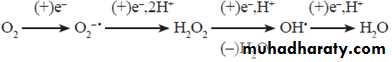Free Radicals and Antioxidants
The outer orbital in an atom or molecule contains two electrons, each spinning in opposite directions. The chemical covalent bond consists of a pair of electrons, each component of the bond donating one electron each.
Definition
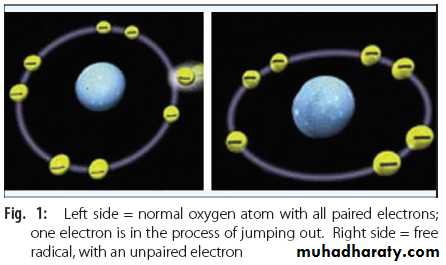
A free radical is a molecule or molecular fragment that contains one or more unpaired electrons in its outer orbital (Fig.1). Free radical is generally represented by a superscript dot, (R· ).
Basically, oxidation means lose of electron, whereas, reduction is gain of electron.
Oxidation reactions ensure that molecular oxygen is completely reduced to water. The products of partial reduction of oxygen are highly reactive and create destruction in the living systems. They are also called reactive oxygen species or ROS. The following are members of this group:
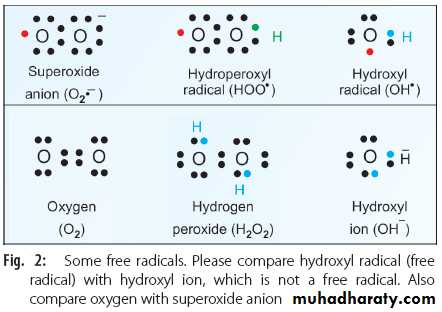
i. Superoxide anion radical (O2–•) (Fig. 2)
ii. Hydroperoxyl radical (HOO• ) (Fig. 2)
iii. Hydrogen peroxide (H2O2) (Fig. 2)
iv. Hydroxyl radical (OH•) (Fig. 2)
v. Lipid peroxide radical (ROO•)
vi. Singlet oxygen ( 1O2)
vii. Nitric oxide (NO• )
viii. Peroxy nitrite (ONOO–•).
Out of this, hydrogen peroxide and singlet oxygen are not free radicals (they do not have superscript dot). However, because of their extreme reactivity, they are included in the group of reactive oxygen species. The sequential univalent reduction steps of oxygen may be represented as:
Important characteristics of the ROS are:
a. Extreme reactivityb. Short lifespan
c. Generation of new ROS by chain reaction
d. Damage to various tissues.
Generation of Free Radicals
i. They are constantly produced during the normal oxidation of foodstuffs, due to leaks in the electron transport chain in mitochondria. About 1–4% of oxygen taken up in the body is converted to free radicals. Mitochondria are major sites for production of superoxide ions from the interaction between co-enzyme Q (ubiquinone) (CoQ) and oxygen in the electron transport chain. Hence a high content of superoxide dismutase (SOD) is needed. Mitochondria also have a high content of glutathione and glutathione peroxidase for preventing lipid peroxidation.
ii. Some enzymes such as xanthine oxidase and aldehyde oxidase form superoxide anion radical or hydrogen peroxide.
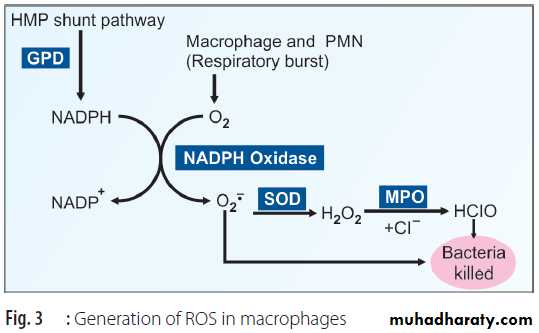
iii. NADPH oxidase in the inflammatory cells (neutrophils, eosinophils, monocytes and macrophages) produces superoxide anion by a process of respiratory burst during phagocytosis (Fig. 3). The superoxide is converted to hydrogen peroxide and then to hypochlorous acid (HClO) with the help of superoxide dismutase (SOD) and myeloperoxidase (MPO). The superoxide and hypochlorous ions are the final effectors of bactericidal action. This is a deliberate production of free radicals by the body. Along with the activation of macrophages, the consumption of oxygen by the cell is increased drastically; this is called respiratory burst.
In chronic granulomatous disease (CGD), the NADPH oxidase is absent in macrophages and neutrophils. In this condition, macrophages ingest bacteria normally, but cannot destroy them. However, streptococci and pneumococci themselves produce H2O2. Therefore, they are destroyed by the myeloperoxidase system of the macrophages. But staphylococci being catalase positive can detoxify H2O2 in the macrophages and therefore, are not destroyed in such persons. Hence, recurrent pyogenic infection by staphylococci are common in chronic granulomatous disease.
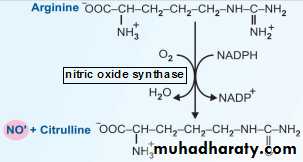
iv. Macrophages also produce NO˙ from arginine by the enzyme nitric oxide synthase. This is also an important antibacterial mechanism.
v. Peroxidation is also catalyzed by lipo-oxygenase in platelets and leukocytes.
vi. Ionizing radiation damages tissues by producing hydroxyl radicals, hydrogen peroxide and superoxide anion from H2O.
H2O -------(gamma, UV radiation)----→ H• + OH•
vii. Light of appropriate wavelengths can cause photolysis of oxygen to produce singlet oxygen.
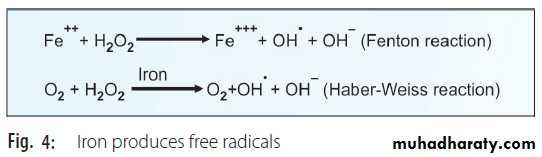
viii. The capacity to produce tissue damage by H2O2 is minimal when compared to other free radicals (by definition, H2O2 is not a free radical). But in presence of free iron, H2O2 can generate OH• (hydroxy radical) which is highly reactive (Fig.4).
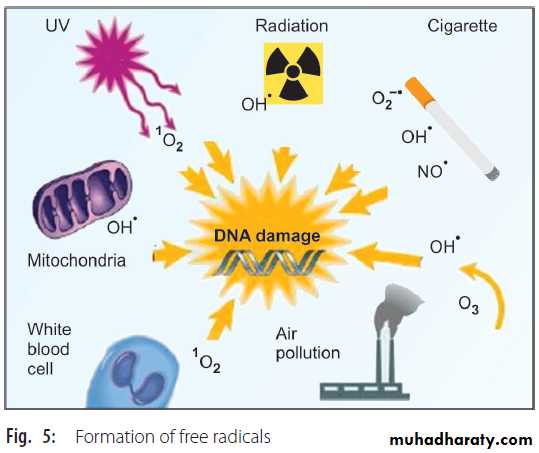
ix. Cigarette smoke contains high concentrations of various free radicals.
x. Inhalation of air pollutants will increase the production of free radicals. These facts are summarized in Figure 5.
Free Radical Scavenger Systems
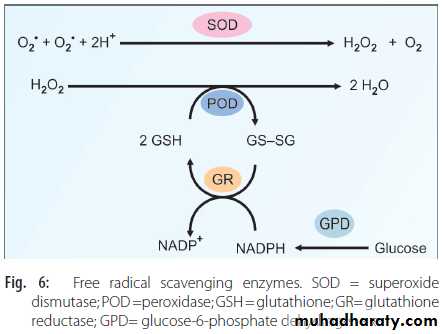
Superoxide Dismutase (SOD)
The reaction is depicted in Figure 6. The mitochondrial SOD is manganese dependent; whereas, cytoplasmic SOD is copper-zinc dependent. A defect in SOD gene is seen in patients with amyotrophic lateral sclerosis
Glutathione Peroxidase
In the next step, the H2O2 is removed by glutathione peroxidase (POD) (Fig. 6). It is a selenium dependent enzyme.
Glutathione Reductase
The oxidized glutathione, in turn, is reduced by the glutathione reductase (GR), in presence of NADPH (Fig. 6). This NADPH is generated with the help of glucose-6-phosphate dehydrogenase (GPD) in PPP pathway. Therefore, in GPD deficiency, the RBCs are liable to lysis, especially when oxidizing agents are administered (drug induced hemolytic anemia).
Catalase
When H2O2 is generated in large quantities, the enzyme catalase is also used for its removal.
Polyphenols
Consumption of polyphenol-rich fruits, vegetables, and beverages is beneficial to human health. Dietary polyphenols represent a wide variety of compounds that occur in fruits, vegetables, wine, tea and chocolate. They contain flavones, isoflavones, flavonols, catechins and phenolic acids. They act as agents having antioxidant, antiapoptotic, antiaging, anticarcinogenic, antiinflammatory and anti-atherosclerotic effects. They are protective against cardiovascular diseases. Researchers suggest that grape polyphenols can prevent brain damage due to alcohol. Oral administration of grape polyphenol extract improve cerebral ischemia induced neuronal damage.Damage Produced by Reactive Oxygen Species
Free radicals are extremely reactive. Their mean effective radius of action is only 30 Angstrom. Their half-life is only a few milliseconds. When a free radical reacts with a normal compound, other free radicals are generated. This chain reaction leads to thousands of events.
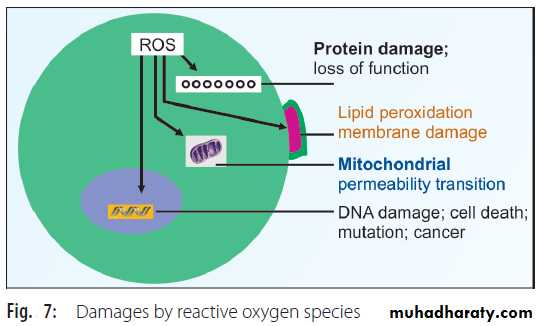
Peroxidation of poly unsaturated fatty acids (PUFA) in plasma membrane leads to loss of membrane functions. Lipid peroxidation and consequent degradation products such as malondialdehyde (CHO-CH2-CHO) are seen in biological fluids. Almost all biological macromolecules are damaged by the free radicals (Fig.7). Thus, oxidation of sulfhydryl group containing enzymes, loss of function and fragmentation of proteins are noticed. Polysaccharides undergo degradation. DNA is damaged by strand breaks. The DNA damage may directly cause inhibition of protein and enzyme synthesis and indirectly cause cell death or mutation and carcinogenesis (Fig.7).
CLINICAL SIGNIFICANCE
Chronic Inflammation
Chronic inflammatory diseases such as rheumatoid arthritis are self-perpetuated by the free radicals released by neutrophils. ROS induced tissue damage appears to be involved in pathogenesis of chronic ulcerative colitis, chronic glomerulonephritis, etc.
Acute Inflammation
At the inflammatory site, activated macrophages produce free radicals. Respiratory burst and increased activity of NADPH oxidase are seen in macrophages and neutrophils.
Respiratory Diseases
Breathing of 100% oxygen for more than 24 hours produces destruction of endothelium and lung edema. This is due to the release of free radicals by activated neutrophils. In premature newborn infants, prolonged exposure to high oxygen concentration is responsible for bronchopulmonary dysplasia. Adult respiratory distress syndrome (ARDS) is characterized by pulmonary edema. It is produced when neutrophils are recruited to lungs which subsequently release free radicals. Cigarette smoke contains free radicals. Soot attracts neutrophils to the site which releases more free radicals, leading to lung damage.
Diseases of the Eye
Retrolental fibroplasia (retinopathy of prematurity) is a condition seen in premature infants treated with pure oxygen for a long time. It is caused by free radicals, causing thromboxane release, sustained vascular contracture and cellular injury.
Cataract formation is related with aging process. Cataract is partly due to photochemical generation of free radicals. Tissues of the eye, including the lens, have high concentration of free radical scavenging enzymes.
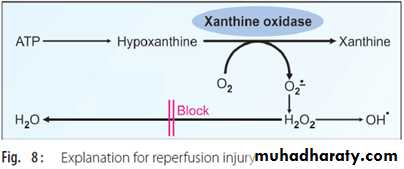
Reperfusion Injury
Reperfusion injury after myocardial ischemia is caused by free radicals. During ischemia, the activity of xanthine oxidase is increased. When reperfused, this causes conversion of hypoxanthine to xanthine and superoxide anion. At the same time, the availability of scavenging enzymes is decreased, leading to aggravation of myocardial injury (Fig. 8). Allopurinol, a xanthine oxidase inhibitor, reduces the severity of reperfusion injury.
Atherosclerosis and Myocardial Infarction
Low density lipoproteins (LDL) are deposited under the endothelial cells, which undergo oxidation by free radicals. This attracts macrophages. Macrophages are then converted into foam cells initiating atherosclerotic plaque formation. Antioxidants offer some protective effect.
Shock Related Injury
Release of free radicals from phagocytes damage membranes by lipid peroxidation. They release leukotrienes from platelets and proteases from macrophages. All these factors cause increased vascular permeability, resulting in tissue edema. Antioxidants have a protective effect.
Skin Diseases
Certain plant products, called psoralens are administered in the treatment of psoriasis and leukoderma. When the drug is applied over the affected skin and then irradiated by UV light, singlet oxygen is produced with clinical benefit.
Carcinogenesis and Treatment
Free radicals produce DNA damage, and accumulated damages lead to somatic mutations and malignancy. Cancer is treated by radiotherapy. Irradiation produces reactive oxygen species in the cells which trigger the cell death.
Aging Process
Reactive oxygen metabolites (ROM) play a key role in degenerative brain disorders such as Parkinsonism, Alzheimer’s dementia and multiple sclerosis. Cumulative effects of free radical injury cause gradual deterioration in aging process.
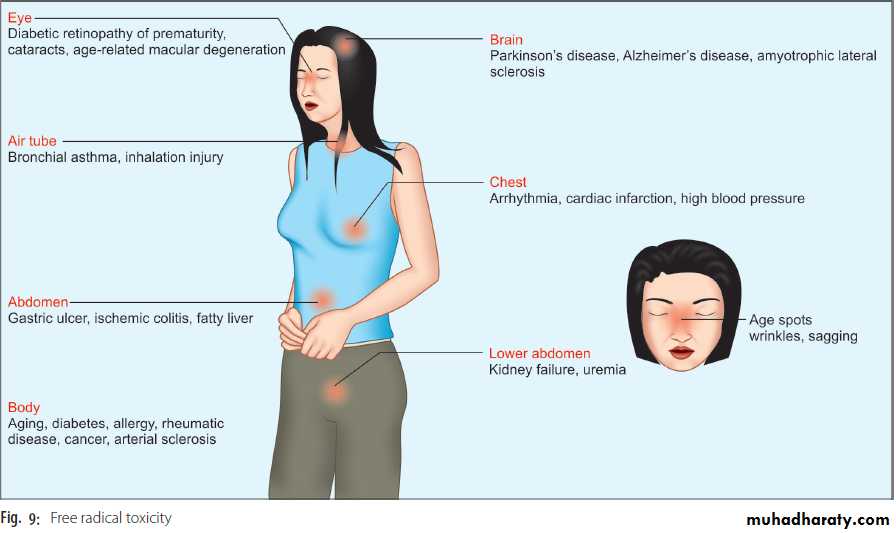
A summary of free radical toxicity is shown in Figure 9.
Lipid Peroxidation
Initiation Phase
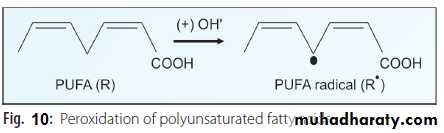
Polyunsaturated fatty acids (PUFA) present in cell membranes are easily destroyed by peroxidation. During the initiation phase, the primary event is the production of R• (carbon centered radical) (PUFA radical) or ROO• (lipid peroxide radical) by the interaction of a PUFA molecule with free radicals generated by other means (Fig.10).
RH + OH• ------→ R• + H2O (Reaction 1-A)
ROOH -------→ ROO• + H+ (Reaction 1-B)
The R• and ROO•, in turn, are degraded to malondialdehyde (3 carbon). It is estimated as an indicator of fatty acid breakdown by free radicals.
Propagation Phase
The carbon centered radical (R• ) rapidly reacts with molecular oxygen forming a peroxyl radical (ROO•) which can attack another polyunsaturated lipid molecule.
R• + O2 → ROO• (Reaction No. 2)
ROO• + RH → ROOH + R• (Reaction No. 3)
The net result of reactions 2 and 3 is the conversion of R• to ROOH (a hydroperoxide). But there is simultaneous conversion of a carbon centered radical to a peroxyl radical, ROO•. This would lead to continuous production of hydroperoxide with consumption of equimolecular quantities of PUFA. One free radical generates another free radical in the neighboring molecule; a “chain reaction” or “propagation” is initiated. Accumulation of such lipid damages lead to the destruction of fine architecture, and integrity of the membranes.
Termination Phase
The reaction would proceed unchecked till a peroxyl radical reacts with another peroxyl radical to form inactive products.
ROO• + ROO• → RO--OR + O2 (Reaction 4-A)
R• + R• → R--R (Reaction 4-B)
ROO• + R• → RO--OR (Reaction 4-C)
Role of Antioxidants
Apart from the scavenging enzymes described earlier, there are two types of antioxidants:a. Preventive antioxidants: They will inhibit the initial production of free radicals. They are catalase,
glutathione peroxidase and ethylenediaminetetraacetate (EDTA).
b. Chain breaking antioxidants: They can inhibit propagative phase. They include superoxide dismutase, uric acid and vitamin E. Alpha tocopherol (T-OH) (vitamin E) would intercept the peroxyl free radical and inactivate it before a PUFA can be attacked.
T-OH + ROO• → TO• + ROOH (Reaction 5)
The phenolic hydrogen of the alpha tocopherol reacts with the peroxyl radical, converting it to a hydroperoxide product. The tocoperoxyl radical thus formed is stable and will not propagate the cycle any further.
The tocoperoxyl radical can react with another peroxyl radical getting converted to inactive products.
TO• + ROO• → inactive products (Reaction 6)
Vitamin E (Alpha tocopherol) acts as the most effective naturally occurring chain breaking antioxidant in
tissues. Only traces of tocopherol are required to protect considerable amounts of polyunsaturated fat (one tocopherol molecule per 1000 lipid molecules). But it is seen from reaction 5 and 6 that while acting as antioxidant, alpha tocopherol is consumed. Hence, it has to be replenished by daily dietary supply.
Antioxidants
1. Vitamin E is the lipid phase antioxidant.
2. Vitamin C is the aqueous phase antioxidant.
3. Ceruloplasmin can act as an antioxidant in extracellular fluid .
4. Caffeine is another effective antioxidant.
5. Cysteine, glutathione, carotenoids, flavonoids and vitamin A are minor antioxidants. Beta carotene can act as a chain-breaking antioxidant, but is less effective than alpha tocopherol. The incidence of heart attack is only 50% in vegetarians compared to non-vegetarians.
6. Food items containing good quantity of antioxidants are:
a) Spices used in ordinary Indian cooking contain highest quantity of antioxidants. b) Curcumin.
c) Fruits and vegetables such as berries, broccoli, spinach and green tea, which contain flavonoids, flavones, isoflavones and anthocyanins. d) Resveratrol present in grapes.
Antioxidants used as Therapeutic Agents
1. Vitamin E
2. Vitamin C
3. Dimethyl thiourea
4. Dimethyl sulfoxide
5. Allopurinol
Commercial Use of Antioxidants
Antioxidants are regularly used in food industry to increase the shelf-life of products. Commercially used food preservatives are Vitamin E, propyl gallate, butylated hydroxy anisole (BHA) and butylated hydroxy toluene (BHT). They prevent oxidative damage of oils, particularly those containing PUFA and prevent rancidity.
Protection Against Ozone
When ozone content in air is high, protection is by uric acid present in the lining of the nasal cavity. Glutathione and ascorbic acid in the proximal and distal respiratory tract additionally reacts with ozone.
Ozone which escapes this antioxidant screen can react with proteins, carbohydrates and lipids to generate lipid peroxides, to generate a chain reaction. A second line of defense is by alpha tocopherol and glutathione. Most individuals are able to protect against small amounts of ozone in the atmosphere, but about 10 – 20% healthy individuals can have respiratory symptoms.