Iron deficiency anemia
Iron metabolismIron is distributed in active metabolic & storage pool.
Total body iron is about 3.5 gm (50 mg/kg) in healthy men & 2.5 gm (40 mg/kg) in women.
The difference relates to women smaller body size, lower androgen level & dearth of stored iron because of iron loss with menses & pregnancy.
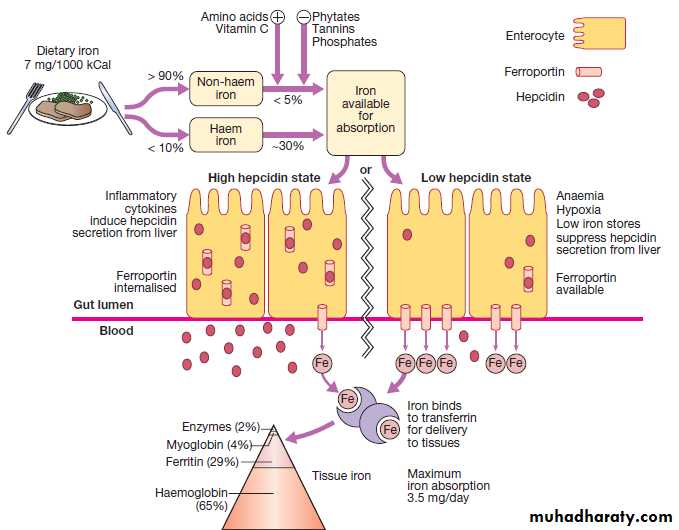
Hemoglobin (2mg, 65%)
Myoglobin (0.2 gm , 4%)2% is found in heme and nonheme enzymes
Ferritin or hemosiderin( 1gm, 25%) in liver, bone marrow, spleen, and muscle
Iron in the body is used primarily for synthesis of Hb & normal erythropoiesis requires 20-25 mg of iron per day.
Requirements for exogenous iron
All iron required for red cells production is acquired through the recycling of iron from senescent RBC.Only 5 % of iron needed for erythropoiesis (1mg/d) is absorbed from GIT to balance losses in urine, stool, sweat & desquamated skin.
Iron is lost from the body only when cells are lost, particularly epithelial cells from GIT , epidermal cells of skin & in menstruating women, RBC.
Small amounts lost in urine & sweat.
Iron absorption
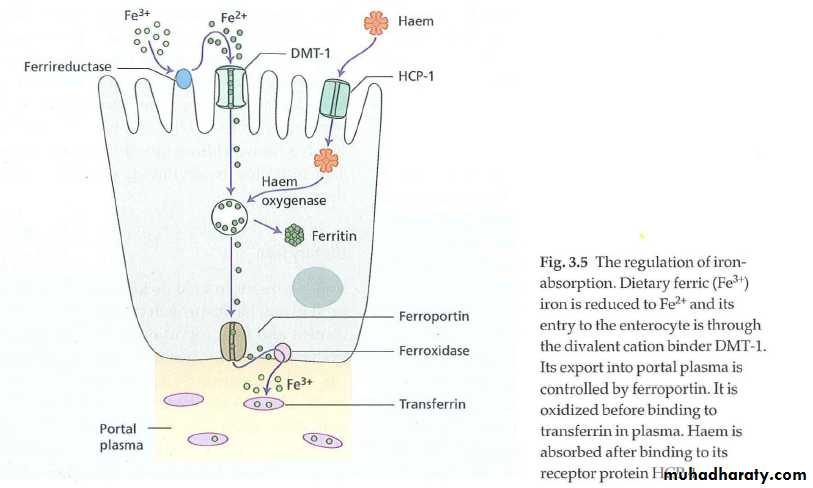
• Sites: maximal absorption occur in duodenum & upper jejunum. The gastric acid reduces insoluble ferric iron to its soluble ferrous state.• Heme iron (meat) is better absorbed than non-heme iron. Non-heme iron absorption is reduced by other food items (e.g. vegetable fiber, phytates & polyphenols; tea tannates, bran) & certain antibiotics (e.g. tetracycline).
Heme iron absorption is not affected by above factors. Beef , lamb, pork, chicken & fish considerbly enhance non heme iron absorption.
Factors influence iron absorption
Decrease iron absorptionIncrease Fe absorption
alkalis, antacids
pancreatic secreations
Ferric iron
Agent that precipitate iron as phytate in grains
-acids: HCL, vit C
-ferrous Fe+2-IDA
-increase demands as in pregnancy, infancy, adolescence, hemolysis, bleeding.
-primary hemochromatosis.
Phytates (grains & vegetables) decrease iron absorption.
Polyphenols present in legumes, tea, coffee & wine also interfere with iron absorption.
Phosphates & phosphoproteins inhibit iron absorption from egg & milk.
Calcium inhibits intestinal iron absorption.
Iron balance is primarily, if no exclusively, achieved by control of absorption rather than by control of excetion.
Iron transport
Transferrin(synthesized in liver) is the principle means for moving iron & is a transport protein with 2 iron binding sites. Transferrin picks up iron from mucosal cells of intestine then deliver iron to receptors on surface of nucleated RBC & reticulocytes, the macrophages then transport the iron free transferrin back to the place. The amount of transferrin is measured directly as TIBC (total iron binding capacity).Iron storage & recycling
Iron not used for erythropioeis is transferred by transferrin to the storage pool which has 2 forms, ferritin & hemosiderin.The most important form is ferritin ( a heterogenous group of proteins surrounding an iron core), which is a soluble & active storage fraction located in liver (in hepatocytes) , bone marrow & spleen (in macrophages); in RBC & in serum.
Iron stored in ferritin is readily available for any body requirements. Circulating ferritin level parallel the size of the body stores (1 ng/ml = 8 mg of iron in storage pool).
The 2nd storage pool of iron is in hemosiderin, which is relatively insoluble & is stored primarily in liver (in kupffer cells) & in marrow (in macrophages).
Because iron absorption is so limited , the body recycles & conserves iron. Transferrin grasps & recycles available iron from aging RBC undergoing phagocytosis by mononucear phagocytosis. This mechanism provides about 97% of daily iron needed (about 25 mg of iron).
Hepcidin
Hepcidin is a polypeptide produced by liver cells, It is both an acute phase protein and the major hormonal regulator of iron homeostasisIt inhibits iron release from macrophages, intestinal epithelial cells.
It increased by inflammation via interleukin 6 (IL-6)
Decreased production of hepcidin occurs in response to iron deficiency, hypoxia and ineffective erythropoiesis.
Hepcidin synthesis and secretion are controlled by three proteins: HFE, hemojuvelin and transferrin receptor 2.
Iron deficiency anemia IDA
Since body stores of iron must be exhausted before red cell production is restricted, anemia is a late stage of iron deficiency.Stages of IDA:
• Depletion of iron (prelatent): In the mildest stage, the reticuloendothelial iron stores are subnormal but there is no biochemical evidence of deficiency. The only physiologic consequence of prelatent deficiency is a compensatory increase in the rate of iron absorption.
2. It may be said to exist when iron stores are exhausted, but the blood hemoglobin level remains above the lower limit of normal. In this stage, certain biochemical abnormalities in iron metabolism are usually detected:
Fatigue although not anemic
S. iron usually low
TIBC increase
3. Iron-deficient erythropoiesis :
Intially normochromic normocytic
Later hypochromic microcytic / tissue changes may be seen.
Causes
Chronic blood loss :• Uterine blood loss (0.5 mg/d)
• GIT blood loss :
• Peptic ulcer
• Esophageal varicies
• Hiatus hernia
• Colonic polyps & diverticulae
• Hemorrhoids
• Chronis aspirin use
• Parasites: hook worm, ancylostoma doudenale
• Malignancy: stomach, colon, esophagus, small intestine.
3. Pulmonary hemosiderosis
4. Urinary tract :hypernephroma, bladder ca, paroxysmal nocturnal Hb uria PNH, parasites (schistosoma, whipworm: trichuris)5. Increase iron requirements
6. Malabsorption
7. Poor diet
8. Regular blood transfusion: 1 cc contain 0.5 mg, iron supplements adviced for those who donate > twice/ year.
Malsbsorption: Gastric acid is required to release iron from food and helps to keep iron in the soluble ferrous state. Achlorhydria in the elderly or that due to drugs such as proton pump inhibitors may contribute to the lack of iron availability from the diet, as may previous gastric surgery. Iron is absorbed actively in the upper small intestine and hence can be affected by coeliac disease .
Physiological demands At times of rapid growth, such as infancy and puberty, iron requirements increase and may outstrip absorption. In pregnancy, iron is diverted to the fetus, the placenta and the increased maternal red cell mass, and is lost with bleeding at parturition
Menstruation:
most common single cause of IDAAverage blood loss 35-80 ml/cycle
Pathogenesis:
• Decrease Hb synthesis
• Decrease cellular proliferation
• Shortend RBC survival.