Aspirin Toxicity
Dr. Shamil AL-NeaimyAspirin
Aspirin is one of the oldest medications that remains a part of current practiceAspirin is a widely prescribed antiplatelet therapy for cardiovascular and cerebrovascular disease
When combined with the fact that aspirin is readily available, aspirin toxicity remains an important clinical problem
Epidemiology
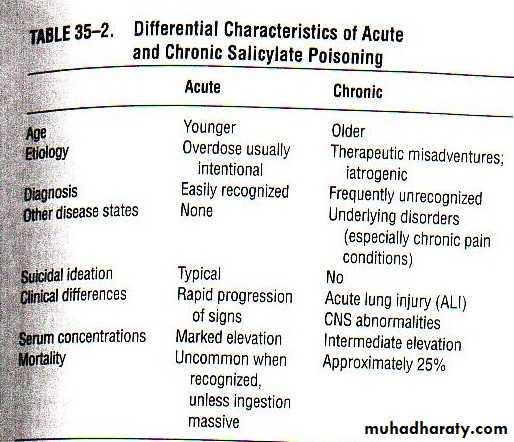
Accounts for 12.6% of all analgesic-related deathsAcute overdoses cause morbidity in 16% and mortality in 1% of cases
Chronic overdoses cause morbidity in 30% and 25% mortality in of cases
Pharmacokinetics
Rapidly absorbed in the stomachReach peak levels in 15-60 minutes
90% bound to albumin in the blood at a dose of 10 mg/dL
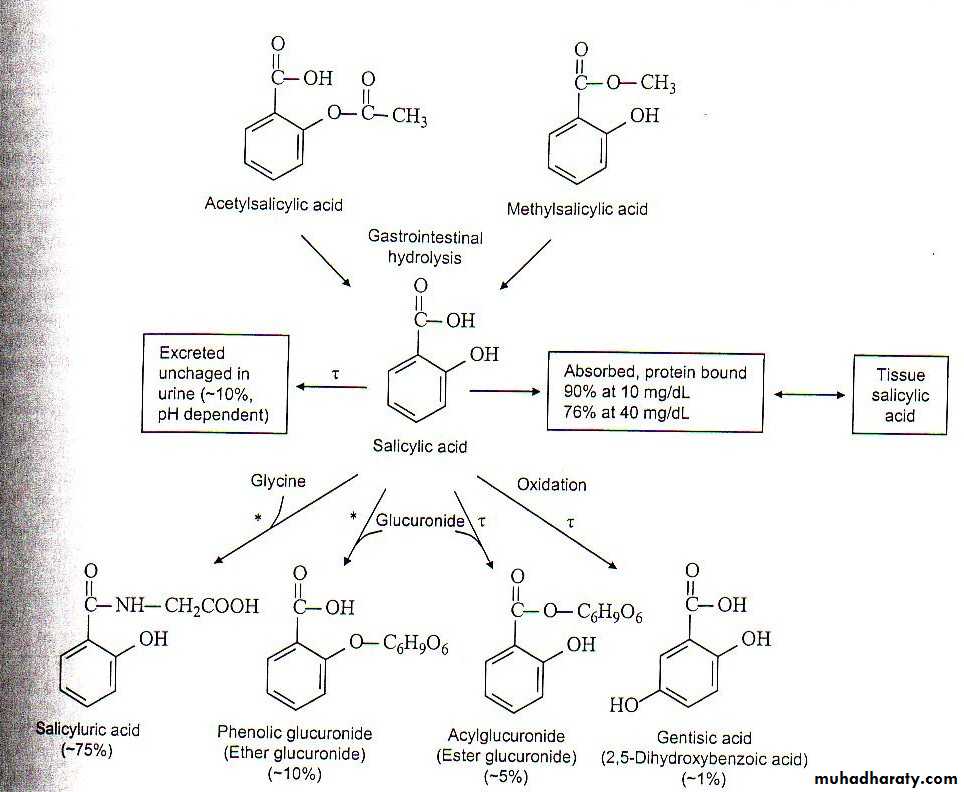
90% metabolized in the liver, 10% unchanged
T1/2 = 15-20 minutes
Metabolites and unchanged drug are filtered and secreted by the kidneys
Toxicokinetics
Absorption may be delayed due to bezoar formation or pylorospasm
Peak blood concentrations may be delayed 2-4 hours
76% bound to albumin at a dose of 40 mg/dL
increased free drug in the blood
Hepatic enzymes become saturated and elimination follows zero-order kinetics
Aspirin Metabolism
Aspirin
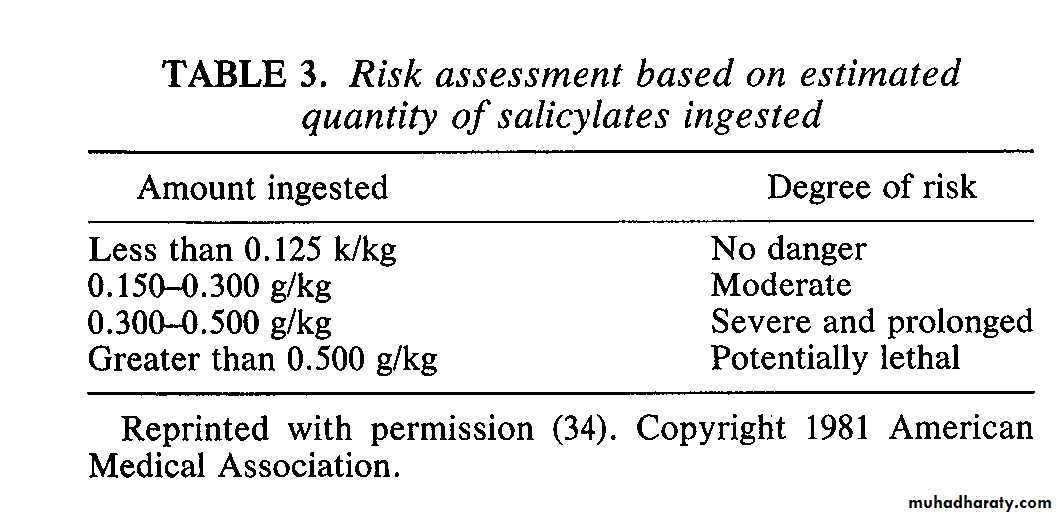
Toxic dose = 1 grain/lbs or 150 mg/kgMinimal lethal dose = 3-4 grains/lbs or 450 mg/kg
Methylsalicylate
Lethal dose in children = 4 cc of 100% MS
Lethal dose in adults = 6 cc of 100% MS
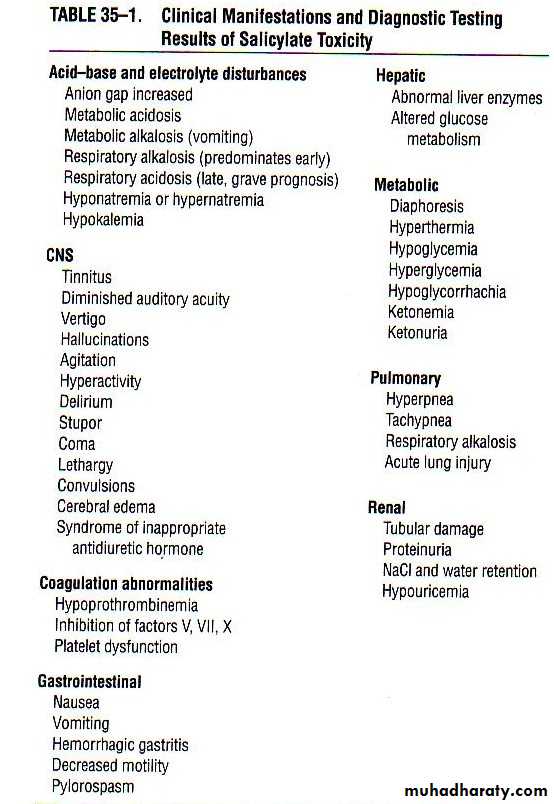
Signs & Symptoms
In salicylate poisoning there generally exists two settings, an acute toxicity and a chronic toxicityIn general, the earliest signs and symptoms of toxicity include nausea, vomiting, diaphoresis, and tinnitus with or without hearing loss
A marked elevation in temperature is a sign of severe toxicity and typically preterminal condition
Factors Influencing Salicylate Toxicity
DoseAge Of Victim
Renal Function
Dehydration
Fever
Pathophysiology
Acid-BaseSalicylates clearly stimulate the respiratory center in the brainstem inducing hyperventilation and respiratory alkalosis
Salicylates are also weak acids and can replace plasma bicarbonate coupled with impaired renal function (due to ASA toxicity) leading to accumulation of sulfuric and phosphoric acids
Path continued
Acid-Base con’tEven though the metabolic acidosis occurs at an early stage, the respiratory alkalosis predominates initially
Glucose Metabolism
Salicylate toxicity appears to produce a discordance between plasma and CSF glucose concentrationsPath continued
Hepatic EffectsThere is an increased entry and oxidation of fatty acids into muscle and liver cells. As a result, the concentrations of plasma free fatty acids, phospholipids, and cholesterol decrease
Neurologic Effects
Toxic doses of salicylates stimulate then depress the CNS. Confusion, dizziness, delirium, psychosis, and then ultimately stupor and coma may occurOtolaryngologic Effects
Large doses (not necessarily toxic) lead to tinnitus, loss of absolute acoustic sensitivity and alterations in perceived sounds
Path continued
Pulmonary Effects
When ALI or edema are present in a patient with salicylate poisoning the following etiologies must be considered:
Aspiration pneumonitis, viral/bacterial infections, postictal and neurogenic ALI and salicylate ALI
Salicylate ALI is a result of adrenergic over activity producing a shift of blood from systemic to the pulmonary circuit
Leading to pulmonary capillary hypertension and then edema
GI Effects
Salicylate toxicity results in nausea and vomiting due to local gastric irritation and stimulation of medullary chemoreceptor trigger zonesPath continued
Renal EffectsAspirin use has not been demonstrated to be associated with either chronic nephrotoxicity or ESRD
Possibly due to inhibition of prostaglandins necessary to maintain renal blood flow
Hematologic Effects
Toxicity effects include hypoprothrombinemia and platelet dysfunction
Musculoskeletal Effects
Pure salicylate overdoses can lead to rhabdomyolysis due to uncoupling of oxidative phosphorylation
Diagnosis
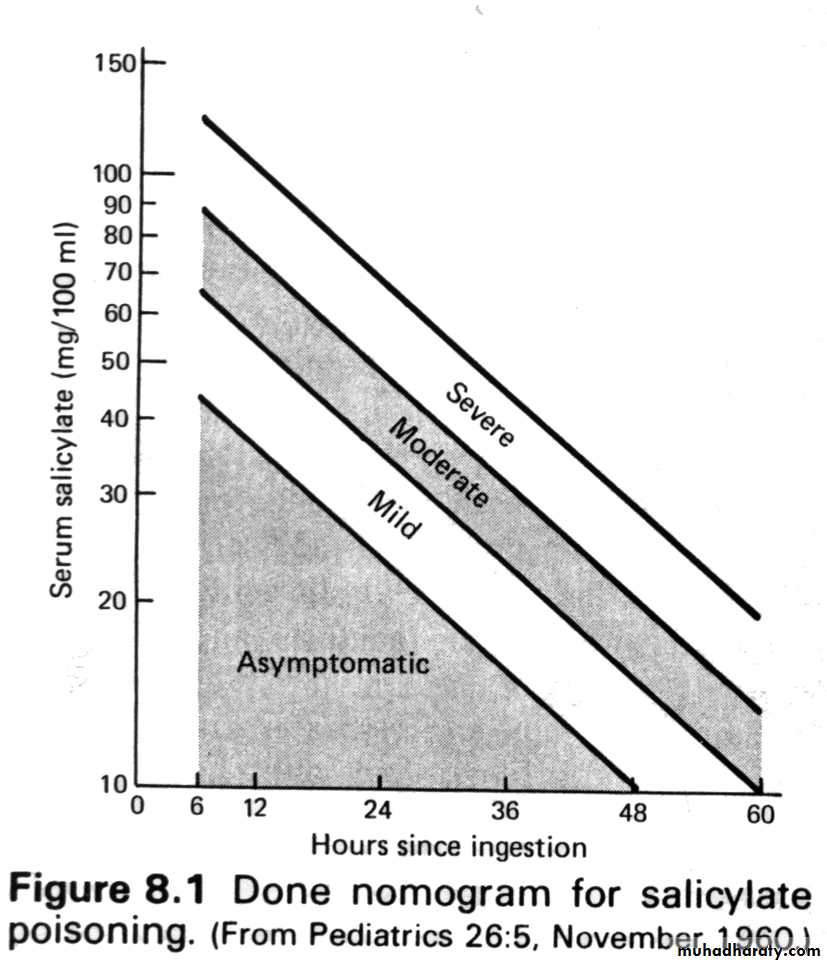
Serum salicylate concentrations and concomitant arterial blood pH values can definitively confirm or exclude toxic salicylate levels
Another test that can be used to rule out ASA toxicity is Trinder spot test
The Trinder spot test is a diagnostic test used to determine exposure to salicylates.The test employs the Trinder reagent which is mixed with a patient's urine.
The color change, resulting from the Trinder reaction, is immediate, enabling rapid bedside assessment.
The test for the Trinder reaction is to mix 1 ml of urine with 1 ml of the Trinder reagent in a test tube.
The test is positive if a color change results. The specific color changes are:
blue or purple positive test
no change negative test
Brown false-positive test caused by the presence of phenothiazines
Diagnosis continued
3 criteria in the ‘point of care’ setting that can rapidly indicate salicylate poisoning are:Positive urine ketones
Increase in fatty acid metabolism
Whole blood glucose and electrolyte determination
Shows decreased bicarbonate and other electrolyte and glucose abnormalities
Whole blood ABG
Shows characteristic acid-base disturbance of salicylate toxicity
Management
Within the initial presentation the use of gastric decontamination (activated charcoal) has shown to reduce the amount of active salicylate by 50-80%
A ratio of 10:1 charcoal to salicylate has the maximum effectiveness
Multiple doses of activated charcoal appears to be superior to single doses
Fluid replacement is very important in the management of salicylate toxicity
Toxicity can induce major fluid losses through tachypnea, vomiting, hypermetabolic state, and insensible perspiration
Management continued
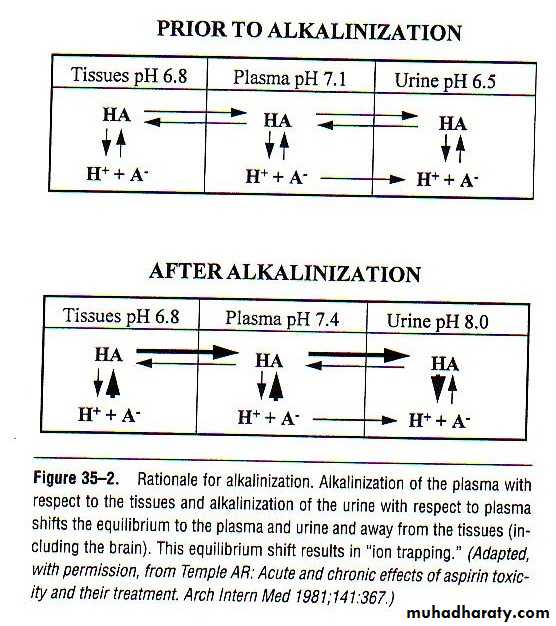
The most important management is through urine alkaliniztionAlkalinization with sodium bicarbonate results in enhanced excretion of ionized acid form of salicylate
Alkalinizing the urine from a pH of 5 to 8 increased renal clearance from 1.3 to 100 ml/min
Do not use acetazolamide due to a concomitant systemic metabolic acidosis and acidemia
Alkalemia via hyperventilation has many risks and contributes to mortality
Management continued
Alkalinization can be achieved with a bolus of 1-2 mEq/kg, followed by an IV infusion of 3 ampules of sodium bicarbonate in 1 L of D5% to run at 1.5-2 times maintenance fluid rangeUrine pH must be maintained at 7.5-8.0 and hypokalemia must be corrected
Hypokalemia is a common complication due to movement of potassium into cells in exchange for hydrogen ions to compensate for the alkalemia
Calcium should also be measured as decreases are a complication of bicarbonate therapy
Management continued
Extracorporeal measures (Hemodialysis)
These are indicated in specific situations due to the ability to remove salicylates as well as correct fluid, electrolyte, and acid-base disorders
While a more comprehensive therapy, alkalinization of the urine reduces salicylate levels much more rapidly
Absolute Indications for Hemodialysis
Renal failureCongestive heart failure
Acute lung injury
Persistent CNS disturbances
Progressive deterioration in vital signs
Severe acid-base or electrolyte imbalance, despite appropriate treatment
Hepatic compromise with coagulopathy
Salicylate concentration (acute) > 100 mg/dL (in the absence of the above)
Summary
Initial assessment of ASA toxicity is important in the elderly, as the clinical manifestations can mimic other common medical complaints in that age groupKnowledge of drug combinations that include ASA in their formulation is important, as patients may not be aware of its presence
Prompt response to a case of ASA toxicity with activated charcoal and alkalinization of the urine is important and can reverse the levels of salicylates rapidly