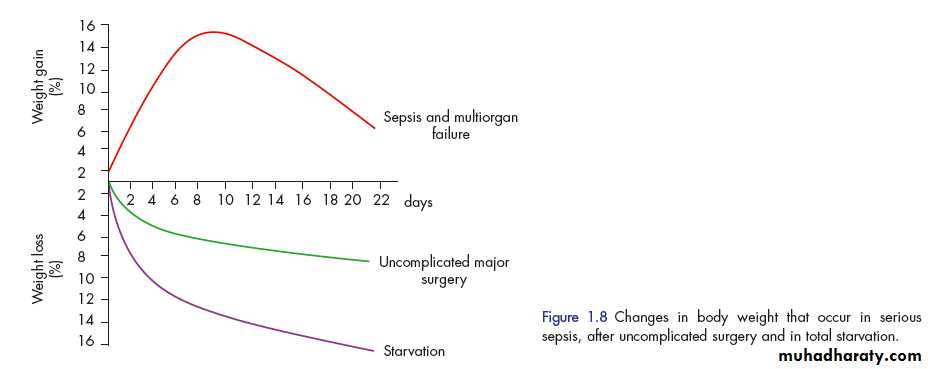
Critically ill patients admitted to the ICU with sepsis or major blunt trauma undergo massive changes.
1. Body weight increases immediately on resuscitation with an expansion of extracellular water by 6-10 litres within 24 hours. .
2.total body protein will diminish by 15 per cent in the next 10 days, and body weight will reach negative balance as the expansion of the extracellular space resolves.
The body weight and nitrogen equilibrium following major elective surgery can be achieved by blocking the neuroendocrine stress response with:
1. epidural analgesia
2. early enteral feeding ,
3. avoidance of excessive administration of intravenous saline.
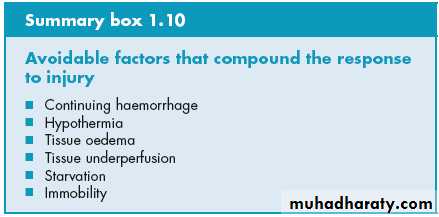
Volume loss
simple haemorrhage the pressor receptors in the carotid artery and aortic arch,theleft atrium, initiate afferent nerve input to the central nervous system (CNS)resulting in the release of both aldosterone and antidiuretic hormone (ADH)Pain can also stimulate ADH release. ADH acts directly on the kidney to cause fluid retention. Decreased pulse pressure stimulates the juxtaglomerular apparatus in the kidney and directly activates the renin–angiotensin system, which in turn increases aldosterone release.
effects of ADH and aldosterone result in the natural oliguria observed after surgery and conservation of sodium and water in the extracellular space. The tendency to water and salt retention is exacerbated by resuscitation with saline-rich fluids. Salt and water retention can result in
1. peripheral oedema.
2. visceral oedema (e.g. stomach). visceral oedema reduced gastric emptying, and prolonged hospital stay
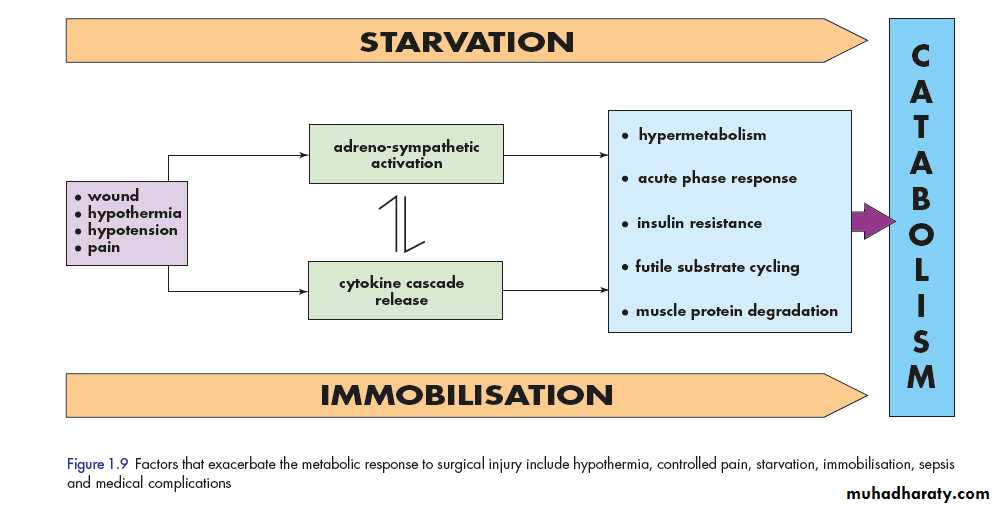
Hypothermia
Hypothermia results in increased elaboration of adrenal steroids and catecholamines. When compared with normothermic controls, even mild hypothermia results in a two- to three-fold increase in postoperative cardiac arrhythmias and increased catabolism. Randomised trials have shown that maintaining normothermia reduces wound infections, cardiac complications and bleeding and transfusion requirements.Tissue oedema
During systemic inflammation, fluid, plasma proteins, leukocytes, macrophages and electrolytes leave the vascular space and accumulate in the tissues. This can1. diminish the alveolar diffusion of oxygen
2. reduced renal function. Increased capillary leak is mediated by a variety of mediators including cytokines, , bradykinin and nitric oxide.
InSystemic inflammation and tissue underperfusion
1. Maintaining normoglycaemia with insulin infusion during critical illness has been proposed to protect the endothelium, thereby contribute to the prevention of organ failure and death.
2.Administration of activated protein C to critically ill patients has been shown to reduce organ failure and death and is thought to act, in part, via preservation of the microcirculation in vital organs.
Starvation
During starvation, the body is faced with an obligate need to generate glucose to sustain cerebral energy metabolism (100g of glucose per day).1. This is achieved in the first 24 hours by mobilising glycogen stores.
2. Thereafter by hepatic gluconeogenesis from amino acids, glycerol and lactate.
The energy of other tissues is sustained by
• mobilising fat from adipose tissue.
2. fat mobilisation is mainly dependent on a fall in circulating insulin levels.
3. the liver converting free fatty acids into ketone bodies, which can serve as a substitute for glucose for cerebral energy metabolism.3. (2 litres ) of intravenous 5 per cent dextrose as intravenous fluids for surgical patients who are fasted provides 100 g of glucose per day and has a significant protein-sparing effect.
4.Avoiding unnecessary fasting.
5. early oral enteral/parenteral nutrition form the platform for avoiding loss of body mass in surgical patients.
Modern guidelines
1. on fasting prior to anaesthesia allow intake of clear fluids up to 2 hours before surgery.
2. Administration of a carbohydrate drink at this time reduces perioperative anxiety and thirst and decreases postoperative insulin resistance.
There is now a strong scientific rationale for avoiding
1. unmodulated exposure to stress.2. prolonged fasting.
3. excessive administration of intravenous (saline) fluids .
(laparoscopic) surgery is a key change in surgical practice that can reduce the magnitude of surgical injury.
CONCEPTS BEHIND OPTIMAL PERIOPERATIVE CARE
modulating the stress/inflammatory response at the time of surgery over periods of months or longer
1. B-blockers and statins have recently been shown to improve long-term survival after major surgery. due to suppression of innate immunity at the time of surgery.
2. the use of epidural analgesia to reduce pain, block the cortisol stress response and attenuate postoperative insulin resistance may, via effects on the body’s protein economy.