
Dr. Ahmed Saleem
FICMS
TUCOM / 3rd Year / 2015
PRINCIPLES OF ONCOLOGY
As the population ages, oncology is becoming a larger portion of surgical practice. The surgeon often is
responsible for the initial diagnosis and management of solid tumors. Knowledge of cancer epidemiology,
etiology, staging, and natural history is required for initial patient assessment, as well as to determination
of the optimal surgical therapy.
Definitions
Metaplasia: Reversible transformation of one type of terminally differentiated cell into another fully
differentiated cell type.
Dysplasia: Potentially premalignant condition characterized by increased cell growth, atypical morphology,
and altered differentiation.
Neoplasia: Autonomous abnormal growth of cells which persists after the initiating stimulus has been
removed.
A neoplasm (Tumor): is a lesion resulting from neoplasia.
Cancer: The name ‘cancer’ comes from the Greek and Latin words for a crab, and refers to the claw-like
blood vessels extending over the surface of an advanced breast cancer.
Benign tumors: Slow growing, usually encapsulated, do not metastasize, do not recur if completely
excised, and rarely endanger life. Effects are due to size and site. Histology: well differentiated, low
mitotic rate, resemble tissue of origin.
Malignant tumors (Cancer): These expand and infiltrate locally, encapsulation is rare, metastasize
to other organs via blood, lymphatics or body spaces, endanger life if untreated. Histology: varying
degrees of differentiation from tissue of origin, pleomorphic (variable cell shapes), high mitotic rate.
Cancer Biology
Cancer cells are psychopaths. They have no respect for the biological principles of normal cellular
organization. Their proliferation is uncontrolled; their ability to spread is unbounded. Their relentless
progress destroys first the tissue and then the person. In order to behave in such an unprincipled fashion,
cells have to acquire a number of characteristics before they are fully malignant. It has been proposed that
there are six essential alterations in cell physiology that dictate malignant growth: self-sufficiency of growth
signals, insensitivity to growth-inhibitory signals, evasion of apoptosis (programmed cell death), potential
for limitless replication, angiogenesis, and invasion and metastasis.
Invasion: is the most important single criterion for malignancy and is also responsible for clinical
signs and prognosis as well as dictating surgical management. Factors that enable tumors to
invade tissues include:
Increased cellular motility.
Loss of contact inhibition of migration and growth.
Secretion of proteolytic enzymes, such as collagenase, which weakens normal connective
tissue bonds.
Decreased cellular adhesion.
Page 1 of 8

Metastasis: is a consequence of the invasion property and is the process by which malignant
tumors spread from their site of origin (primary tumor) to form secondary tumors at distant
sites. The routes of metastasis are:
Hematogenous: via the bloodstream.
Lymphatic: to local, regional, and systemic nodes.
Transcelomic: across pleural, pericardial, and peritoneal cavities.
Implantation: during surgery or along biopsy tracks.
Genetic abnormalities in tumors:
Two genetic mechanisms of carcinogenesis are proposed:
Oncogenes: Enhanced expression of stimulatory dominant genes.
Tumor suppressor genes: Inactivation of recessive inhibitory genes.
Examples include BRCA1, p53, k-ras, APC, DCC.
Malignant transformation:
The characteristics of the cancer cell arise as a result of mutation. Only very rarely is a single
mutation sufficient to cause cancer; multiple mutations are usually required. Cancer is usually
regarded as a clonal disease. Once a cell has arisen with all the mutations necessary to make it fully
malignant, it is capable of giving rise to an infinite number of identical cells, each of which is fully
malignant. These cells divide, invade, metastasize and destroy but, ultimately, each is the direct
descendant of that original, primordial, transformed cell.
Tumor growth:
Tumor doubling time depends on cell cycle time, growth function, and cell loss fraction. In tumors
such as leukemia, the doubling time remains remarkably constant: the cell mass increases
proportionally with time. This is exponential growth. In solid tumors, doubling time slows as size
increases. This is referred to as Gompertzian growth.
Etiology of Cancer
Both inheritance and environment are important determinants of cancer development. Although
environmental factors have been implicated in more than 80 per cent of cases, this still leaves plenty of
scope for the role of genetic inheritance (Inherited cancer syndromes).
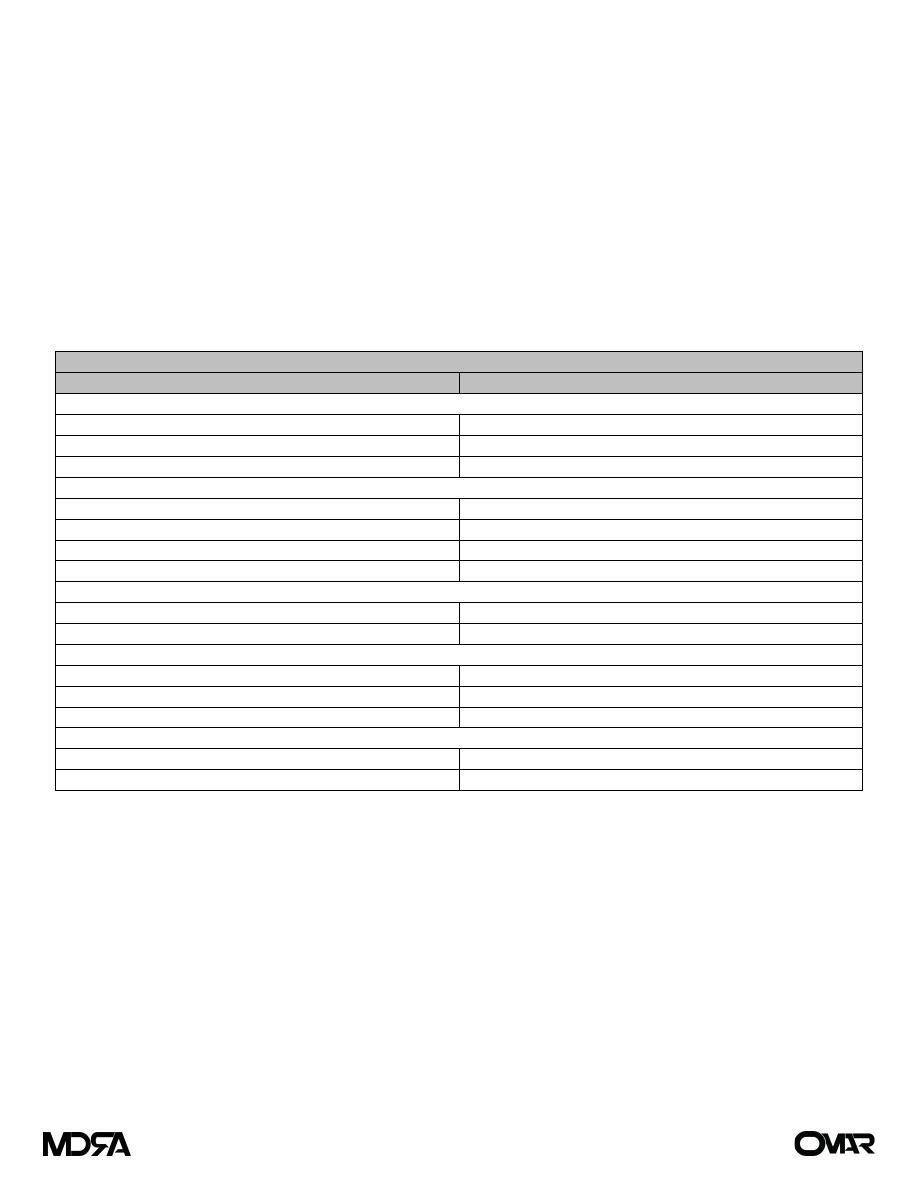
Examples of Inherited syndromes associated with cancer
Syndrome
Gene(s) implicated
Associated tumors
Familial adenomatous polyposis (FAP)
APC gene
Colorectal cancer under the age of 25
years, Papillary carcinoma of the
thyroid, Cancer of the ampulla of
Vater, Hepatoblastomas
Hereditary non-polyposis colorectal cancer
(HNPCC)
DNA mismatch repair genes
(MLH1, MSH2, MSH6)
Colorectal cancer (typically in the 40s
and 50s) Endometrium, stomach,
hepatobiliary
Li–Fraumeni syndrome
p53
Sarcomas, Leukemia, Osteosarcomas
Brain tumors,
Adrenocortical carcinomas
Familial breast cancer
BRCA1, BRCA2
Breast cancer, Ovarian cancer
Papillary serous carcinoma of the
peritoneum
Prostate cancer
Page 2 of 8
Carcinogenesis is the process that results in malignant neoplasm formation. Usually more than one
carcinogen is necessary to produce a tumor, a process which may occur in several steps (Multistep
hypothesis).
Initiators: produce a permanent change in the cells, but do not themselves cause cancer, e.g.
ionizing radiation: this change may be in the form of gene mutation.
Promoters: stimulate clonal proliferation of initiated cells, e.g. dietary factors and hormones: they
are not mutagenic.
Latency: is the time between exposure to carcinogen and clinical recognition of tumor due to:
Time taken for clonal proliferation to produce a significant cell mass.
Time taken for exposure to multiple necessary carcinogens.
Persistence: is when clonal proliferation no longer requires the presence of initiators or promoters
and the tumor cells exhibit autonomous growth.
Common carcinogens
Known carcinogen
Type of cancer
Chemicals
Polyaromatic hydrocarbons
Lung cancer (smoking), skin cancers
Aromatic amines
Bladder cancer (rubber and dye workers)
Alkylating agents
Leukemia
Viruses
HIV
Kaposi’s sarcoma, lymphoma
Epstein-Barr virus
Burkitt’s lymphoma, nasopharyngeal cancer
Human papilloma virus
Squamous papilloma (wart), cervical cancer
Hepatitis B virus
Liver cell carcinoma
Radiation
UV light
Malignant melanoma, basal cell carcinoma
Ionizing radiation
Particularly breast, bone, thyroid, marrow
Biological agents
Hormones, e.g. estrogens
Breast and endometrial cancer
Mycotoxins, e.g. aflatoxins
Liver cell carcinoma
Parasites, e.g. schistosoma
Bladder cancer
Miscellaneous
Asbestos
Mesothelioma and lung cancer
Nickel
Nasal and lung cancer
Management of Cancer
The traditional approach to cancer concentrates on diagnosis and active treatment. This is a very limited
view that, once active treatment is complete, there is little more do be done. Prevention is forgotten and
rehabilitation ignored.
Prevention: Cancer prevention can be divided into three categories: (a) primary prevention (i.e.,
prevention of initial cancers in healthy individuals), (b) secondary prevention (i.e., prevention of
cancer in individuals with premalignant conditions), and (c) tertiary prevention (i.e., prevention of
second primary cancers in patients cured of their initial disease).
The systemic or local administration of therapeutic agents to prevent the development of
cancer, called chemoprevention, e.g. Tamoxifin in breast cancer.
Page 3 of 8

In selected circumstances, the risk of cancer is high enough to justify surgical prevention. These
high-risk settings include hereditary cancer syndromes such as hereditary breast-ovarian cancer
syndrome, as well as some nonhereditary conditions such as chronic ulcerative colitis.
Screening
Screening involves the detection of disease in an asymptomatic population in order to improve
outcomes by early diagnosis.
Criteria for screening:
The disease
Recognizable early stage
Treatment at an early stage more effective than at a later stage
Sufficiently common to warrant screening
The test
Sensitive and specific
Acceptable to the screened population
Safe
Inexpensive
The program
Adequate diagnostic facilities for those with a positive test
High-quality treatment for screen-detected disease to minimize morbidity and mortality
Screening repeated at intervals if the disease is of insidious onset
Benefit must outweigh physical and psychological harm
Diagnosis and histopathological classification
Precise diagnosis is crucial to the choice of correct therapy; the wrong operation, no matter how
superbly performed, is useless. An unequivocal diagnosis is the key to an accurate prognosis. Only
rarely can a diagnosis of cancer confidently be made in the absence of tissue for pathological or
cytological examination. Cancer is a disease of cells and, for accurate diagnosis, the abnormal cells
need to be seen, then can be classified according to the following:
Tissue of origin: Organ and tissue type.
Behavior: Benign or malignant.
Primary or secondary.
Grading: is the process of assessing the degree of differentiation of a malignant tumor.
Staging is the process of assessing the extent of local and systemic spread of a malignant tumor or
the identification of features which are risk factors for spread.
The objectives of staging a tumor are:
To plan appropriate treatment (loco-regional and/or systemic) for the individual patient.
To give an estimate of the prognosis.
To compare similar cases when assessing outcomes or designing clinical trials.
The commonest system is the internationally agreed TNM (tumor, nodes, metastases) classification.
The International Union against Cancer (UICC) is responsible for it, and is compatible with, and
relates to, the American Joint Committee on Cancer (AJCC) system for stage grouping of cancer.
Specific staging systems also exist for some tumors (e.g. Duke’s staging in colorectal cancer).
Page 4 of 8
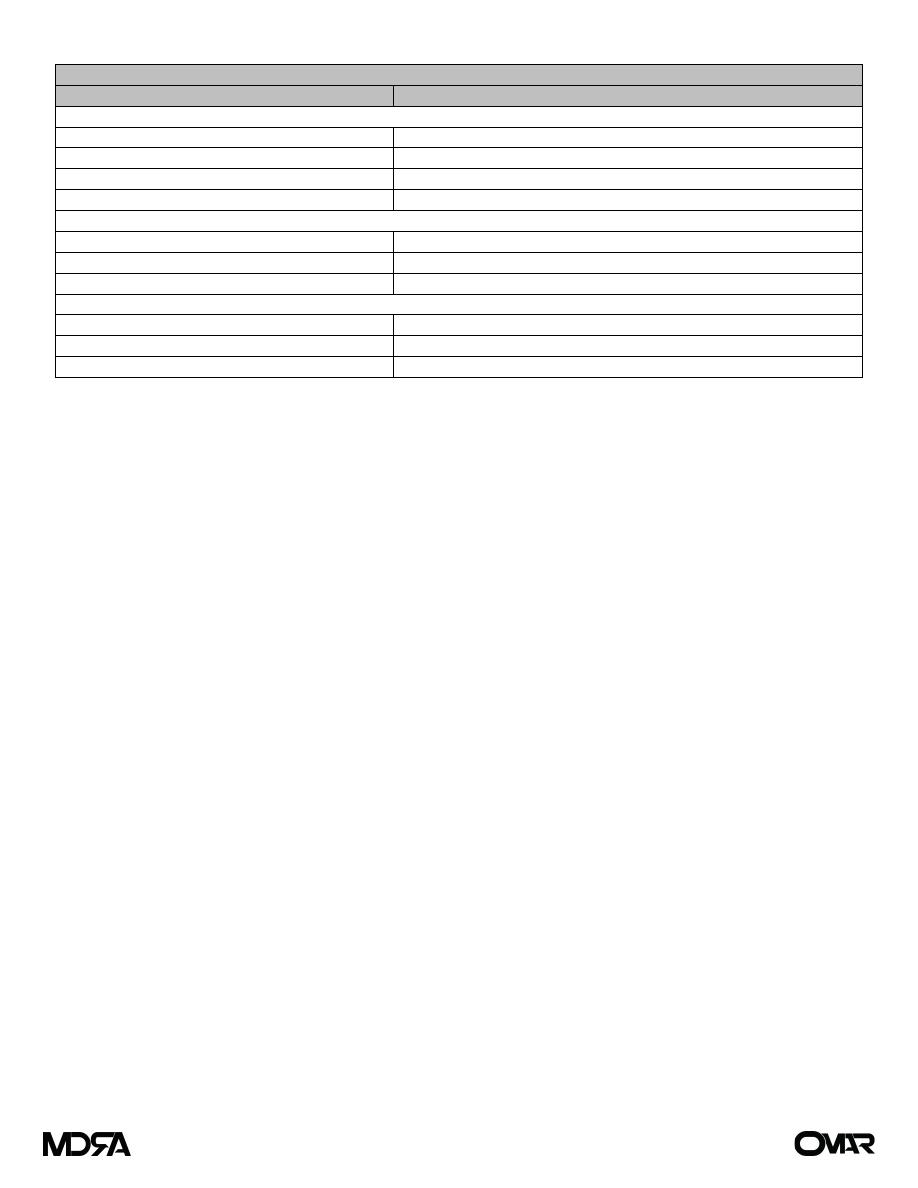
Basic form of TNM classification
Classification
Interpretation
Primary tumor (T)
TX
Primary tumor cannot be evaluated
T0
No evidence of primary tumor
Tis
Tumor in situ
T1, T2, T3, T4
Size and extent of primary tumor
Regional lymph nodes (N)
NX
Regional lymph nodes cannot be evaluated
N0
No regional lymph node involvement
N1, N2, N3
Number and location of involved lymph nodes
Distant metastasis (M)
MX
Distant metastasis cannot be evaluated
M0
No distant metastasis
M1
Distant metastasis
Tumor markers
Tumor markers are complex molecules, often proteins that can be detected by a variety of techniques,
including chemical, immunological, or bioactivity testing. Most are molecules normally produced by normal
cells in small amounts, but which may be produced in increased amounts by tumor cells due to changes in
cellular function (e.g. increased production, increased gene expression, decreased degradation, increased
release).
Testing
is most commonly via serum measurements or testing tissue specimens.
Common uses include:
Screening (detection of subclinical disease).
Diagnosis (including differentiation of tumor origin in metastatic disease).
Monitoring response to treatment.
Monitoring for development of recurrence.
Non-tumor related elevations in tumor marker levels (reducing the specificity of these tests for tumors)
may occur due to:
Increased production/release due to inflammation, infection, trauma, or surgery.
Decreased removal/destruction due to renal or liver disease.
Common examples include:
AFP (alpha-fetoprotein).
β-HCG (beta-human chorionic gonadotrophin).
CEA (carcinoembryonic antigen).
LDH (lactic dehydrogenase).
PSA (prostate-specific antigen).
Surgical treatment of cancer
For most solid tumors, surgery remains the definitive treatment and the only realistic hope of cure.
However, surgery has several roles in cancer treatment including diagnosis, removal of primary
disease, removal of metastatic disease, palliation, prevention and reconstruction.
Page 5 of 8

Non-surgical treatment of cancer
The principles underlying the non-surgical management of cancer:
First, the spatial distribution of the effects of therapies has to be considered: surgery and
radiotherapy are local or, at best, loco-regional treatments; chemotherapeutic drugs offer a
therapy that is systemic.
Second, the intent underlying the treatment. Occasionally, radiotherapy, chemotherapy or the
combination of the two may be used with curative intent. More usually, chemotherapy or
radiotherapy is used to lower the risk of recurrence after primary treatment with surgery, so-
called adjuvant therapy.
Radiation therapy:
Physical Basis:
Radiation therapy is delivered primarily as high-energy photons (gamma rays and x-rays) and
charged particles (electrons). Gamma rays are photons that are released from the nucleus of a radioactive
atom. X-rays are photons that are created electronically, such as with a clinical linear accelerator. Currently,
high-energy radiation is delivered to tumors primarily with linear accelerators. X-rays traverse the tissue,
depositing the maximum dose beneath the surface, and thus spare the skin. Gamma rays typically are
produced by radioactive sources used in brachytherapy. The dose of radiation absorbed correlates with the
energy of the beam. The basic unit is the amount of energy absorbed per unit of mass (joules per kilogram)
and is known as a gray (Gy). One gray is equivalent to 100 rads, the unit of radiation measurement used in
the past.
Biologic Basis: Until about 20 years ago, it was assumed that the biological effects of radiation
resulted from radiation induced damage to the DNA of dividing cells. Nowadays, it is known that,
although this undoubtedly explains some of the biological effects of radiation, it does not provide a
full explanation. Radiation can, both directly and indirectly, influence gene expression: over 100
radiation-inducible effects on gene expression have now been described. These changes in gene
expression are responsible for a considerable proportion of the biological effects of radiation upon
tumors and normal tissues. In this sense, radiotherapy is a precisely targeted form of gene therapy
for cancer.
Radiation Therapy Planning:
Define the target to treat.
Design the optimal technical set up to provide uniform irradiation of that target.
Choose that schedule of treatment that delivers radiation to that target so as to maximize the
therapeutic ratio. One of the main problems with assessing a therapeutic ratio for a given
schedule of radiation is that there is dissociation between the acute effects on normal tissues
and the late damage. The acute reaction is not a reliable guide to the adverse consequences of
treatment in the longer term.
Page 6 of 8
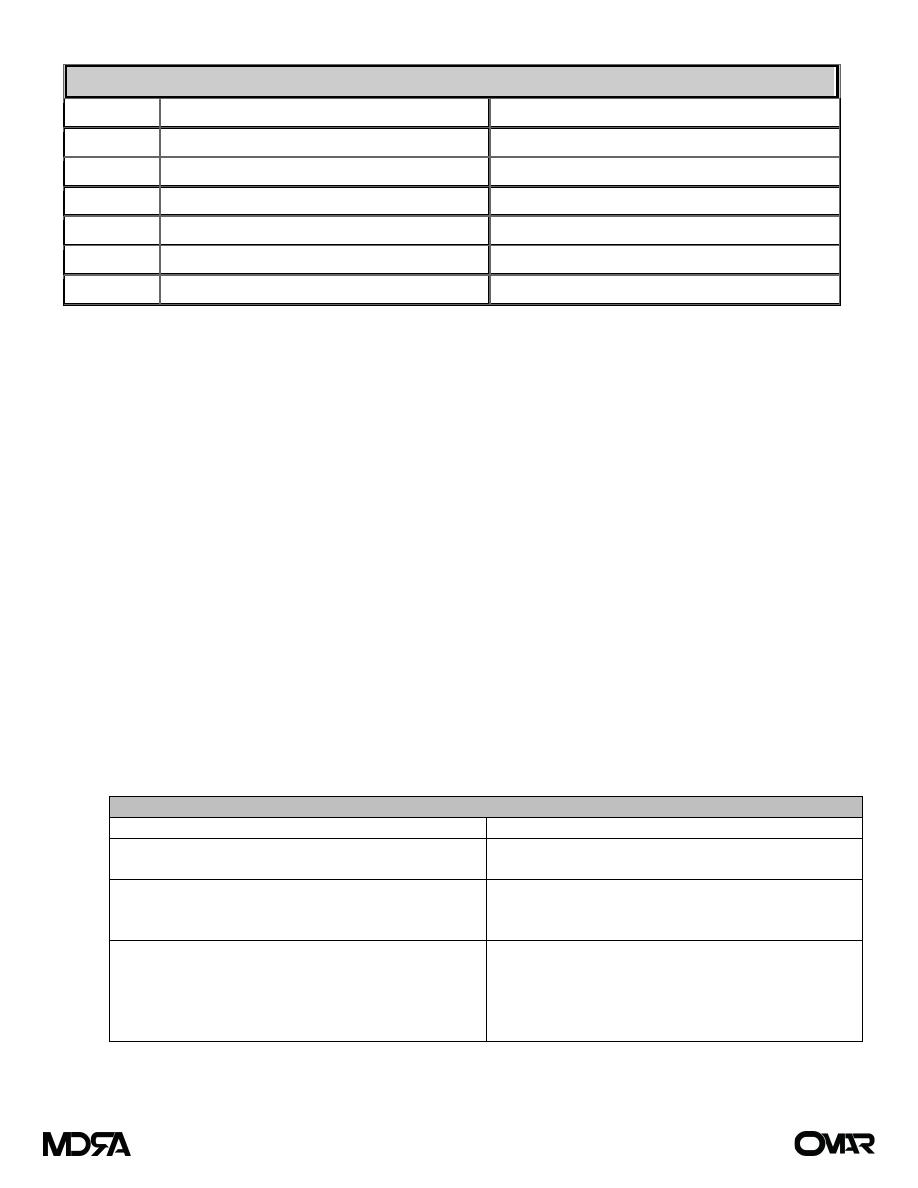
Effects of Radiation
Organ
Acute Changes (2 to 3 weeks)
Chronic Changes (weeks to years)
Skin
Erythema, wet or dry desquamation, epilation
Telangiectasia, subcutaneous fibrosis, ulceration
GI tract
Nausea, diarrhea, edema, ulceration, hepatitis Stricture, ulceration, perforation, hematochezia
Kidney
—
Nephropathy, renal insufficiency
Bladder
Dysuria
Hematuria, ulceration, perforation
Gonads
Sterility
Atrophy, ovarian failure
Eye
Conjunctivitis
Cataract, keratitis, optic nerve atrophy
Chemotherapy
Cytotoxic drugs kill rapidly-growing cells by damaging their DNA and/or by interfering with DNA synthesis or
cell division.
The relationship between dose and response and the principle of selective toxicity:
Cytotoxic drugs show no intrinsic specificity for cancer cells versus normal tissues: in addition to
killing tumor cells, they damage normal tissues that are rapidly dividing, including the normal bone
marrow, gut lining and hair follicles. An element of selectivity can be introduced by careful
adjustment of dose and schedule to maximize damage to the tumor while allowing recovery of
normal tissues.
Classification of chemotherapeutic drugs:
Chemotherapeutic agents can be classified according to the phase of the cell cycle during which they
are effective. Cell-cycle phase–nonspecific agents (e.g., alkylating agents) have a linear dose-
response curve, such that the fraction of cells killed increases with the dose of the drug. In contrast,
the cell-cycle phase–specific drugs have a plateau with respect to cell killing ability, and cell kill will
not increase with further increases in drug dose.
Chemotherapeutic Agents
Mechanism of action
Examples
Drugs that interfere with mitosis
Vincristine
Taxanes
Drugs that interfere with DNA synthesis
(anti-metabolites)
5-Fluorouracil (5-FU)
Methotrexate
6-Mercaptopurine
Drugs that directly damage DNA or
interfere with its function
Mitomycin C
Cisplatinum
Doxorubicin
Cyclophosphamide
Etoposide
Page 7 of 8

Combination Chemotherapy:
To maximize the chance that a tumor will respond to therapy, cytotoxic drugs are often used in
combination. The principles of combination chemotherapy are that the selected drugs should:
Be active against the tumor when used alone.
Have different mechanisms of action, to maximize tumor cell kill.
Have a different spectrum of side-effects, to minimize toxicity to the patient.
Toxicity of Chemotherapy:
The dose of chemotherapeutic drugs is limited by its toxic effects on normal tissues. Some of these
effects are manifest acutely, within minutes to weeks of administration, and may necessitate
adjustment of dose or schedule. Some effects may be delayed for months or years, in some cases
long after completion of the therapy that caused them, that is, when it is too late for dose
modification or cessation of treatment.
Acute toxicity
Extravasation of the drug and tissue damage
Bone marrow toxicity (Sepsis and Bleeding)
Gastrointestinal toxicity
Alopecia
Neuropathy
Long-term toxicity
Cardiotoxicity
Pulmonary toxicity
Carcinogenesis
Gonadal damage
Page 8 of 8
