RENAL PHYSIOLOGY…Proff Amjad Fawzi
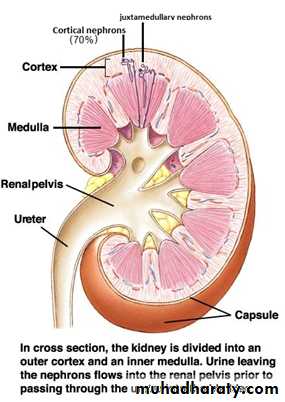
Anatomy of the kidney: A frontal section through a kidney reveals three distinct regions: cortex, medulla, and pelvis (figure 1).The kidneys have several functions including the following:
[1] Kidneys regulate water and electrolytes balance: Intake of water and many electrolytes usually are governed mainly by a persons's eating and drinking habits, necessating the kidneys to adjust their excretion rates. If intake is less than excretion, the amount of that substance in the body will decrease, and vice versa.[2] Kidneys responsible for excretion of metabolic waste products like urea (from the metabolism of amino acids), creatinine (from muscle creatine), uric acid (from nucleic acid), bilirubin (the end product of Hb breakdown), metabolites of various hormones and foreign chemicals like drugs and toxins. Wastes must be eliminated as rapidly as they are produced to prevent the toxic effects of their accumulation.
[3] Kidneys play essential role in regulation of arterial pressure both in long-term regulation (through excretion of variable amounts of sodium and water) and in short-term regulation (through secretion of vasoactive substances such as renin).
[4] Kidneys contribute to acid-base regulation (along with the lungs and body buffers) through excreting acids and by regulating the body buffer stores.
[5] Kidneys responsible for regulation of erythrocyte production from the bone marrow by secreting erythropoietin which stimulate the bone marrow to produce erythrocytes.
[6] Kidneys regulate 1,25- dihydroxy vit D3 production which is essential in regulation of Ca and phosphate.
Juxtamedullary nephron (30%)
Figure 1:Figure 1:
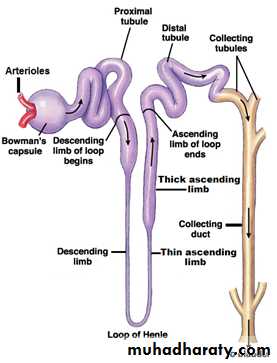
Figure(2): The nephron.
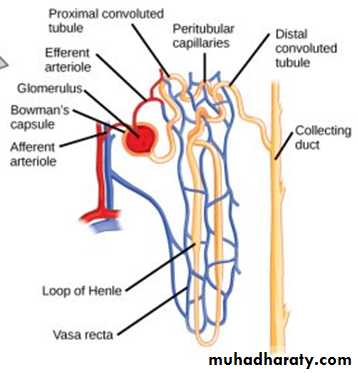
(1)Nephron: It is the basic functional unit of the kidney and capable of forming urine by itself (figure 2). There are about 1 million nephrons in each kidney in human. Kidneys cannot regenerate new nephrons and their number decrease with aging, each nephron consists of:
[1] Bowman's capsule: It is the invaginated blind end of the tubule that encased the glomerulus (which is a branching capillaries). The pressure in the glomerular capillaries is higher than that in other capillary beds.
The membrane of the glomerular capillaries is called the glomerular membrane.
In general, this membrane have three layers; endothelial layer of the capillary itself, a basement membrane (basal lamina), and a layer of epithelial cells (podocytes). Another type of cells is also present between the basal lamina and the endothelium called mesangial cells, which are contractile cells and play a role in the regulation of glomerular filtration. The permeability of the glomerular membrane is from 100-500 times as great as that of the usual capillary. The tremendous permeability of the glomerular membrane is caused by the presence of thousands of small holes which are called fenestrae in the endothelial cells, by the presence of large spaces in the basement membrane, and by incontinuity of the cells that form the epithelial layer which are finger-like projections that forms slits between themselves called slit-pores.
[2] Proximal convoluted tubules: They lie in the renal cortex along with the glomerulus. The epithelial cells of the proximal tubule are highly metabolic cells, with large number of mitochondria to support extremely rapid active transport processes and they are interdigitated with one another and are united by apical tight junction but contain lateral intercellular space. It contains a brush border due to the presence of microvilli.
Reabsorption in the proximal tubule is essentially isotonic; i.e. the osmolality of fluid in all parts of the proximal tubule is approximately to that of plasma. Overall, 65% of the filtered Na and water, almost all the filtered glucose , amino acids , organic acids, and small amount of protein which is present, as well as much of the K, Ca, phosphate and urea are reabsorbed in the proximal tubule.
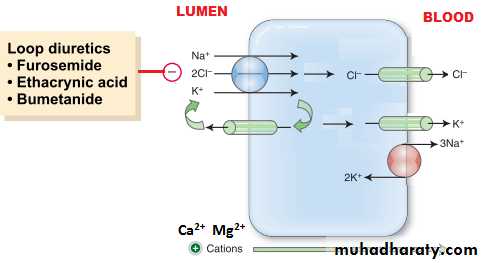
[3] Loops of Henle: The nephrons with their glomeruli located in the outer portion of the renal cortex have short loops of Henle (cortical nephrons, 70%), where as those with glomeruli in the juxtamedullary region of the cortex (juxtamedullary nephrons, 30%) have long loop extending down into medullary pyramids(figure 1). The epithelial cells of the thin descending segment of the loop of Henle are very thin with no brush border and very few mitochondria. They are highly permeable to water but much less permeable to urea, sodium and most other ions. The epithelial cells of the ascending thick segment are similar to those of the proximal tubules except that they have less brush border and much tighter tight junction and the cells adapted for strong active transport of sodium, potassium, and chloride ions. On the other hand, the thick segment cells are adapted for strong active transport of Na+, K+, and 2Cl- ions and is almost entirely impermeable to both water and urea. The active reabsorption of Cl ions creates intraluminal positivity which enhances the movement of Na and other ions passively down the electrical gradient from the lumen of the tubule to the inside of the tubular epithelial cell. This active transport of Cl ions can be inhibited by drugs such as diuretic (frusemide, ethacrynic acid, and bumetanide) which consequently abolish the intraluminal positivity,eventually the passive absorption of Na ions ceases.
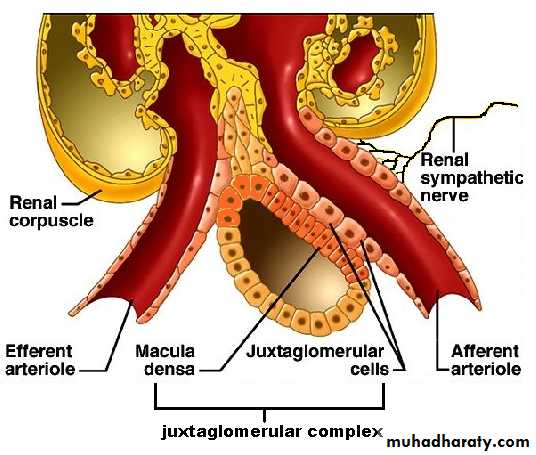
This thick ascending segment ascends all the way back to the same glomerulus from which the tubule originated and passes tightly through the angle between the afferent and efferent arterioles. The cells of this portion of the thick ascending segment which are in complete attachment with the epithelial cells of the afferent and efferent arterioles are called Macula densa. The specialized smooth muscle cells of the afferent arterioles that come in contact with the macula densa are called juxtaglomerular cells (JG cells) which contain renin granules. Macula densa and JG cells plus few granulated cells between them are collectively known as juxtaglomerular complex or apparatus
(Figure 3) which has a dense adrenergic neural innervations.
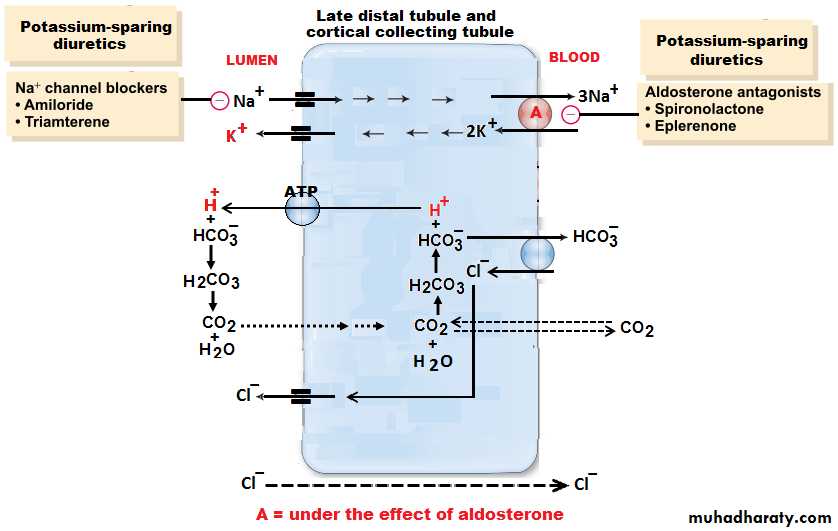
[4] Distal convoluted tubule: They lie in the renal cortex. The first half of the distal tubule (the diluting segment) has almost the same characteristics as the thick segment of ascending limb of the loop of Henle. It absorbs most of the ions but is almost entirely impermeable to both water and urea. The second half of the distal tubule and the cortical collecting tubule are similar to each other and both are entirely impermeable to urea (similar to first half), reabsorb sodium ions in an exchange with K ions under the effect of aldosterone, and permeable to water only in the presence of antidiuretic hormone.
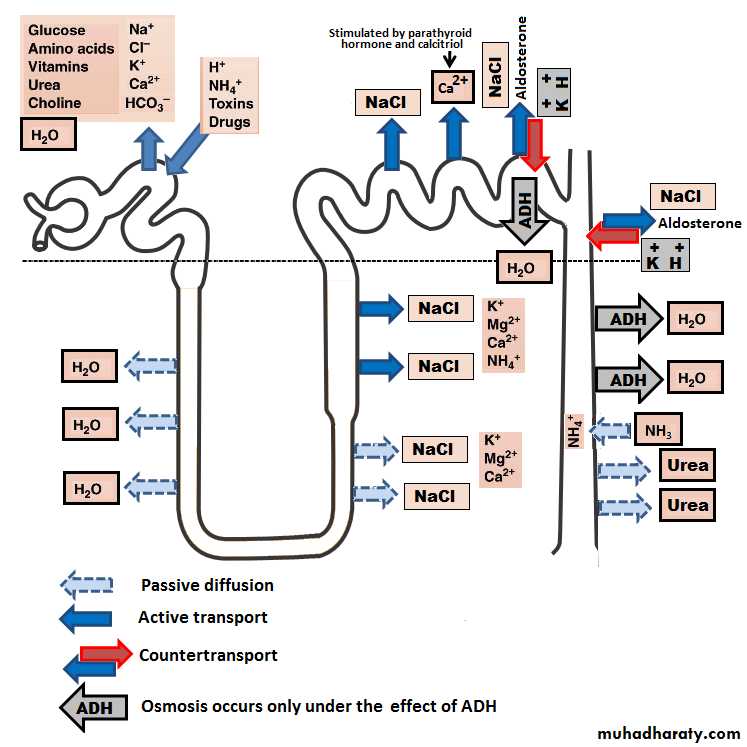
[5] Collecting tubules and ducts: About eight distal tubules coalesce to form the collecting tubule which turns once again away from the cortex and passes downward into medulla where it becomes the collecting ducts. The epithelium of the collecting ducts is made up of principal cells (P cells) which are involved in Na ions reabsorption and vasopressin-stimulated water reabsorption and intercalated cells (I cells) which are concerned with acid-secretion and bicarbonate ions transport(figure 4).
Figure(4): Transport across nephrone.
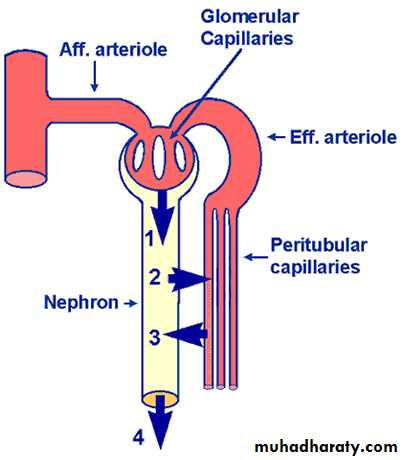
(2)Blood vessels: In resting adult, the renal blood flow(RBF) is about 21% of the total cardiac output (about 1 L/minute).Arterial system of the kidney is technically a portal system, because branches twice in the following arrangement: Interlobar artery → arcuate artery → Interlobular artery → Afferent arteriole → branching capillariesin Bowman's capsule (glomerulus) → Efferent arterioles → branching around the tubules so called (Peritubular capillaries) → arcuate vein → interlobular vein (Figure 5).
In the juxtamedullary glomeruli, long efferent arterioles extend from the glomeruli down into the outer medulla and then divide into specialized long and straight capillary loops called vasa recta extended downward into the medulla to lie side by side with the lower parts of thin segments of juxtamedullary loops of Henle all the way to the renal papillae. Then, like the loop of Henle, they also loop back toward the cortex and empty into the cortical veins. This specialized network of capillaries in the medulla plays an essential role in the formation of concentrated urine.
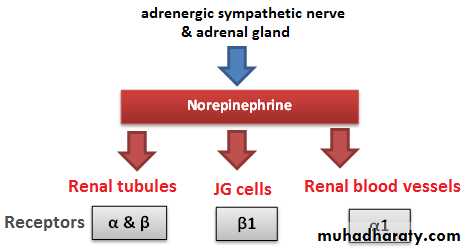
(3)Nerve supply: The kidney has a rich adrenergic sympathetic nerve supply distributed to the:
[A] Vascular smooth muscle to cause vasoconstriction(α1 receptors) .
[B] Juxtaglomerular cells to cause renin secretion(β1 receptors)
[C] Tubular cells to stimulate Na and water reabsorption(α and β receptors).
Figure(5): Renal blood vessels. Figure(6): Renal nerve supply.
Urine formation: Three processes are involved in forming urine: glomerular filtration, tubular reabsorption, and tubular secretion.
Figure(7): processes of rine formation.
Glomerular filtration.
Tubular reabsorption.
Tubular secretion.
Glomerular Function
Glomerular filtration rate (GFR): It is the amount of fluid that filtrate through the glomerulus into Bowman's capsule each minute in all nephrons of both kidneys which is about 125 ml/min or 180 L/day in males (10% lower in female). The high GFR of the glomerular membrane is due to very high permeability of the capillaries of the glomerulus, which is about 100-500 times as great as that of the usual capillary. Yet, despite the tremendous permeability of the glomerular membrane, it has an extremely high degree of selectivity. The selectivity of the glomerular membrane depends on:1. Size of the molecules: Neutral substance with effective molecular diameter of less than 4 nm are freely filtrated, and those with diameter more than 8 nm (80 A), filtration is zero, between these two values filtration is inversely proportional with diameter.
2. The electrical charges of the molecules: The inner side of the pores of the glomerular membrane is negatively charged repelling other negatively charged molecules that tend to pass through pores. For these two reasons(size &charge), the glomerular membrane is almost completely impermeable to all plasma proteins but is highly permeable to all other dissolved substances in normal plasma.
The composition of the glomerular filtrate is the same as plasma except that it has no significant amount of proteins.
Filtration fraction:is the fraction of the renal plasma flow that becomes glomerular filtrate. Since the normal plasma flow through both kidneys is 650 ml/min and the normal GFR is 125 ml/min, the average filtration fraction is about 20%.
Measurement of GFR: GFR can be measured by calculating the clearance of a substance which fulfills the following criteria: [A] Freely filtered. [B] Neither reabsorbed nor secreted by the tubules. [C] Not metabolized or stored in the kidney. [D] Not toxic and not affecting the GFR. A substance known as Inulin fulfill the above criteria. Therefore, it can be used to measure GFR.
Clearance: Is the volume of blood cleared of a substance per unit time.
Inulin clearance = [Urine volume (mL/min) x Concentration of inulin in urine (mg/dL)] / Plasma inulin concentration (mg/dL) = [1.1 x 29] / 0.25 = 128 mL/min.However, measurement of endogenous creatinine clearance is more suitable because it does not need intravenous infusion as in inulin.
Para-aminohippuric acid (PAH) clearance is used as a measure of renal plasma flow (RPF) because it is like inulin but also cleared from the plasma by a single passage through the kidney and the remaining PAH in the plasma after the glomerular filtrate is formed, is secreted into the tubules by the proximal tubule.
Factors that affect GFR:
(I):The filtration pressure is the net pressure forcing fluid through glomerular membrane.
It is determined by:
[A] Glomerular capillary hydrostatic pressure: affected by:
1.Renal blood flow: Increase blood flow through the nephrons greatly increases the GFR.
2. Afferent arteriolar constriction: decrease the glomerular pressure and decrease the GFR, and vice versa.
3. Efferent arteriolar constriction: A slight efferent arteriolar constriction increases the glomerular pressure causing slight increase in GFR. However, moderate and severe efferent arteriolar constriction causes a decrease in the GFR because large portion of plasma will filter out which increase the plasma colloid osmotic pressure and so a decrease in the GFR.
[B] A change in Bowman's capsule hydrostatic pressure: Urinary tract obstruction by stone for example, reduces GFR and vice versa.
[C] A change in glomerular capillary colloid osmotic pressure: A decrease in the glomerular capillary colloid osmotic pressure increases GFR and vice versa.
[D] An increase in the Bowman's colloid osmotic pressure: Damage of glomerular membrane in renal disease causes filtration of protiens and consequently increases GFR.
(II): The capillary filtration coefficient which is the product of the permeability which (affected by renal diseases) and filtering surface area & thickness (affected by contraction or relaxation of mesangial cells) in response to various substances and eventual changes in the GFR.
Autoregulation of GFR and Renal Blood Flow:
It is the feedback intrinsic mechanisms by which the kidneys normally keep the renal blood flow and GFR relatively constant, despite marked changes in arterial blood pressure. GFR (125 ml/min) and renal blood flow (1200 ml/min) normally they have to remain relatively constant for both kidneys even the blood pressure changes from 75-160 mm Hg. This is because that even a 5% too great or too little rate of glomerular filtration can have considerable effects in causing either excess loss of solutes and water into the urine, or at the other extreme too little of necessary excretion of waste products. There are specialized negative feedback mechanisms for autoregulation:
[A] Myogenic mechanism: When the arterial pressure rises, renal blood flow increases so it stretches the wall of the arteriole, and this in turn causes a secondary contraction of the arteriole therefore decreases the renal blood flow and GFR back toward normal. Conversely, when the pressure falls too low, an opposite myogenic response allows the artery to dilate and therefore increases the flow and GFR.
[B] Tubuloglomerular feedback mechanism: A negative feedback control mechanism which appears to be responsible for autoregulation of GFR and Renal Blood Flow (RBF). It occurs through JG complex by utilizing information regarding distal tubular fluid flow rate.
[1] The afferent arteriolar vasodilator feedback mechanism: A low GFR causes overreabsorption of Na and Cl ions therefore decreases the ions concentration at the macula densa which initiates a signal (not completely understood) to dilate the afferent arteriole which result to an increased GFR back toward the required level.
[2] The efferent arteriolar vasoconstrictor feedback mechanism: Too few Na and Cl ions at the macula densa are believed to cause JG cells to release renin and this inturn causes the formation of angiotensin II which constricts mainly the efferent arterioles (much more than the afferent arterioles). Therefore, the constriction of the efferent arterioles causes the pressure in the glomerulus to rise leading to increase in GFR back to normal.
Glomerulotubular Balance: It is the ability of the tubules to increase reabsorption rate in response to increased tubular load ,( poorly understood mechanism), which means that when the GFR increases, the rate of tubular reabsorption increases in exact proportion to avoid large fluctuations in urinary excretion. Glomerulotubular balance is especially good in the proximal tubules and loop of henle and less effective in the more distal segments of the tubular system.
Basic Mechanism of Absorption and Secretion in the Tubules
Unlike glomerular filtration , tubular reabsorption is a highly selective process.
[1] Proximal tubule
Normally about 65% of the filtered load of Na and water and a slightly lower percentage of filtered Cl are reabsorbed here.fig 4.
Na ions are reabsorbed by passive diffusion, facilitated diffusion, or by co-transport with glucose and amino acids, and by counter-transport in an exchange with H ions. All depending primarily on the electrochemical gradient of Na ions created by active Na-K pump.
Cl ions are reabsorbed by passive and co-transport mechanisms.
Water is reabsorbed by osmosis.
Ca, K, Mg, sulfate, phosphate, urate, acetoacetate ions, and vitamins, all these are completely or almost completely actively reabsorbed in the proximal tubule.
Proteins are absorbed through the brush border of the proximal tubule by the process of pinocvtosis.
The concentration of Na and the total osmolarity remains relatively constant (isotonic) water reabsorption proportional to Na reabsorption.
The proximal tubule is also the site for secretion of organic acids and bases (bile salts, oxalate, urate, Catecholamines),drugs, toxins and PAH.
[2] Loop of Henle
The descending thin limb loop of Henle is highly permeable to water and about 20% of the filtered water is reabsorbed therefore, filtrate becomes hypertonic.
About 25% of filtered loads of Na, Cl, and K (and other ions such as Ca, HCO3- , Mg) are reabsorbed in the thick ascending limb loop of Henle.
In the thick ascending loop of Henle, Na ions are reabsorbed by secondary active Na-K-2C1 cotransport and by Na-H secondary active counter-transport mechanisms.
Because the thick segment of the ascending loop of Henle is impermeable to water, most of the water is delivered toward the distal tubule and filtrate becomes hypotonic.
[3] Distal tubule
The early part of the distal tubule (called the diluting segment) has the same reabsorptive characteristics of the thick segment of the ascending limb of the loop of Henle, i.e. it reabsorbs most of the ions.
The second half of the distal and cortical collecting tubule have the following functional characteristics:
Impermeable to urea.
Reabsorb Na ions in an exchange with K ions (Na-K ATPase pump) under the effect of the hormone aldosterone.
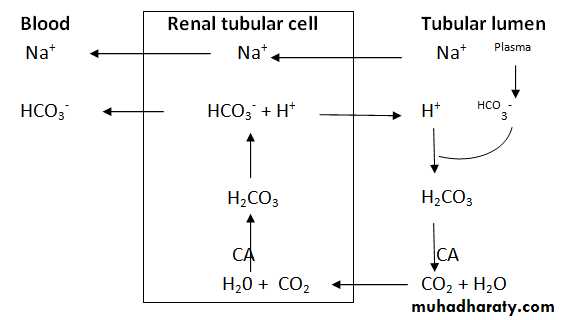
Secretion of H ions (by H-ATPase pump) after being generated inside the cell by the action of carbonic anhydrase on water and CO2 to form carbonic acid which then dissociates into H ions and HCO3- ions. Then the available HCO3- ions are reabsorbed across the basolateral membrane(Figure8).
Figure 8: H ion secretion.
In addition, the permeability of the late distal tubule and cortical collecting duct to water is controlled by ADH (vasopressin). With high level of ADH, these tubular segments are permeable to water, but in the absence of ADH, they are virtually impermeable to water.
[4] Medullary collecting duct: It is the final site for urine processing, and reabsorb less than 10% of the filtered water and Na. It plays an extremely important role in determining the final urine output of water and solutes (urine volume and composition).
• Its permeability to water is under the control of ADH similar to the cortical collecting duct.
• It is permeable to urea (unlike the cortical collecting duct).
• It is capable of secreting H ions against concentration gradient.
Factors Affect the Rate of Reabsorption of Fluid
These factors play a significant role in determining the urine output.[1] Osmotic diuresis: An important example on the osmotic diuresis is the diabetes mellitus in which the proximal tubules fail to reabsorb all the glucose, as normally occurs. Instead, the nonabsorbed glucose passes the entire distance through the tubules and carries with it a large portion of the tubular water. Osmotic diuresis also occurs when substances that are poorly or cannot be reabsorbed by the tubules are filtered in excessive quantities from the plasma into glomerular filtrate. Examples of such substances are sucrose, mannitol, and urea.
[2] Plasma colloid osmotic pressure: A sudden increase in plasma colloid osmotic pressure decreases the GFR and therefore increases tubular reabsorption (slow filtration rate means more time for reabsorption).
[3] Sympathetic stimulation: causes constriction of the afferent arterioles…..decreases glomerular pressure …….decreased GFR …….increases reabsorption .
[4] Arterial pressure: Under normal condition (when the renal autoregulatory mechanism is intact), a change in blood pressure causes a slight change in water and Na excretion. Unlike in renal diseases (when the renal autoregulatory mechanism is impaired), small increase in arterial pressure often causes marked increase in urinary excretion of Na and water. This results from two separate effects: (A) the increase in arterial pressure increases glomerular pressure, which in turn increases GFR, thus leading to increased urine output. (B) The increase in arterial pressure also increases the peritubular capillary pressure, thereby decreasing tubular reabsorption.
[5] Hormonal control:
ADH: When excess antidiuretic hormone is secreted by the posterior pituitary gland, the effect is to increase the water permeability of the distal tubule, collecting tubule and collecting duct with a consequent decrease of urinary output.Aldosterone: Act on the principal cells of the cortical collecting tubule to increase Na reabsorption and to increase K secretion.
Angiotensin II:
Stimulates aldosterone secretion, which in turn increases Na and water reabsorption.It constricts the efferent arterioles and consequently increases Na and water reabsorptive forces(↓hydrostatic and↑osmotic pressure in the peritubular capillary).
It act directly especially on the proximal tubule to increase Na and water reabsorption (by stimulating Na-K ATPase pump at the basolateral membrane of the tubular cell) and Na-H exchange (at the luminal side of the tubular cell).
Atrial natriuretic peptide: It is released from specific cells of the cardiac atria upon distension as a result of plasma volume expansion. It inhibits the reabsorption of Na and water by the renal tubules especially in the collecting ducts with consequent increase in the urinary output.
Parathyroid hormone: It increases the reabsorption of Ca and Mg ions from the ascending limb of loop of Henle and distal tubule. It inhibits the reabsorption of phosphate from the proximal tubule.
Tubular load of a Substance: Is the total amount of the substance that filters through the glomerular membrane into tubules per minute.
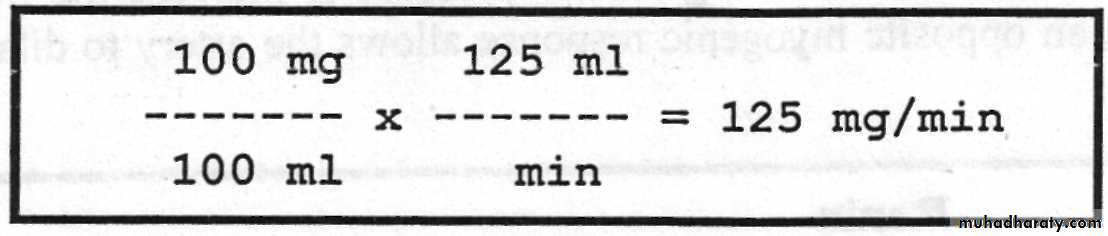
Tubular load = conc. of the substance in the filtrate x GFR. It is expressed in mg/min. For example, if the plasma concentration of glucose is 100 mg/100 ml plasma, so the tubular load of glucose is equal to :
Tubular Transport Maximum (Tm): It is the maximum rate for actively reabsorbing or secreting substance by the tubule. For example, the Tm for glucose average 320 mg/min for adult human being, and if the tubular load becomes greater than 320 mg/min, the excess above this amount is not reabsorbed but instead passes on into the urine. The serum level of the substance below which none of it appears in the urine and above which progressively larger quantities appear is called the threshold concentration of that substance. For example, glucose begins to spill into the urine when its tubular load exceeds 320 mg/min. The threshold concentration of glucose in plasma that gives this tubular load is 180 mg/100 ml when kidneys are operating at their normal glomerular filtration rate of 125 ml/min.
Renal Mechanisms for Controlling Urine Concentration
The kidneys can excrete urine with an osmolarity as low as 50mOsmol/l , a concentration that is only about sixth the osmolarity of normal extracellular fluid) 300mOsmol/l(. Conversely, when there is a deficit of water and extracellular fluid osmolarity is high, the kidney can excrete urine with a concentration of about 1200 to 1400 mOsmol/1. Equally important, the kidney can excrete a large volume of dilute urine or a small, volume of concentrated urine without major changes in rates of excretion of solutes such as Na and K. This ability to regulate water excretion independently of solute excretion is necessary for survival, especially when fluid intake is limited. When the osmolality of the body fluids fall too low, i.e. when the fluids become too dilute, the kidney automatically excrete a great excess of water in urine causing the urine to be diluted and therefore increasing the body fluid osmolality back toward normal. Conversely, when the osmolality of body fluids is too great, the kidney automatically excrete an excess of solutes in urine causing the urine to be concentrated thereby reducing the body fluid osmolality again back toward normal.[1]Renal mechanism for excreting diluted urine: As fluid flows through the proximal tubule, solutes and water are reabsorbed in equal proportions, so that little change in osmolarity occurs, that is the proximal tubule fluid remains isotonic to the plasma, with an osmolarity of about 300 mOsmol/1. As fluid passes down the descending loop of Henle, water is reabsorbed by osmosis and the tubular fluid reaches equilibrium with the surrounding interstitial fluid of the renal medulla, which is very hypertonic, of about 1200 mOsmol/1, i.e. four times the osmolarity of the original glomerular filtrate. Therefore, the tubular fluid becomes more concentrated as it flows into the inner medulla. In the ascending limb of the loop of Henle, especially the thick segment, Na, K, and Cl are avidly reabsorbed. However, this portion of the tubular segment is impermeable to water. Therefore, the tubular fluid becomes more dilute as it flows up the ascending loop of Henle into the early distal tubule, with the osmolarity decreasing progressively to about 100 mOsmol/1 by the time the fluid enters the early distal tubular segment. Thus, the fluid leaving the early distal tubular segment is hypotonic with an osmolarity of only about one third the osmolarity of plasma. As the dilute fluid in the early distal tubule passes into the late distal tubule, cortical collecting tubule, and collecting duct, there is additional reabsorption of NaCl. This portion of the tubule is also impermeable to water (in the absence of ADH), and additional reabsorption of solutes in the presense of aldosterone causes the tubular fluid to become even more diluted with an osmolarity of about 50 mOsmol/1.
[2]Renal mechanisms for excreting concentrated urine: The basic requirements for forming a concentrated urine are:
[A] A high level of ADH: Which increases the permeability of the distal tubules and collecting ducts to water, thereby allowing these tubular segments to avidly reabsorb water. The signal that tells the kidney whether to excrete a diluted or a concentrated urine is the hormone called antidiuretic hormone (ADH) or vasopressin that is secreted from the posterior pituitary gland. When the body fluids are too concentrated, the posterior pituitary gland secretes large amount of ADH, which causes the kidney to excrete excessive amounts of solutes but to conserve water in the body. Conversely, in the absence of ADH the kidney excretes dilute urine, thus removing excessive amount of water from the body fluids and causing them to become concentrated once again.
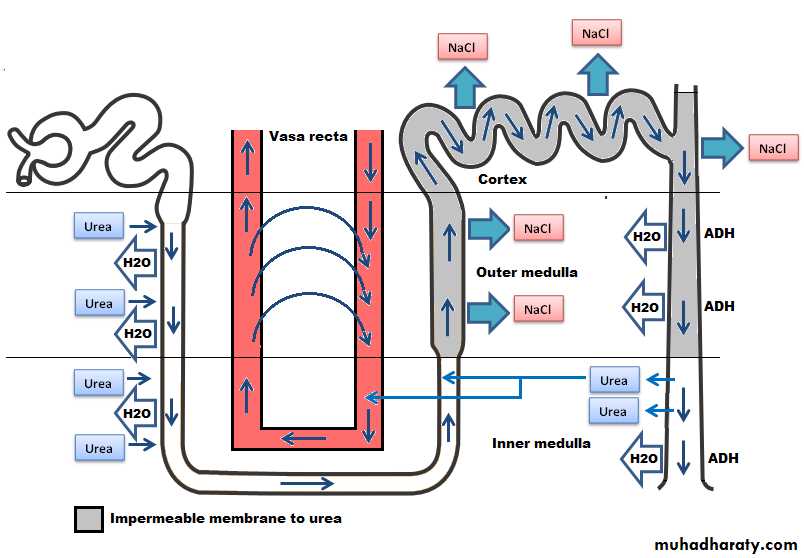
Figure(9): Counter current system.
[B] A high osmotic gradient along the renal medullary interstitial fluid(the countercurrent system):Which means 300 mOsmol/1 at the cortex, about 800 mOsmol/1 at the outer medulla, and as high as 1200-1400 mOsmol/l. at the inner medulla. This gradient is produced by the countercurrent system [the inflow runs parallel to, counter to (opposite to), and in close proximity to the outflow for some distane]. This occurs for both the loop of Henle(countercurrent multiplier) and the vasa recta of the renal medulla (countercurrent exchanger).
The increasing osmotic gradient along the medullary interstial fluid is PRODUCED by the operation of each loop of Henle as a countercurrent multiplier:
Active transport of Na and CI from thick ascending limb lumen to the interstium creating a high osmotic gradient between the interstial fluid and the fluid in the thin descending loop of Henle which is permeable to water.
Movement of water by osmosis from the thin descending loop of Henle to the medullary interstial fluid.
However, such medullary osmotic gradient is MAINTAINED by the action of vasa recta as a countercurrent exchanger which prevent the solutes from being washed out to the circulation by two factors:
Solutes diffuse passively into the descending vessel which carry blood toward medulla and leaves the ascending vessel which carry the blood toward the cortex. Water moves passively in the opposite direction. Thus the solute will recircle in the medulla without loss while the water bypass it.
The medullary blood flow through vasa recta is very slight in quantity (2%) of the total renal blood flow.
Role of urea: Urea plays an important role in producing and maintaining the high medullary osmotic gradient necessary for the urine concentration process. Urea moved passively out of the proximmal tubule, increasingly concentrated in the tubular fluid as water is removed from the loop and distal tubule. However, at the medullary collecting duct, urea moves into the interstitial fluid thus adding to the hyperosmolarity of the interstitium (Figure 9).The fundamental role of urea in contributing to urine concentrating ability is evidenced by the fact that people who fed a high protein diet, yielding large amounts of urea as a nitrogenous waste product, can concentrate their urine much better than people whose protein intake and urea production are low.
Urea excretion: The rate of urea excretion is determined by:
The concentration of urea in the plasma.
The GFR.
In general, between 40-60% of the filtered urea is excreted with urine. In many renal diseases the GFR falls below normal however, the body continues to form large quantities of urea which results in elevated blood urea. Many other waste products that must be excreted by the kidneys obey the same principles for excretion such as creatinine and uric acid.
The kidneys can excrete urea with minimum quantities of water and this can be achieved by two mechanisms:
By the presence of ADH.
Recirculation of urea from the collecting duct into the thin limb of the loop of Henle so that it passes upward through distal tubule, collecting tubule and then collecting duct again. In this way, urea can recirculate through these terminal portions of the tubular system several times in a way to concentrate urea in the medullary interstitium before it is excreted (urea cycle).
Na Excretion:
[A] About 65% of Na is actively reabsorbed with Cl and water at the proximal tubule while about 25% is actively reabsorbed at the thick portion of ascending limb of Henle but not associated with water because this segment is not permeable to water. The rest 10% is reabsorbed at the distal tubule, cortical collecting tubule, and the collecting ducts is controlled by aldosterone.
[B] In the presence of large amounts of aldosterone, almost all tubular Na are reabsorbed in exchange for from these portions of the tubular system by active transport process coupled at least partially with active transport of K into the cells in opposite direction (i.e. K being exchanged for Na and therefore, none of Na enters the urine).
Potassium Excretion: To maintain normal body K balance, only one eighth of the total daily tubular load of K can be excreted which must be carefully controlled.
About 65% of K are actively reabsorbed at the proximal tubule and 25% at thick portion of the ascending limb of loop of Henle, leaving only about 8% of the original filtered K to enter the distal tubule and cortical collecting tubules to be actively reabsorbed, consequently, all the filtered K is actually reabsorbed back to the blood, which would eventually be lethal because of the toxic effects of K accumulation in the body. Therefore, K secretion in the distal segments of the tubular system (under the efect of aldosterone in an exchange to Na ions) is the principal means by which the tubular system controls the rate of K loss in the urine.
There are three factors that determine the rate of tubular secretion of K:
Aldosterone: In the presence of aldosterone, Na ions reabsorbed from the tubular fluid in an exchange with K ions.Plasma K concentration: As a result of high plasma K concentration, the rate of K transport from the interstitial fluid to the tubular lumen increased markedly according to electrochemical gradient.
Distal tubular Na load: As a result of high Na ions entered the distal tubules, this increases the rate of Na reabsorption in an exchange to K ions.
H+ Secretion: All epithelial cells of the renal tubule, apart from the thin segment of the loop of Henle, secrete H+ into the tubular fluid in an exchange with Na+ ions and HCO3- into the extracellular fluid by secondary active transport.
The H+ comes from intracellular dissociation of H2CO3 , and the HCO3- that is formed diffuses into the interstitial fluid. Thus, for each H+ ion secreted, one Na+ and one HCO3- ion enter the interstitial fluid. The sodium ion and HCO3- then are transported together from the epithelial cell into the extracellular fluid. The mechanism by which this occurs is illustrated in figure (8). Since the chemical reactions for secretion of H+ begin with CO2, the greater the CO2 concentration, the more rapidly the reaction proceed, and the greater becomes the rate of H + secretion. Therefore, any factors that increase the CO2 concentration in the extracellular fluids, such as decreased respiration or increased metabolic rate, also, increase the rate of H+ secretion and vice versa.
Regulation of Extracellular Fluid (ECF) Volume: The basic mechanism for fluid volume control is the same as the basic mechanism for arterial pressure control.
(1) Volume receptor reflexes: The volume receptors are stretch receptors located in the walls of the right and left atria. When the blood volume becomes excessive, it causes increased pressure in the two atria. Stretching of the atrial walls causes the following responses:
(A). Neural reflexes: The resultant stretch of the atrial walls transmits nerve signals into the brain, and these in turn elicit two important renal responses that accelerate the return of blood volume to normal within hours.
1. The sympathetic nervous signals to the kidneys are inhibited, which causes a moderate increase in the rate of urinary output.
2. Inhibiton of ADH secretion by the posterior pituitary gland allows the kidneys to excrete increased quantities of water.
(B). Atrial Natriuretic Factor (ANF) release: Excess blood volume stretches the atrial walls and this promotes the release of ANF that in turn causes renal Na excretion and concomitant water loss and reduction of the blood volume.
(2). Cardiac output mechanism: The final determination of the precise level to which the blood volume will be adjusted is still the function of the cardiac output level. This is because the volume receptors adapt completely within one to three days and become no longer active.
A slight increase in blood volume increases cardiac output markedly…….increases arterial pressure markedly…….stimulate baroreceptors…….activate essentially the same central nervous system reflexes as the atrial volume receptors which lead to increase urinary output....…decreases the ECF volume..…decreases the cardiac output back to normal.
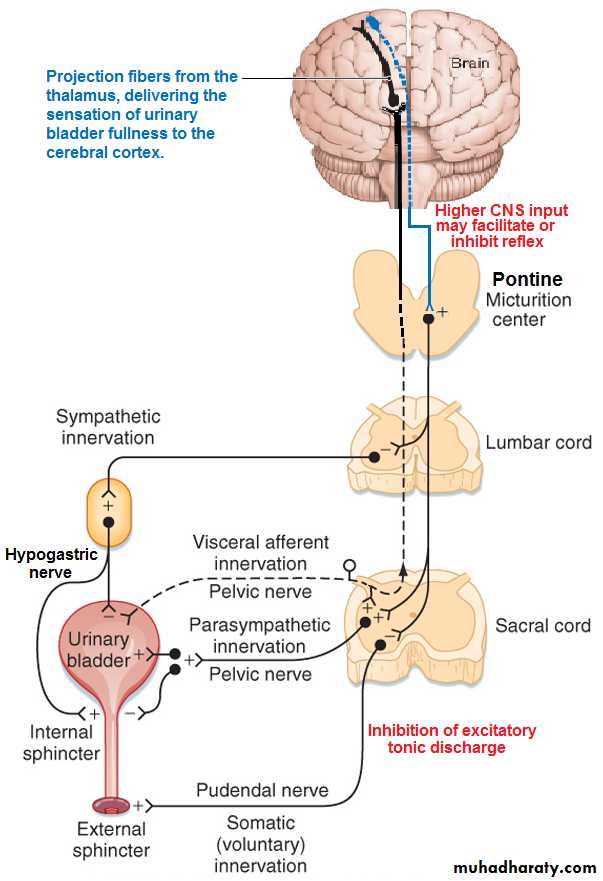
MICTURITION: Is the process by which the urinay bladder empties when it becomes filled. The principal nerves supply to the bladder are:
(1). The pelvic nerves (S-2 and S-3) are sensory and motor. Sensory nerve fibers detect the degree of stretch of the bladder wall then motor parasympathetic nerve fibers innervate the detrusor muscle to evacuate the bladder.
(2). Motor somatic fibers are also transmitted to the bladder through pudendal nerve to innervate the skeletal muscle fibers of external bladder sphincter.
Figure(10): Micturition reflex.
(3).The hypogastric nerve fibers (L2) carry the sympathetic fiber mainly to the blood vessel and have very little to do with micturition, but they do mediate the contraction of the bladder muscle that prevents semen from entering the bladder during ejaculation. Some sensory fibers (for pain and fullness sensation) also pass by way of the sympathetic nerves.
The Micturition Reflex: As the bladder fills.....stimulating stretch receptors in the bladder wall . ….. at a bladder volume of 150 ml ……first urge to void is felt …..at volume 300-400 ml….. marked sense of fullness……..sensory signals (pelvic nerves) are conducted to the sacral segments of the spinal cord then back again to the bladder through the parasympathetic fibers in these same nerves initiating micturition contraction of the detrusor muscles.
Once a micturition reflex begins, it is self-regenerative and the high pressure inside the bladder forces the bladder neck to open against its tonic contraction. Stretching the bladder neck exacerbates the intensity of the micturition reflex and also activates another reflex. This reflex passes to the sacral portion of the spinal cord and then back through the pudendal nerve to the external sphincter to inhibit it. If this inhibition is more potent than the voluntary constrictor signals from the brain, then urination will occur. Then after a few seconds to more than a minute, the reflex begins to fatigue allowing rapid reduction in bladder contraction.
The micturition reflex can be controlled by higher brain centers in the following ways:
[1] The higher centers keep the micturition reflex partially inhibited all the time except when it is desired to micturate.
[2] When the time to urinate arrives, the cortical centers can (a) facilitate the sacral micturition centers to help initiate a micturition reflex, (b) Inhibit the external urinary sphincter so that urination can occur (Figure 10).
Figure(10): Micturition reflex. (10):
Abnormalities of Micturition:
[1] The atonic bladder: It is due to destruction of the sensory fibers from the bladder to the spinal cord which prevents transmission of stretch signals from the bladder and therefore, prevents micturition reflex contraction. Therefore, instead of emptying periodically, the bladder fills to capacity and overflows a few drops at a time through urethra (overflow dribbling or incontinence).[2] The autotmtic bladder: If the spinal cord is damaged above the sacral region but the sacral cord segments are still intact, typical micturition reflexes can still occur. However, they are no longer controlled by the brain.
[3] The neurogenic bladder: This condition derives from partial damage in the spinal cord or the brain stem that interrupts most of the inhibitory signals. It results in frequent and relatively uncontrolled micturation. Therefore, facilitatory impulses passing continually down the cord keep the sacral centers so excitable that even a small quantity of urine will elicit an uncontrolled micturition reflex, and thereby promote frequent urination.
Diuretics: A substance that increases the rate of urine output by decreasing the rate of Na reabsorption from the tubules, which in turn causes natriuresis (increased Na output) and this in turn causes diuresis (increased water output or also called polyuria). Because the renal tubular reabsorption of many solutes, such as K, Cl, Mg, and Ca, is also influenced secondarily by Na reabsorption, many diuretics raises renal output of these solutes as well.
Osmotic diuretic: Inhibit water reabsorption mainly in the proximal tubules (glucose,sucrose,manitol).
Loop diuretics(frusemide): inhibit Na-K-Cl co-transport at thick ascending loop of Henle.
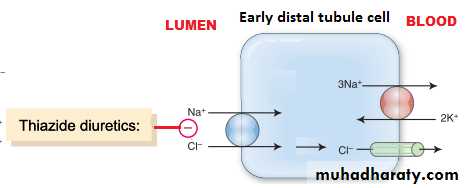
Thiazide diuretics: inhibit Na-Cl co-transport in luminal membrane at early distal tubule.
Carbonic anhydrase inhibitor(acetazolamide)inhibit H secretion which reduce Na reabsorption at proximal tubule.
Aldosterone antagonist(spironolactone) inhibit action of aldosterone on tubular receptors ,decrease Na reabsorption and decrease K secretion at collecting duct. They are called K sparing diuretics.
Sodium channel blockers(triamterene,ameloride) in the luminal membrane of collecting tubules.