
Dr. Ahmed Saleem
FICMS
TUCOM / 3rd Year / 2015
SHOCK
Shock is the most common and therefore the most important cause of death of surgical patients. Death
may occur rapidly due to a profound state of shock, or be delayed due to the consequences of organ
ischemia and reperfusion injury. It is important therefore to understand the pathophysiology, diagnosis
and priorities in management of shock and hemorrhage.
Shock is a systemic state of low tissue perfusion which is inadequate for normal cellular respiration. With
insufficient delivery of oxygen and glucose, cells switch from aerobic to anaerobic metabolism. If perfusion
is not restored in a timely fashion, cell death ensues.
Pathophysiology
Cellular
As perfusion to the tissues is reduced, cells are deprived of oxygen and must switch from aerobic to
anaerobic metabolism. The product of anaerobic respiration is not carbon dioxide but lactic acid.
When enough tissue is underperfused, the accumulation of lactic acid in the blood produces a
systemic metabolic acidosis. As glucose within cells is exhausted, anaerobic respiration ceases and
there is failure of sodium/potassium pumps in the cell membrane and intracellular organelles.
Intracellular lysosomes release autodigestive enzymes and cell lysis ensues. Intracellular contents,
including potassium are released into the blood stream.
Microvascular
As tissue ischemia progresses, changes in the local milieu result in activation of the immune and
coagulation systems. Hypoxia and acidosis activate complement and prime neutrophils, resulting in
the generation of oxygen free radicals and cytokine release. These mechanisms lead to injury of the
capillary endothelial cells. These, in turn, further activate the immune and coagulation systems.
Damaged endothelium loses its integrity and becomes ‘leaky’. Spaces between endothelial cells
allow fluid to leak out and tissue edema ensues, exacerbating cellular hypoxia.
Systemic
Cardiovascular: As preload and afterload decrease, there is a compensatory baroreceptor
response resulting in increased sympathetic activity and release of catecholamines into the
circulation. This results in tachycardia and systemic vasoconstriction.
Respiratory: The metabolic acidosis and increased sympathetic response result in an increased
respiratory rate and minute ventilation to increase the excretion of carbon dioxide (and so
produce a compensatory respiratory alkalosis).
Renal: Decreased perfusion pressure in the kidney leads to reduced filtration at the glomerulus
and a decreased urine output. The renin–angiotensin–aldosterone axis is stimulated, resulting in
further vasoconstriction and increased sodium and water reabsorption by the kidney.
Endocrine: As well as activation of the adrenal and renin–angiotensin systems, vasopressin is
released from the hypothalamus in response to decreased preload and results in
vasoconstriction and resorption of water in the renal collecting system. Cortisol is also released
from the adrenal cortex contributing to the sodium and water resorption and sensitizing the cells
to catecholamines.
Page 1 of 6

Ischemia–reperfusion syndrome
During the period of systemic hypoperfusion, cellular and organ damage progresses due to the
direct effects of tissue hypoxia and local activation of inflammation. Further injury occurs once
normal circulation is restored to these tissues. The acid and potassium load that has built up can
lead to direct myocardial depression, vascular dilatation and further hypotension. The cellular and
humoral elements activated by the hypoxia (complement, neutrophils, microvascular thrombi) are
flushed back into the circulation where they cause further endothelial injury to organs such as the
lungs and the kidneys. This leads to acute lung injury, acute renal injury, multiple organ failure and
death. Reperfusion injury can currently only be attenuated by reducing the extent and duration of
tissue hypoperfusion.
Classification of shock
There are numerous ways to classify shock, but the most common and most clinically applicable is one
based on the initiating mechanism. All states are characterized by systemic tissue hypoperfusion and
different states may coexist within the same patient.
Hypovolemic shock
Hypovolemic shock is due to a reduced circulating volume. Hypovolemia may be due to hemorrhagic
or non-hemorrhagic causes. Non-hemorrhagic causes include poor fluid intake (dehydration),
excessive fluid loss due to vomiting, diarrhea, urinary loss (e.g. diabetes), evaporation, or ‘third-
spacing’ where fluid is lost into the gastrointestinal tract and interstitial spaces, as for example in
bowel obstruction or pancreatitis. Hypovolemia is probably the most common form of shock and to
some degree is a component of all other forms of shock. Absolute or relative hypovolemia must be
excluded or treated in the management of the shocked state, regardless of cause.
Obstructive shock
In obstructive shock there is a reduction in preload due to mechanical obstruction of cardiac filling.
Common causes of obstructive shock include cardiac tamponade, tension pneumothorax, massive
pulmonary embolus or air embolus. In each case, there is reduced filling of the left and/or right sides
of the heart leading to reduced preload and a fall in cardiac output.
Distributive shock
Distributive shock describes the pattern of cardiovascular responses characterizing a variety of
conditions, including septic shock, anaphylaxis and spinal cord injury. Inadequate organ perfusion is
accompanied by vascular dilatation with hypotension, low systemic vascular resistance, inadequate
afterload and a resulting abnormally high cardiac output. In anaphylaxis, vasodilatation is due to
histamine release, while in high spinal cord injury there is failure of sympathetic outflow and
adequate vascular tone (neurogenic shock).
The cause in sepsis is less clear but is related to the release of bacterial products (endotoxin) and the
activation of cellular and humoral components of the immune system. There is maldistribution of
blood flow at a microvascular level with arteriovenous shunting and dysfunction of cellular
utilization of oxygen. In the later phases of septic shock there is hypovolemia from fluid loss into
interstitial spaces and there may be concomitant myocardial depression, complicating the clinical
picture.
Page 2 of 6

Cardiogenic shock
Cardiogenic shock is due to primary failure of the heart to pump blood to the tissues. Causes of
cardiogenic shock include myocardial infarction, cardiac dysrhythmias, valvular heart disease, blunt
myocardial injury and cardiomyopathy. Cardiac insufficiency may also be due to myocardial
depression due to endogenous factors (e.g. bacterial and humoral agents released in sepsis) or
exogenous factors, such as pharmaceutical agents or drug abuse. Evidence of venous hypertension
with pulmonary or systemic edema may coexist with the classical signs of shock.
Endocrine shock
Endocrine shock may present as a combination of hypovolemic, cardiogenic or distributive shock.
Causes of endocrine shock include hypo- and hyperthyroidism and adrenal insufficiency.
Hypothyroidism causes a shock state similar to that of neurogenic shock due to disordered vascular
and cardiac responsiveness to circulating catecholamines. Cardiac output falls due to low inotropy
and bradycardia. There may also be an associated cardiomyopathy. Thyrotoxicosis may cause a high-
output cardiac failure. Adrenal insufficiency leads to shock due to hypovolemia and a poor response
to circulating and exogenous catecholamines. Adrenal insufficiency may be due to pre-existing
Addison’s disease or be a relative insufficiency due to a pathological disease state, such as systemic
sepsis.
Presentation
Compensated shock
As shock progresses, the body’s cardiovascular and endocrine compensatory responses reduce flow
to non-essential organs to preserve preload and flow to the lungs and brain. In compensated shock,
there is adequate compensation to maintain central blood volume and preserve flow to the
kidneys, lungs and brain. Apart from a tachycardia and cool peripheries (vasoconstriction,
circulating catecholamines), there may be no other clinical signs of hypovolemia. However, this
cardiovascular state is only maintained by reducing perfusion to the skin, muscle and
gastrointestinal tract. There is a systemic metabolic acidosis and activation of humoral and cellular
elements within the underperfused organs. Although clinically occult, this state will lead to multiple
organ failure and death if prolonged due to the ischemia–reperfusion syndrome. Patients with occult
hypoperfusion (metabolic acidosis despite normal urine output and cardiorespiratory vital signs) for
more than 12 hours have a significantly higher mortality, infection rate and incidence of multiple
organ failure.
Decompensation
Further loss of circulating volume overloads the body’s compensatory mechanisms and there is
progressive renal, respiratory and cardiovascular decompensation. In general, loss of around 15 per-
cent of the circulating blood volume is within normal compensatory mechanisms. Blood pressure is
usually well maintained and only falls after 30–40 per cent of circulating volume has been lost.
Page 3 of 6
Pitfalls in clinical examination
It is important to recognize the limitations of the clinical examination and to recognize patients who are in
shock despite the absence of classic signs.
Capillary refill
Most patients in hypovolemic shock will have cool, pale peripheries, with prolonged capillary refill
times. However, the actual capillary refill time varies so much in adults that it is not a specific
marker of whether a patient is shocked, and patients with short capillary refill times may be in the
early stages of shock. In distributive (septic) shock, the peripheries will be warm and capillary refill
will be brisk, despite profound shock.
Tachycardia
Tachycardia may not always accompany shock. Patients who are on beta-blockers or who have
implanted pacemakers are unable to mount a tachycardia. A pulse rate of 80 in a fit young adult
who normally has a pulse rate of 50 is very abnormal. Furthermore, in some young patients with
penetrating trauma, where there is hemorrhage but little tissue damage, there may be a paradoxical
bradycardia rather than tachycardia accompanying the shocked state.
Blood pressure
It is important to recognize that hypotension is one of the last signs of shock. Children and fit young
adults are able to maintain blood pressure until the final stages of shock by dramatic increases in
stroke volume and peripheral vasoconstriction. These patients can be in profound shock with a
normal blood pressure. Elderly patients who are normally hypertensive may present with a ‘normal’
blood pressure for the general population but be hypovolemic and hypotensive relative to their
usual blood pressure. Beta-blockers or other medications may prevent a tachycardic response.
The diagnosis of shock may be difficult unless one is alert to these pitfalls.
Resuscitation
Immediate resuscitation manoeuvres for patients presenting in shock are to ensure a patent airway and
adequate oxygenation and ventilation. Once ‘airway’ and ‘breathing’ are assessed and controlled, attention
is directed to cardiovascular resuscitation.
Timing and nature of resuscitation: They will depend on the type of shock and the timing and severity of
the insult. If there is initial doubt about the cause of shock, it is safer to assume the cause is hypovolaemia
and begin with fluid resuscitation, and then assess the response.
In patients who are actively bleeding: It is counterproductive to institute high-volume fluid therapy
without controlling the site of hemorrhage. Increasing blood pressure merely increases bleeding
from the site while fluid therapy cools the patient and dilutes available coagulation factors. Thus
operative hemorrhage control should not be delayed and resuscitation should proceed in parallel
with surgery.
In patients with bowel obstruction and hypovolemic shock: They must be adequately resuscitated
before undergoing surgery otherwise the additional surgical injury and hypovolaemia induced
during the procedure will exacerbate the inflammatory activation and increase the incidence and
severity of end-organ insult.
Page 4 of 6
1) Fluid therapy:
In all cases of shock, regardless of classification, hypovolemia and inadequate preload must be
addressed before other therapy is instituted. Administration of inotropic or chronotropic agents to
an empty heart will rapidly and permanently deplete the myocardium of oxygen stores and
dramatically reduce diastolic filling and therefore coronary perfusion. Patients will enter the
unresuscitatable stage of shock as the myocardium becomes progressively more ischemic and
unresponsive to resuscitative attempts. First-line therapy, therefore, is intravenous access and
administration of intravenous fluids. Access should be through short, wide-bore catheters that
allow rapid infusion of fluids as necessary. Long, narrow lines, such as central venous catheters, have
too high a resistance to allow rapid infusion and are more appropriate for monitoring than fluid
replacement therapy.
Type of fluids
:
There is no ideal resuscitation fluid, and it is more important to understand how
and when to administer it. In most studies of shock resuscitation there is no overt difference in
response or outcome between crystalloid solutions (normal saline, Hartmann’s solution, Ringer’s
lactate) or colloids (albumin or commercially available products), which are more expensive and
have worse side-effect profiles. Most importantly, the oxygen carrying capacity of crystalloids
and colloids is zero. If blood is being lost, the ideal replacement fluid is blood, although
crystalloid therapy may be required while awaiting blood products.
Dynamic fluid response:
The shock status can be determined dynamically by the cardiovascular
response to the rapid administration of a fluid bolus. In total, 250–500 mL of fluid is rapidly given
(over 5–10 minutes) and the cardiovascular responses in terms of heart rate, blood pressure and
central venous pressure are observed.
2) Vasopressor and inotropic support:
Vasopressor or inotropic therapy is not indicated as first-line therapy in hypovolemia; because
administration of these agents in the absence of adequate preload rapidly leads to decreased
coronary perfusion and depletion of myocardial oxygen reserves.
Vasopressor agents (phenylephrine, noradrenaline) are indicated in distributive shock states (sepsis,
neurogenic shock) where there is peripheral vasodilatation, and a low systemic vascular resistance,
leading to hypotension despite a high cardiac output. Where the vasodilatation is resistant to
catecholamines (e.g. absolute or relative steroid deficiency) vasopressin may be used as an
alternative vasopressor.
In cardiogenic shock, or where myocardial depression complicated a shock state (e.g. severe septic
shock with low cardiac output), inotropic therapy may be required to increase cardiac output and
therefore oxygen delivery. The inodilator dobutamine is the agent of choice.
Monitoring
Monitoring for patients in shock
Minimum
ECG
Pulse oximetry
Non-invasive blood pressure
Urine output
Additional modalities
Central venous pressure
Invasive blood pressure
Cardiac output
Base deficit and serum lactate
Page 5 of 6
Consequences *
Unresuscitatable shock
Patients who are in profound shock for a prolonged period of time become ‘unresuscitatable’. Cell death
follows from cellular ischemia and the ability of the body to compensate is lost. There is myocardial
depression and loss of responsiveness to fluid or inotropic therapy. Peripherally there is loss of the ability to
maintain systemic vascular resistance and further hypotension ensues. The peripheries no longer respond
appropriately to vasopressor agents. Death is the inevitable result. This stage of shock is the combined result
of the severity of the insult and delayed, inadequate or inappropriate resuscitation in the earlier stages of
shock. Conversely, when patients present in this late stage, and have minimal responses to maximal therapy,
it is important that the futility of treatment is recognized and valuable resources are not wasted.
Multiple organ failure
As techniques of resuscitation have improved, more and more patients are surviving shock. Where
intervention is timely and the period of shock is limited, patients may make a rapid, uncomplicated recovery.
However, the result of prolonged systemic ischemia and reperfusion injury is end-organ damage and multiple
organ failure. Multiple organ failure is defined as two or more failed organ systems. There is no specific
treatment for multiple organ failure. Management is supporting of organ systems with ventilation,
cardiovascular support and hemofiltration/dialysis until there is recovery of organ function. Multiple organ
failure currently carries a mortality of 60 per cent; thus prevention is vital by early aggressive identification
and reversal of shock.
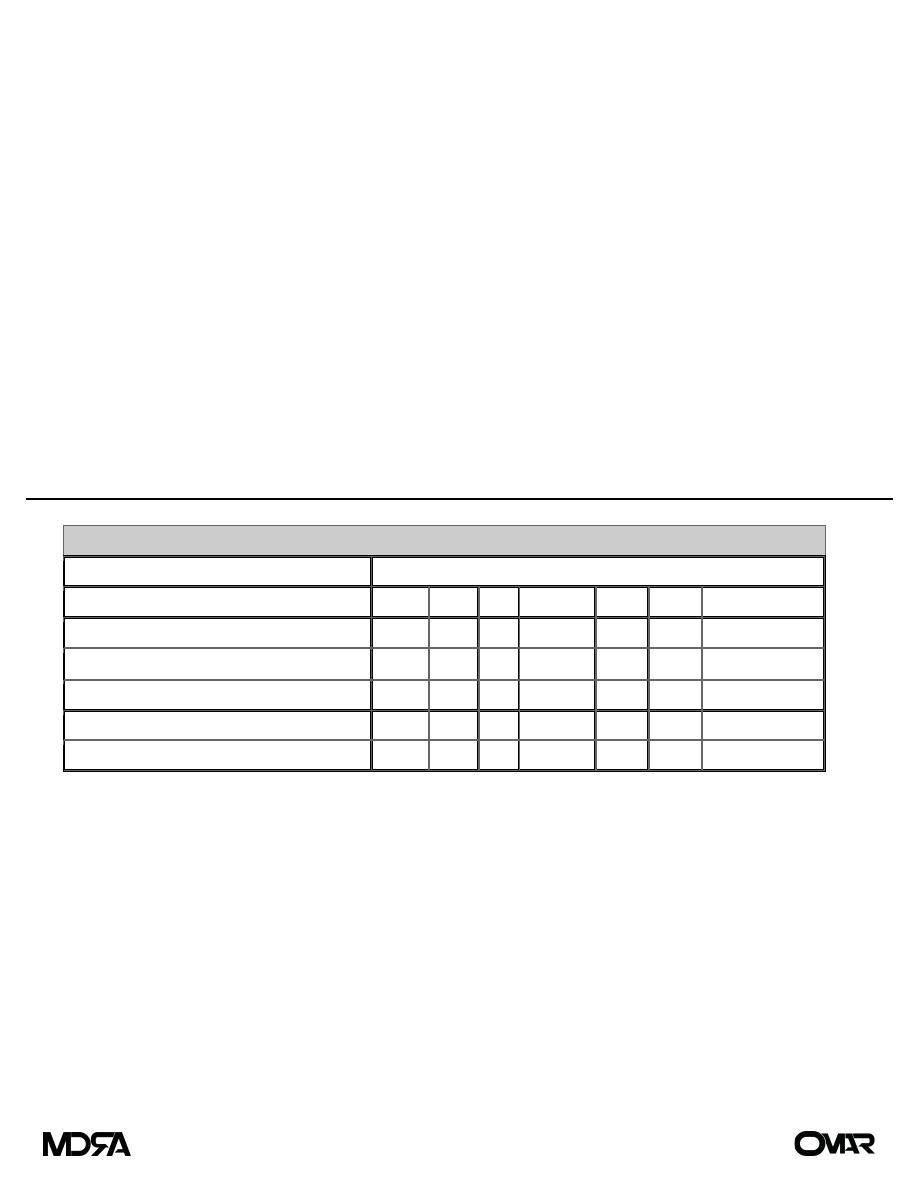
Solutions for Parenteral Administration
Electrolyte Composition (mEq/L)
Solution
Na
CL
K
HCO
3
–
Ca
Mg
mOsm
Extracellular fluid
142
103
4
27
5
3
280–310
Lactated Ringer's
130
109
4
28
3
273
0.9% Sodium chloride
154
154
308
D
5
0.45% Sodium chloride
77
77
407
D5W
253
Both lactated Ringer's solution and normal saline are considered isotonic and are useful in replacing GI
losses and correcting extracellular volume deficits. Lactated Ringer's is slightly hypotonic in that it contains
130 mEq of lactate. Lactate is used rather than bicarbonate because it is more stable in IV fluids during
storage. It is converted into bicarbonate by the liver after infusion, even in the face of hemorrhagic shock.
Sodium chloride is mildly hypertonic, containing 154 mEq of sodium that is balanced by 154 mEq of
chloride. The high chloride concentration imposes a significant chloride load on the kidneys and may lead to
a hyperchloremic metabolic acidosis. Sodium chloride is an ideal solution; however, for correcting volume
deficits associated with hyponatremia, hypochloremia, and metabolic alkalosis. The less concentrated
sodium solutions, such as 0.45% sodium chloride, are useful for replacement of ongoing GI losses as well as
for maintenance fluid therapy in the postoperative period. This solution provides sufficient free water for
insensible losses and enough sodium to aid the kidneys in adjustment of serum sodium levels. The addition
of 5% dextrose (50 g of dextrose per liter) supplies 200 kcal/L, and dextrose is always added to solutions
containing <0.45% sodium chloride to maintain osmolality and thus prevent the lysis of red blood cells that
may occur with rapid infusion of hypotonic fluids. The addition of potassium is useful once adequate renal
function and urine output are established.
Page 6 of 6
