
COORDINATION COMPLEXES
Prof.Dr. Amer A. Taqa
Prof.Dr. Amer A. Taqa
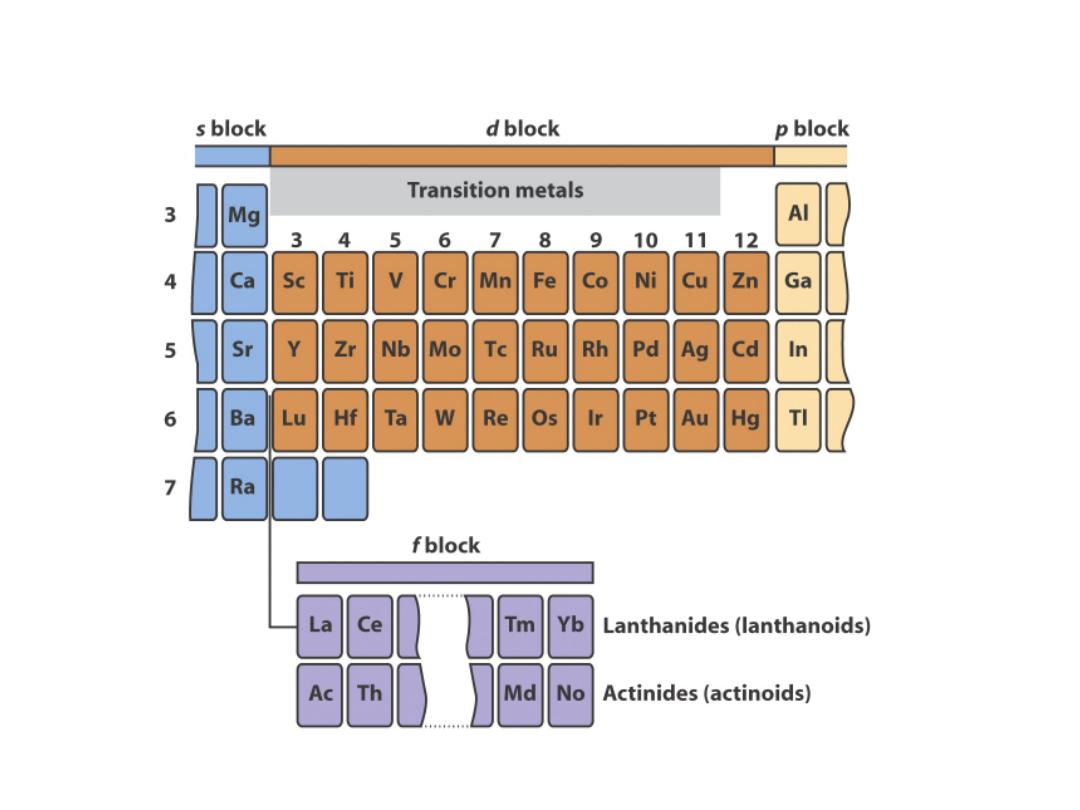
The d block metal form
coordination complexes
with molecules and ions
Prof.Dr. Amer A. Taqa
Prof.Dr. Amer A. Taqa
Prof.Dr. Amer A. Taqa

19.1 Coordination complexes
What is the electronic basis of the color of metal complexes?
Prof.Dr. Amer A. Taqa

Color and Magnetism
• e
‐
in partially filled d sublevel absorbs visible light
•
moves to slightly higher energy d orbital
Magnetic properties due to unpaired electrons
Prof.Dr. Amer A. Taqa

Prof.Dr. Amer A. Taqa
Prof.Dr. Amer A. Taqa

Coordination complex: A structure containing a metal (usually a metal ion)
bonded (coordinated) to a group of surrounding molecules or ions.
Ligand
(ligare is Latin, to bind): A ligand is a molecule or ion that is directly
bonded to a
metal ion
in a coordination complex
Coordination sphere
: A metal and its surrounding ligands
A ligand uses a lone pair of electrons (Lewis base) to bond to the
metal ion (Lewis acid)
Prof.Dr. Amer A. Taqa
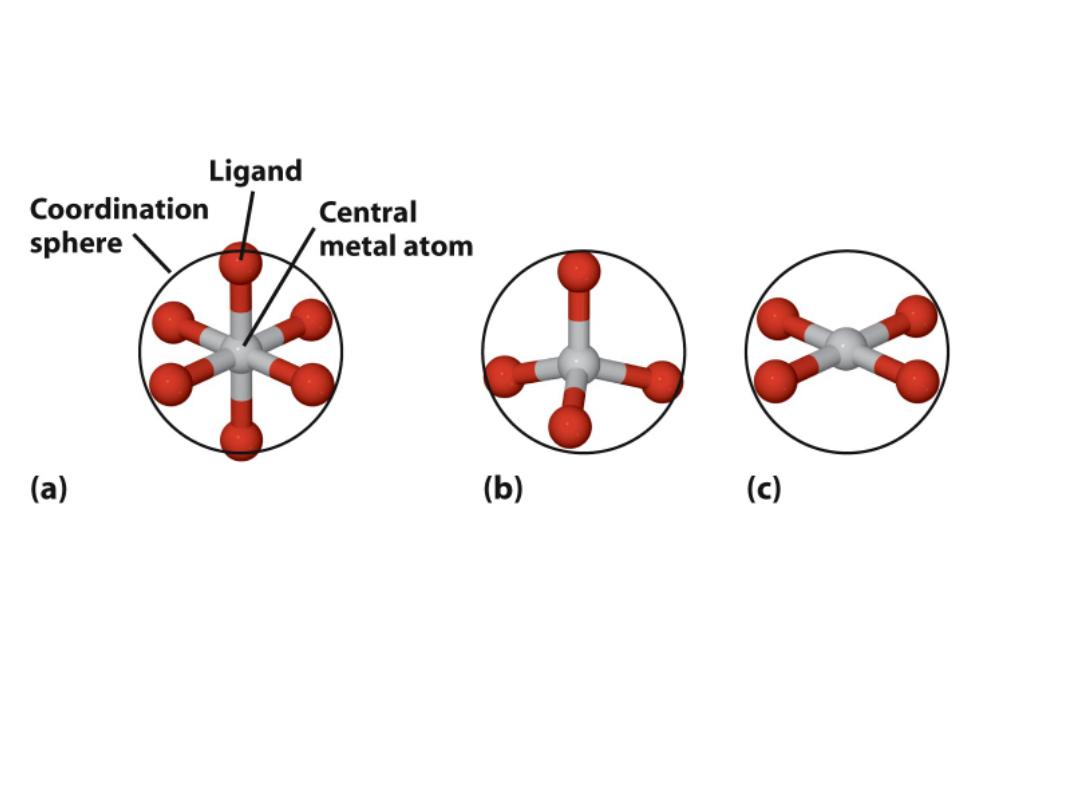
Complex ions: Three common structural types
Octahedral:
Most important
Tetrahedral
Square planar
What determines why a metal takes one of these shapes?
Prof.Dr. Amer A. Taqa
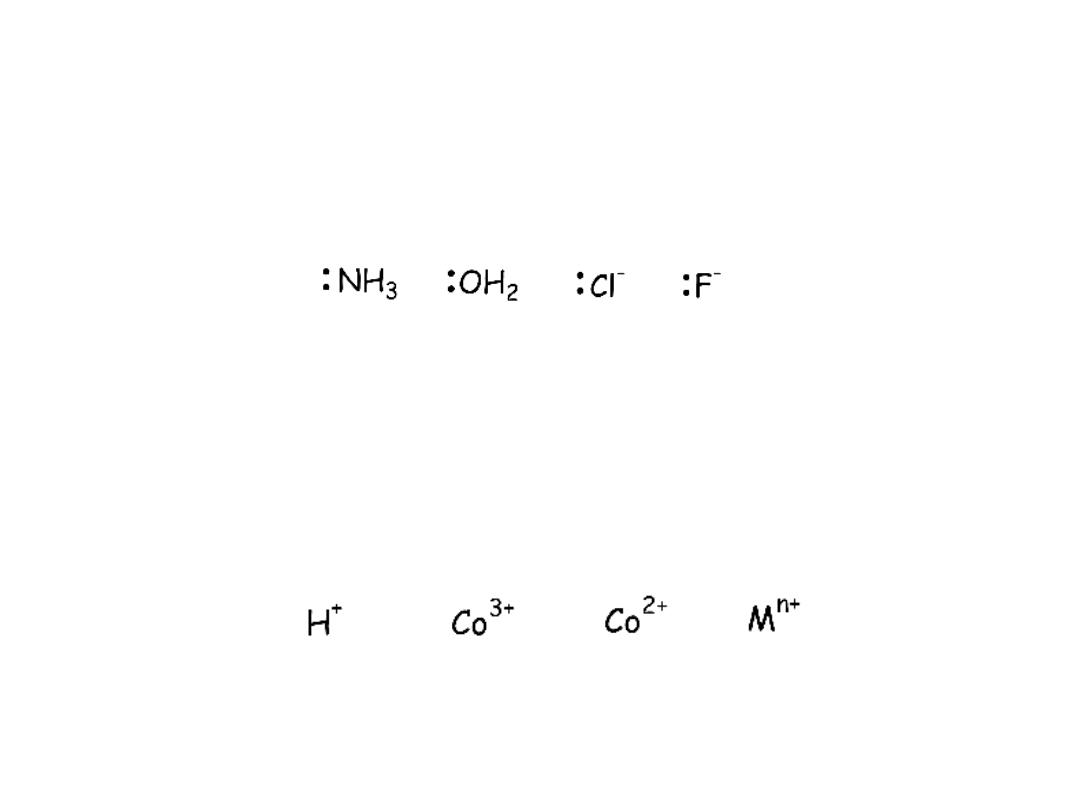
Lewis
acids and bases
A
Lewis base
is a molecule or ion that
donates
a lone pair of electrons to
make a bond
A
Lewis acid
is a molecule of ion that
accepts
a lone pair of
electrons to make a bond
Examples:
Examples:
Prof.Dr. Amer A. Taqa
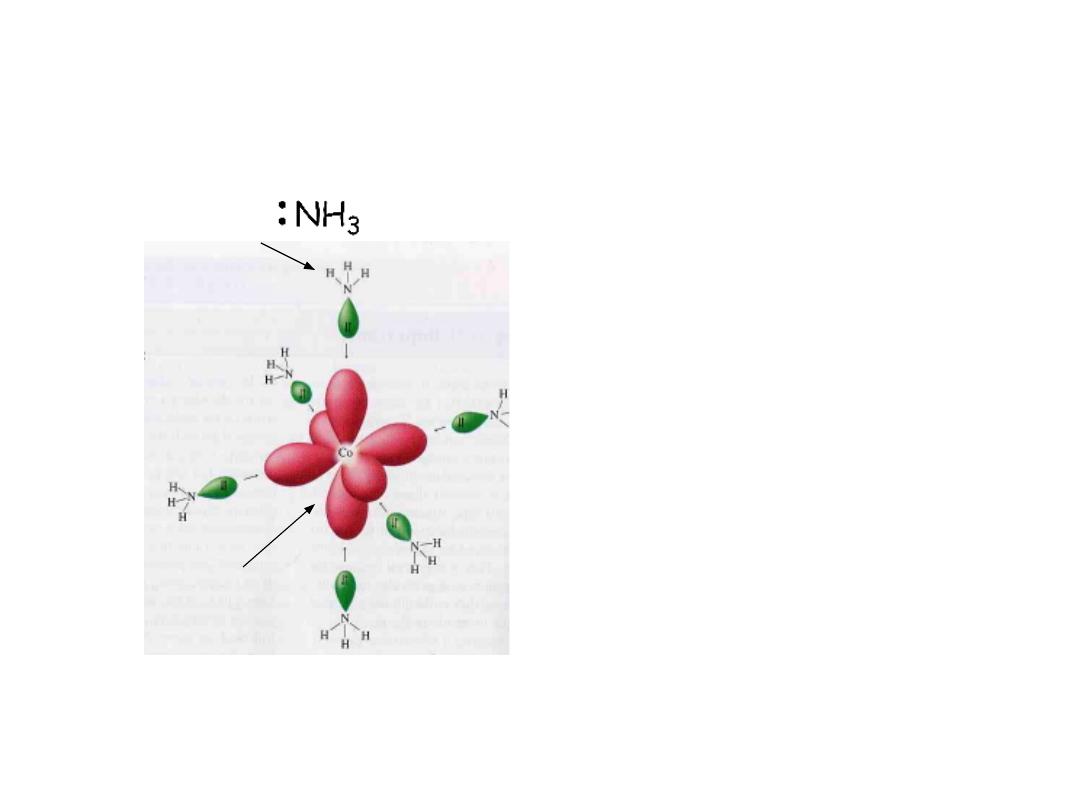
Lewis acid: Co
3+
Lewis base:
Coordination complex: Lewis base
(electron pair donor) coordinated to
a Lewis acid (electron pair acceptor)
Coordination complex: Ligand (electron
donor) coordinated to a metal
(electron acceptor)
The formation of a coordinate complex is a
Lewis acid-base reaction
The number of ligand bonds to the central metal atom is termed the
coordination number
Prof.Dr. Amer A. Taqa
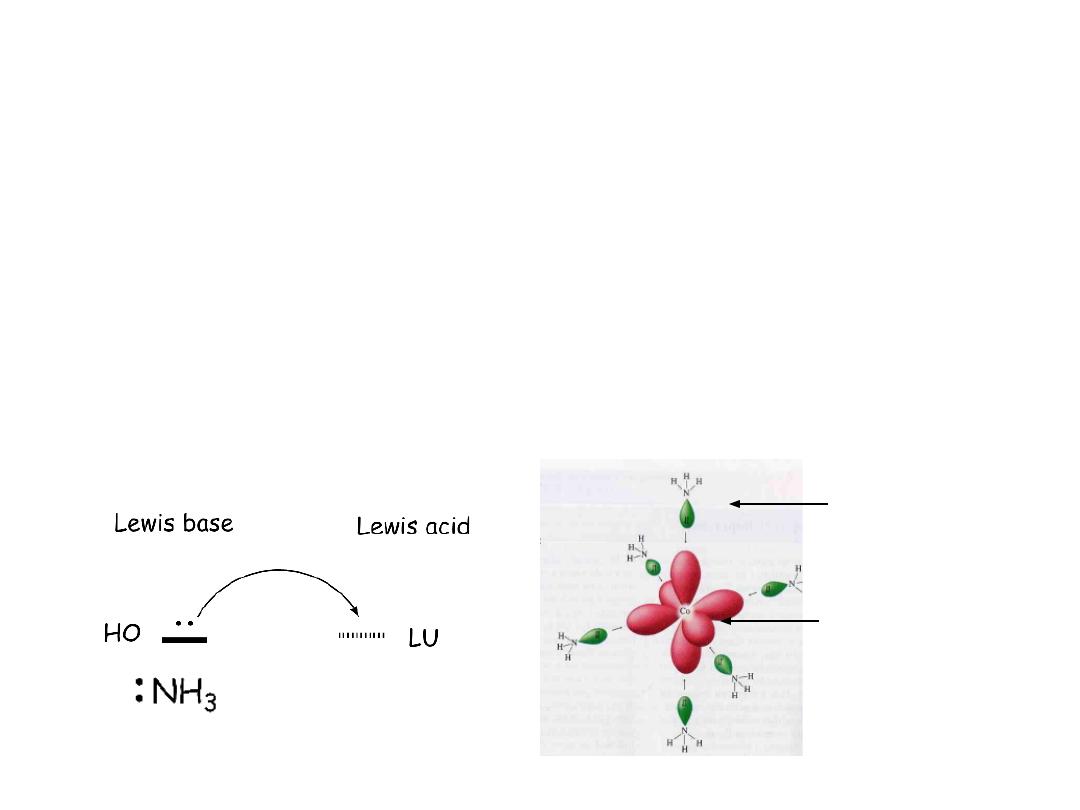
The basic idea is that the ligand (Lewis base) is providing electron
density to the metal (Lewis acid)
In terms of theory we visualize the coordination as the transfer of
electrons from the orbital of the Lewis base to the lowest unoccupied
orbital of the Lewis acid
Co
3+
The bond from ligand to metal is covalent (shared pair), but both
electrons come from the ligand (
coordinate covalent
bond)
Lewis
base
Lewis
acid
Prof.Dr. Amer A. Taqa
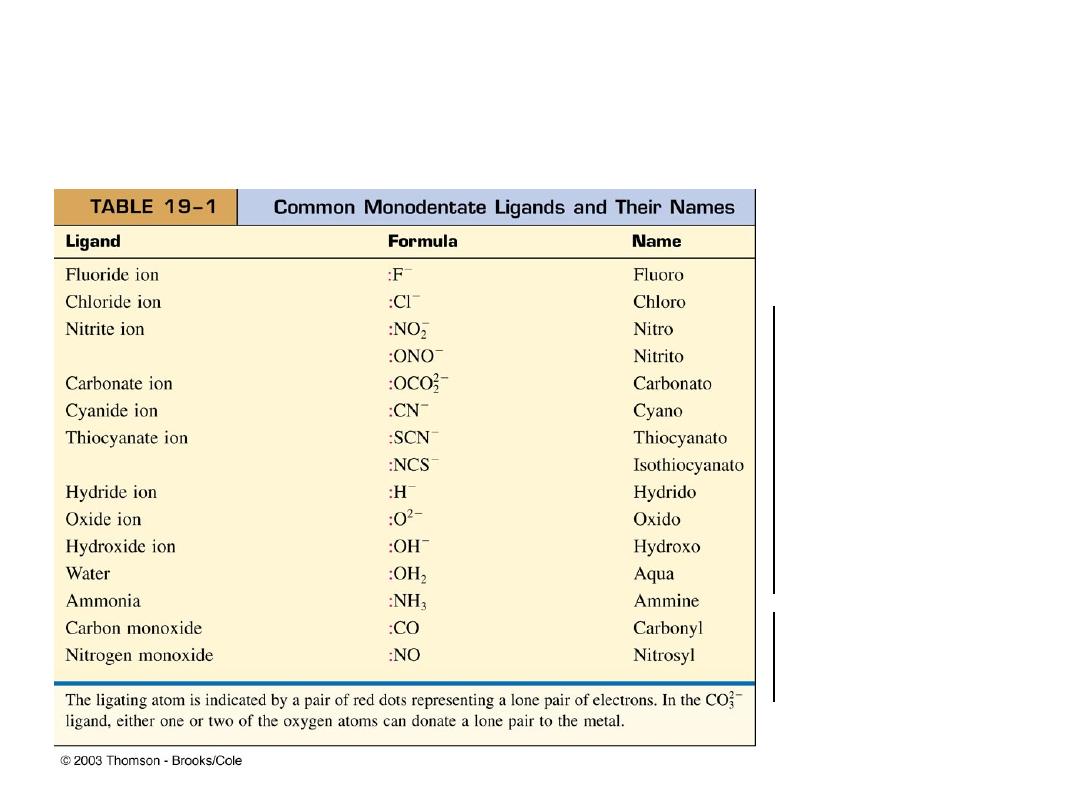
Types of Ligands (electron pair donors:
Monodentate (one tooth) Ligands
Latin: “mono” meaning one and “dens” meaning tooth
Anions
Molecules with
lone pairs
Prof.Dr. Amer A. Taqa
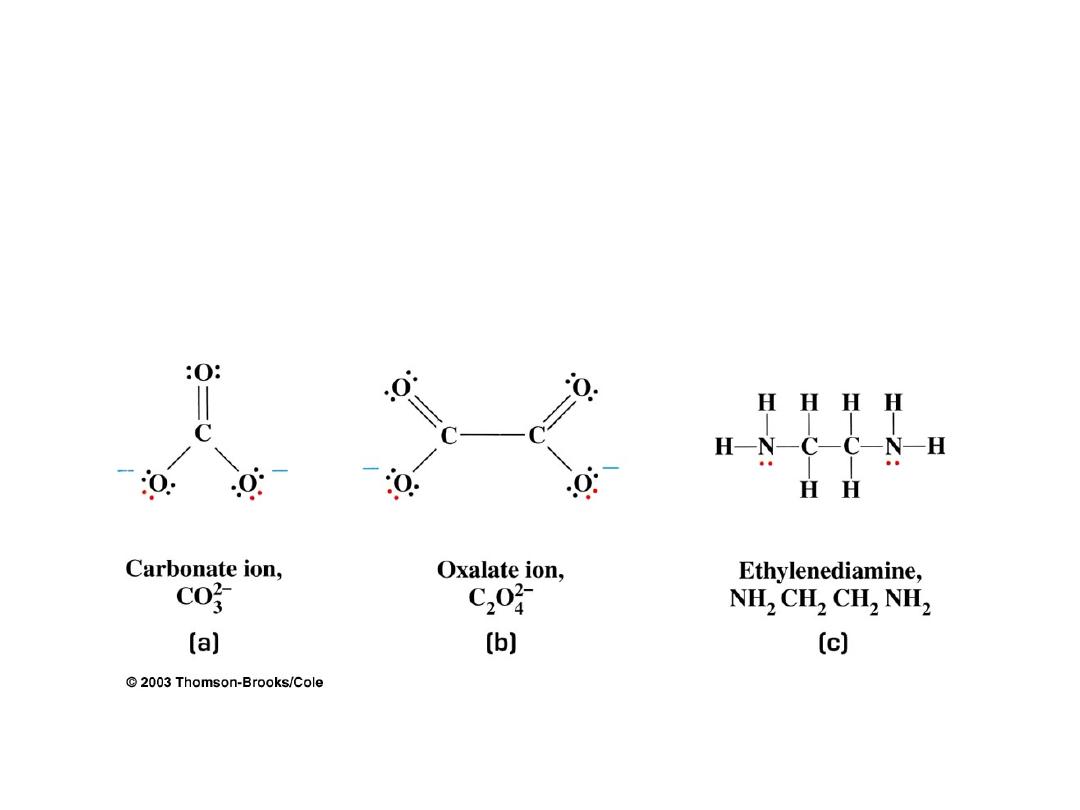
Types of Ligands: Bidentate (two tooth) Ligands
Some common bidentate (chelates):
(
en
)
Prof.Dr. Amer A. Taqa
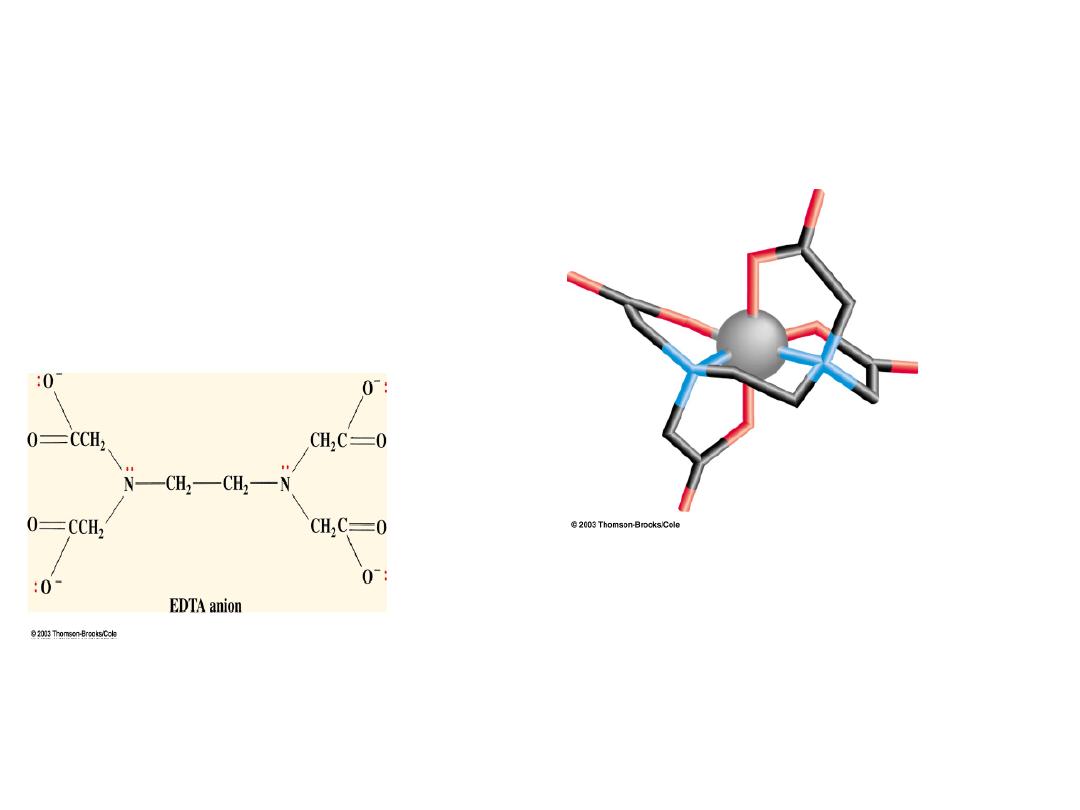
Types of Ligands: Ethylenediaminetetraacetate ion (EDTA): a polydentate
chelating
ligand
Chelate from
Greek
chela, “claw”
EDTA wraps around the metal ion at
all 6 coordination sites producing an
exceedingly tight binding to the
metal
Prof.Dr. Amer A. Taqa

Alfred Werner
Switzerland
University of Zurich
Zurich, Switzerland
b. 1866
(in Mulhouse, then Germany)
d. 1919
The Nobel Prize in Chemistry 1913
"in recognition of his work on the
linkage of atoms in molecules by which
he has thrown new light on earlier
investigations and opened up new
fields of research especially in
inorganic chemistry"
Alfred Werner: the father of the
structure of coordination complexes
Prof.Dr. Amer A. Taqa
Prof.Dr. Amer A. Taqa
Prof.Dr. Amer A. Taqa
Prof.Dr. Amer A. Taqa
Prof.Dr. Amer A. Taqa
(2) Brackets
[]
are used to indicate all of the atomic composition of the
coordinate complex: the
central metal atom
and the
ligands
. The symbol
for the
central metal atom
of the complex is first within the brackets
(3)
Species
outside of the
[]
are not coordinated to the metal but are
require to maintain a charge balance
(1) A coordination compounds is a
neutral
species consisting of a
coordinate complex and uncoordinated ions required to maintain the
charge balance
[
Co
(NH
3
)
6
]
Cl
3
Composition of complex
Free species
[
Co
(NH
3
)
6
]
3+
3 Cl
-
Prof.Dr. Amer A. Taqa

Werner‛s explanation of coordination complexes
Metal ions exhibit
two
kinds of valence:
primary
and
secondary
valences
The
primary
valence is the oxidation number (positive charge) of the
metal (usually 2+ or 3+)
The
secondary
valence is the number of atoms that are directly bonded
(coordinated) to the metal
The secondary valence is also termed the “coordination number” of the
metal in a coordination complex
Prof.Dr. Amer A. Taqa

Exemplar of primary and secondary valence: [Co(NH
3
)
6
]Cl
3
[Co(NH
3
)
6
]
3+
What is the atomic composition
of the complex?
What is the net charge of the
complex?
[Co(NH
3
)
6
]Cl
3
How do we know the charge is 3+ on
the metal?
3+ is required to balance the three
Cl
-
ions
The secondary valence of [Co(NH
3
)
6
]Cl
3
is
The primary valence of [Co(NH
3
)
6
]Cl
3
is
3 (charge on Co
3+
)
6 (six ligands)
Prof.Dr. Amer A. Taqa

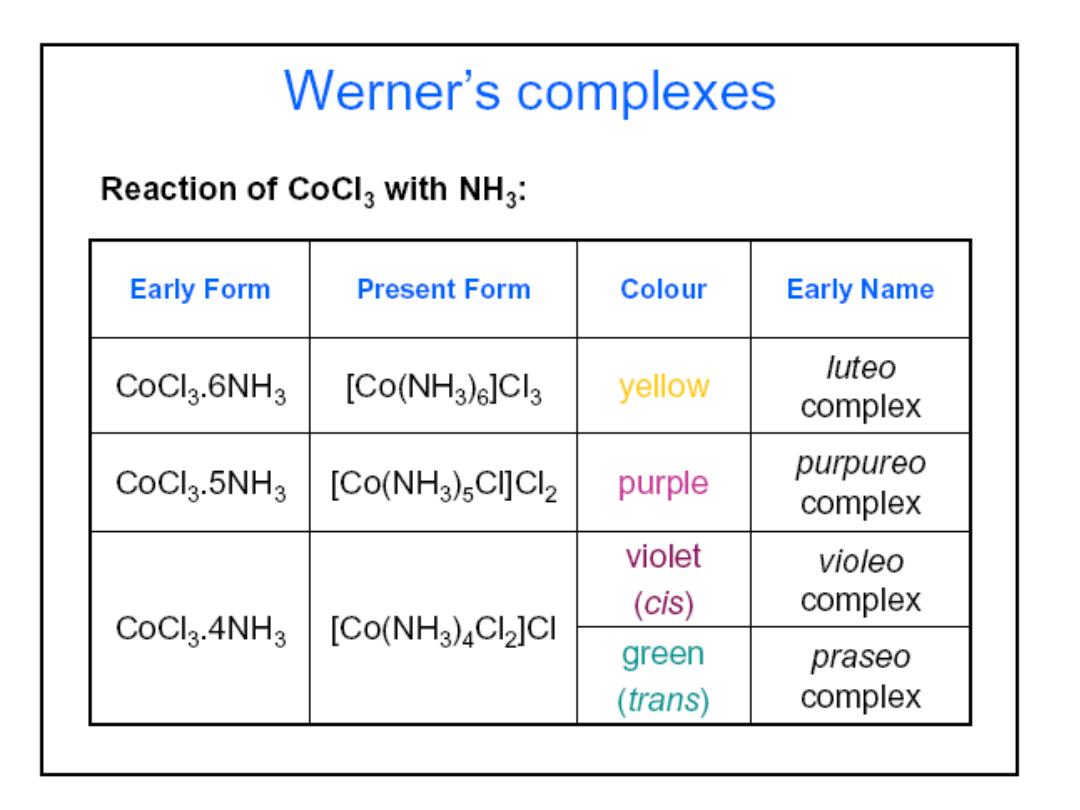
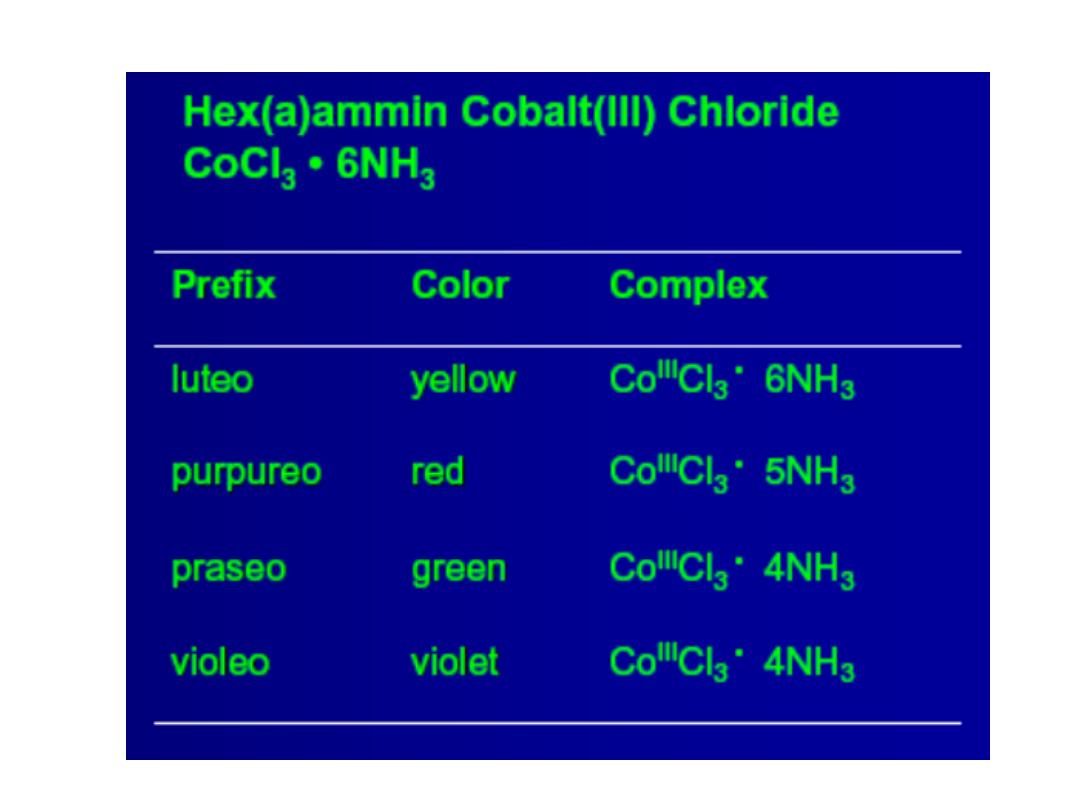
Structures of Coordination Complexes: The ammonia
complexes of Co(III) = Co
3+
CoCl
3
.
6NH
3
CoCl
3
.
5NH
3
CoCl
3
.
4NH
3
CoCl
3
.
3NH
3
In all of these complexes there are no free NH
3
molecules
(No reaction with acid)
3 “free” Cl
-
ions
Orange-Yellow
2 “free” Cl
-
ions
Purple
1 “free” Cl
-
ions
Green
0 “free” Cl
-
ions
Green
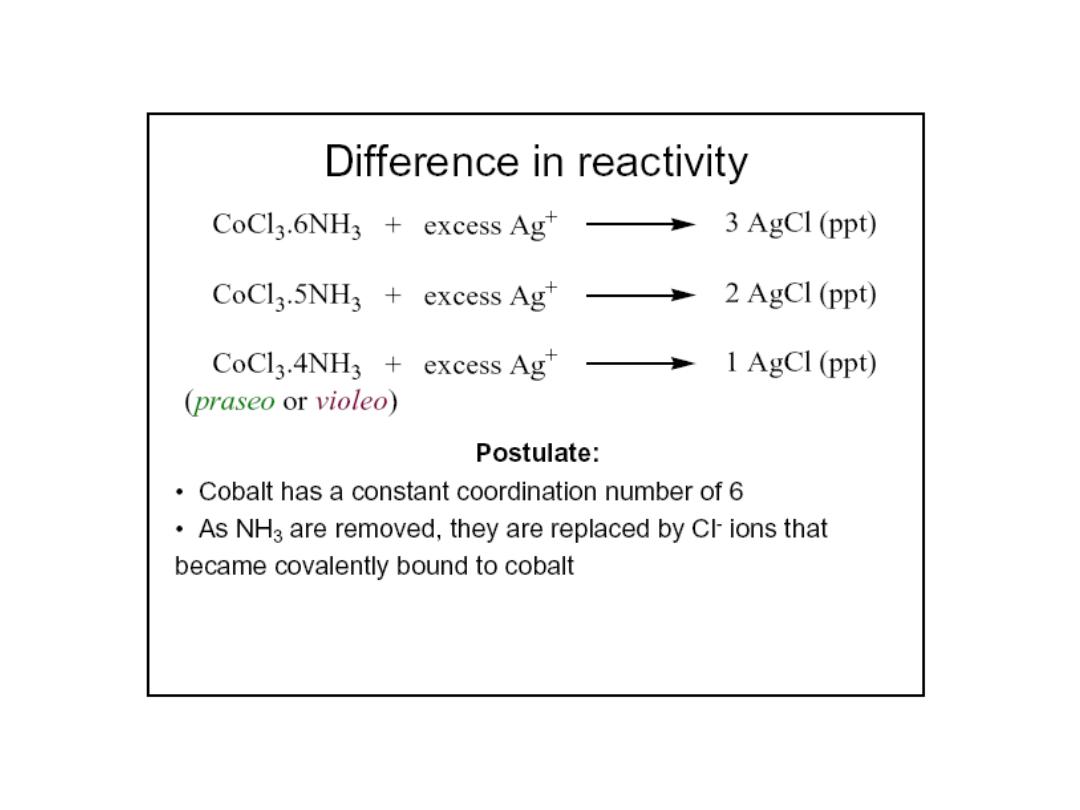
How did Werner deduce the structure of coordination complexes?
Ions
released
Compositio
n
Colo
r
Prof.Dr. Amer A. Taqa

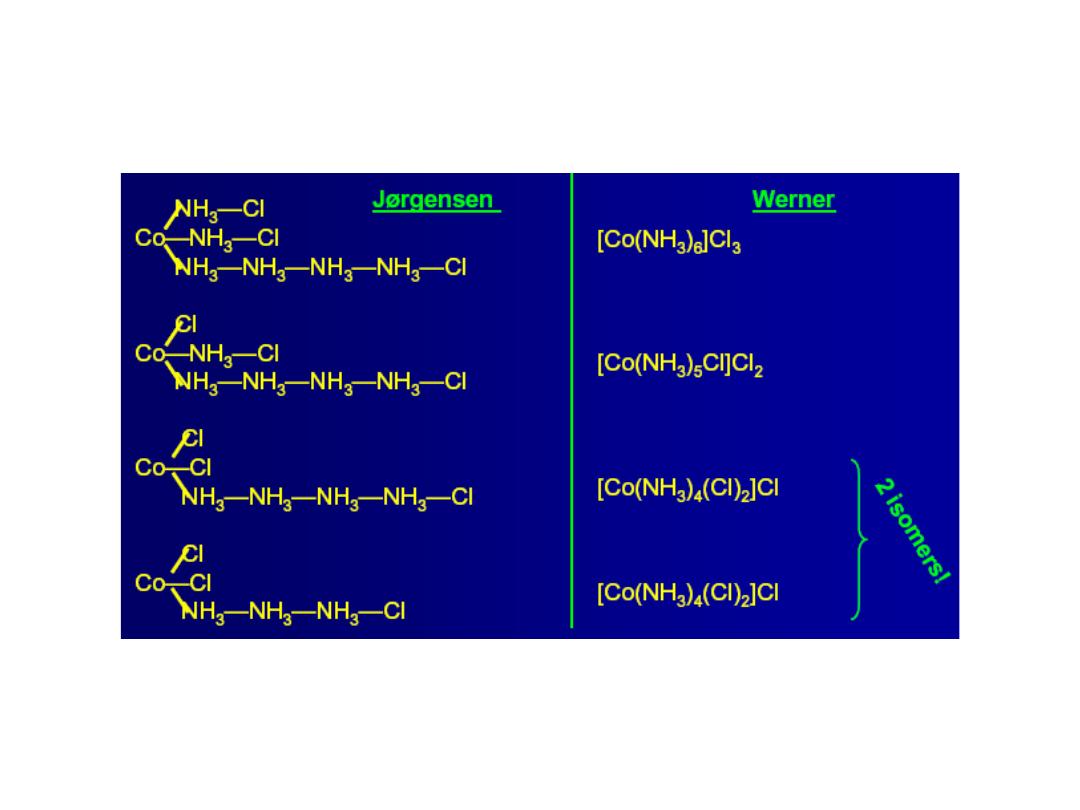
Compound 1:
CoCl
3
.
6NH
3
= [Co(NH
3
)
6
]
3+
(Cl
-
)
3
= [Co(NH
3
)
6
](Cl)
3
Conclude: 3 free Cl
-
ions,
complex = [Co(NH
3
)
6
]
3+
Compound 2:
CoCl
3
.
5NH
3
= [Co(NH
3
)
5
Cl]
2+
(Cl
-
)
2
= [Co(NH
3
)
5
Cl](Cl)
2
Conclude: 2 free Cl
-
ions,
complex = [Co(NH
3
)
5
Cl]
2+
Compound 3:
CoCl
3
.
4NH
3
= [Co(NH
3
)
4
Cl
2
]
1+
(Cl
-
) = [Co(NH
3
)
4
Cl
2
](Cl)
Conclude: 1 free Cl
-
ion,
complex = [Co(NH
3
)
4
Cl
2
]
1+
Compound 4:
CoCl
3
.
3NH
3
=
[Co(NH
3
)
3
Cl
3
] = complex
No free Cl
-
ions, both Cl
-
and NH
3
in sphere
“free” Cl
-
is not in sphere; all NH
3
molecules are is in sphere
Prof.Dr. Amer A. Taqa
Prof.Dr. Amer A. Taqa
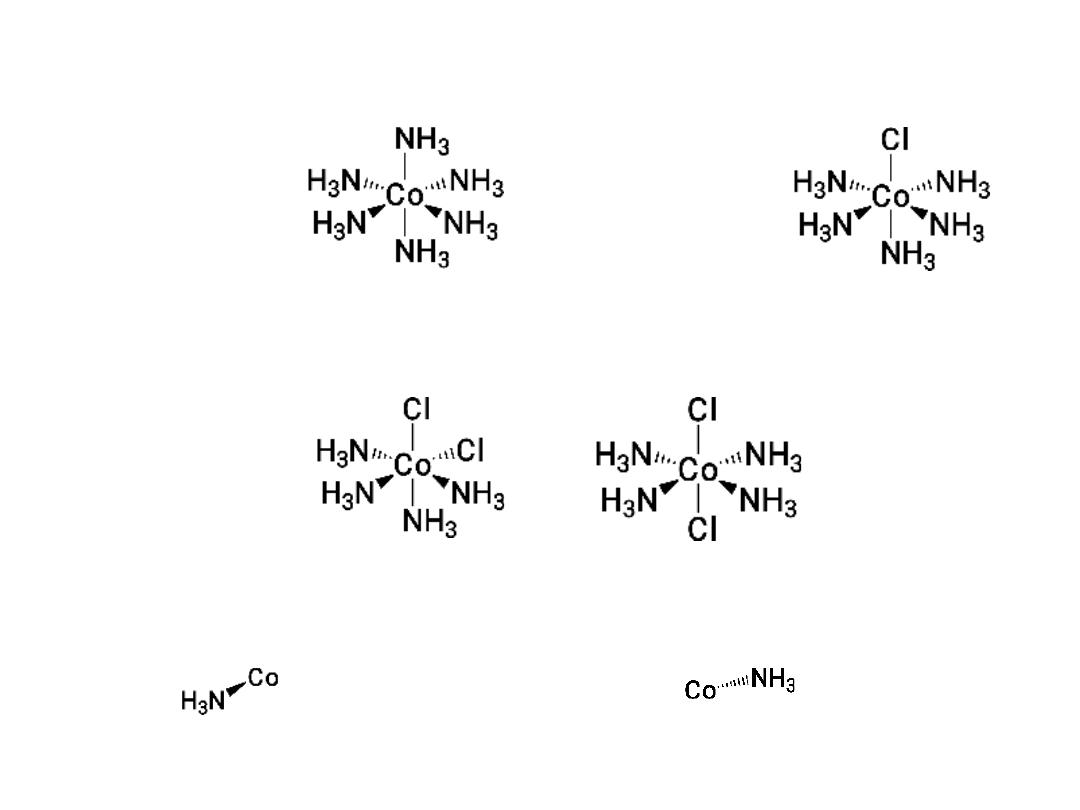
CoCl
3
.
6NH
3
CoCl
3
.
5NH
3
CoCl
3
.
4NH
3
Isomers!
Coordination complexes: Three dimensional structures
Cl
-
Cl
-
Cl
-
Cl
-
Cl
-
Cl
-
Cl
-
Bond toward
you
Bond away from
you
Prof.Dr. Amer A. Taqa
Prof.Dr. Amer A. Taqa
Prof.Dr. Amer A. Taqa
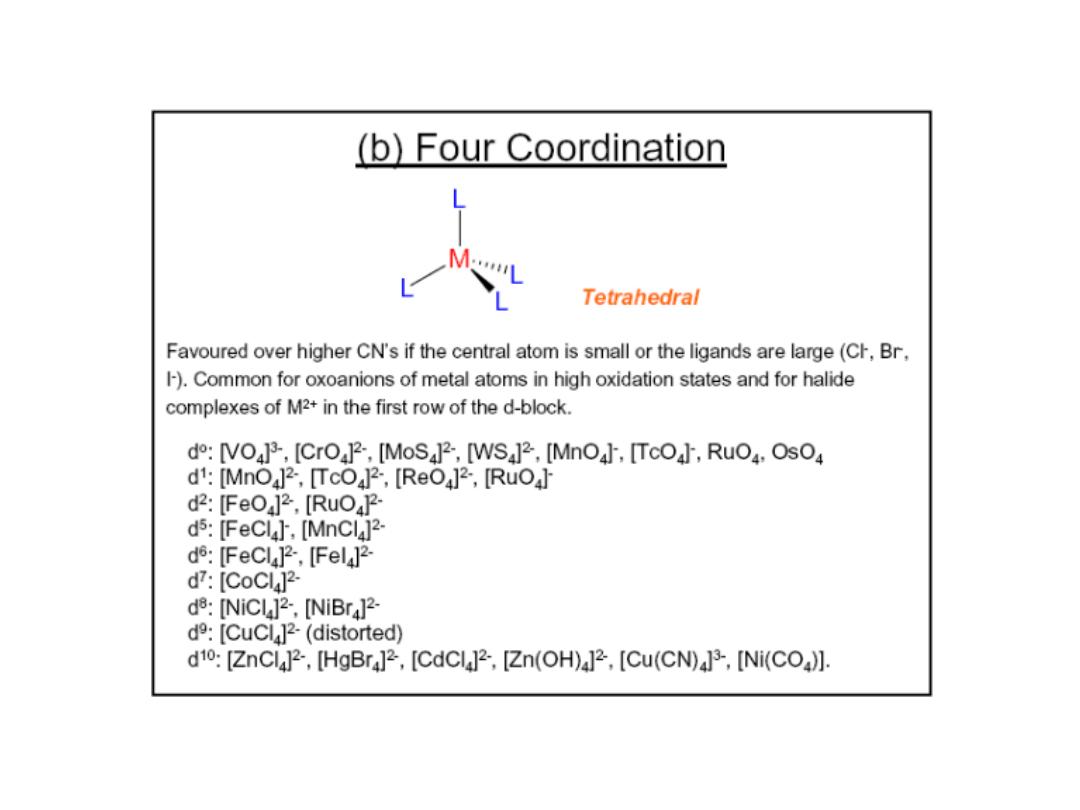
Prof.Dr. Amer A. Taqa
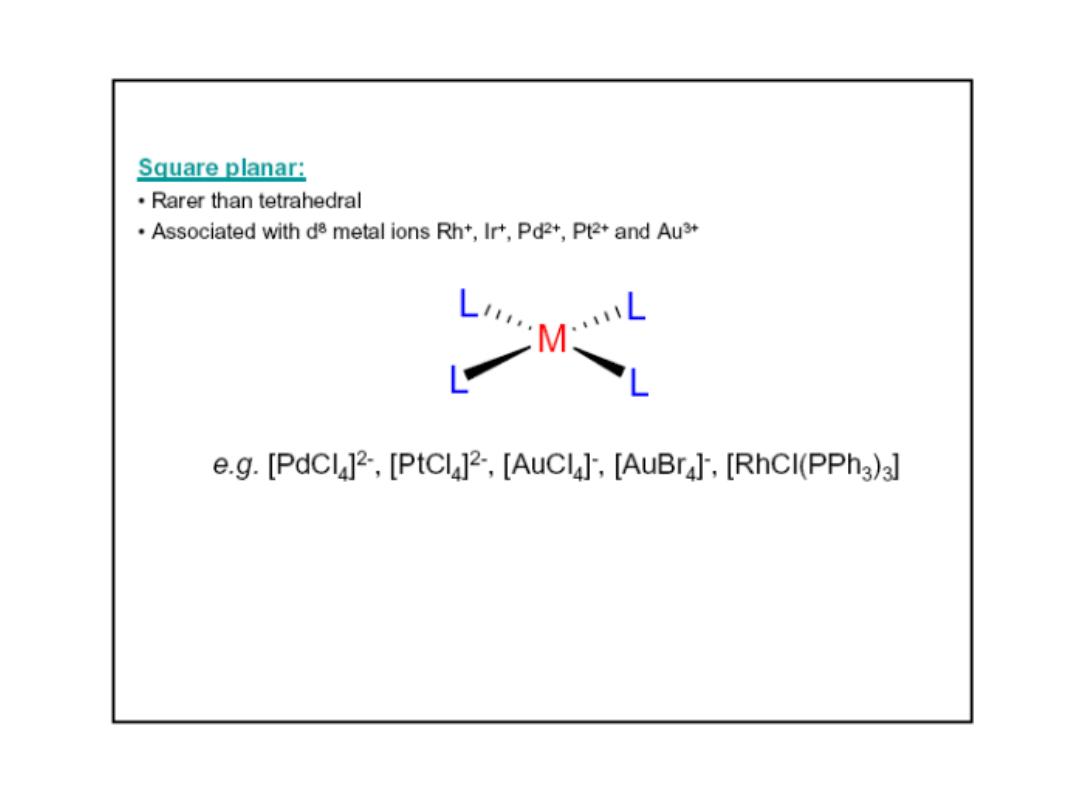
Prof.Dr. Amer A. Taqa
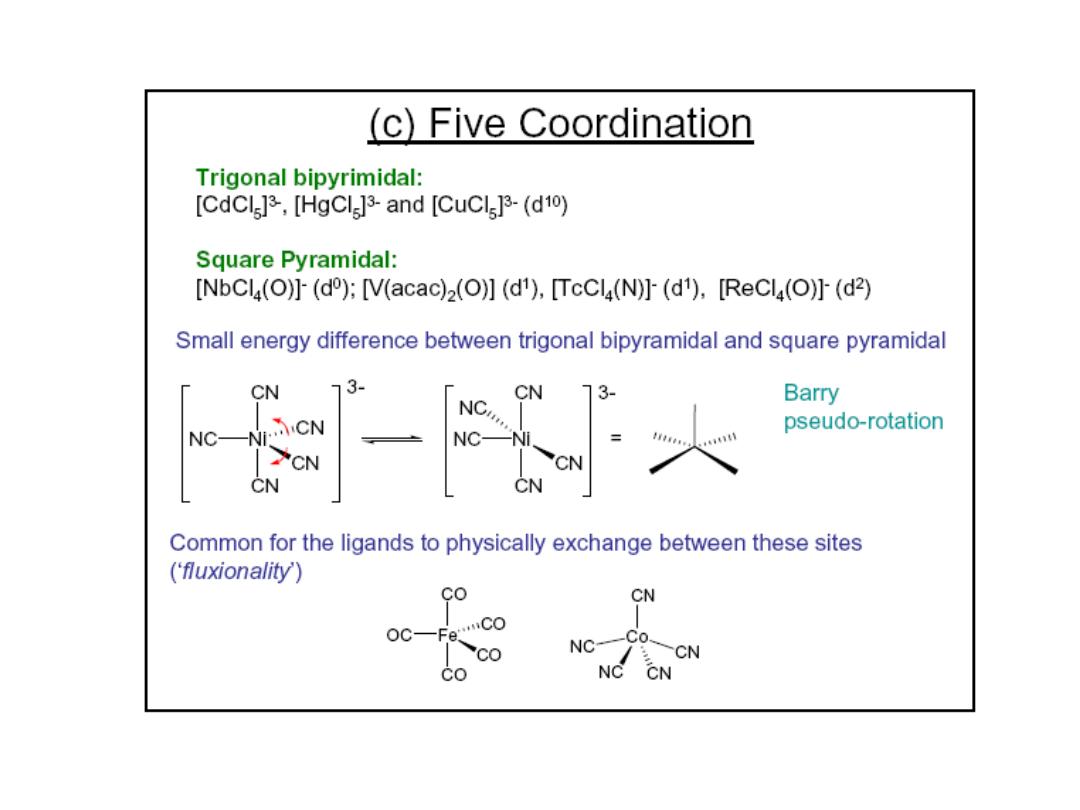
Prof.Dr. Amer A. Taqa
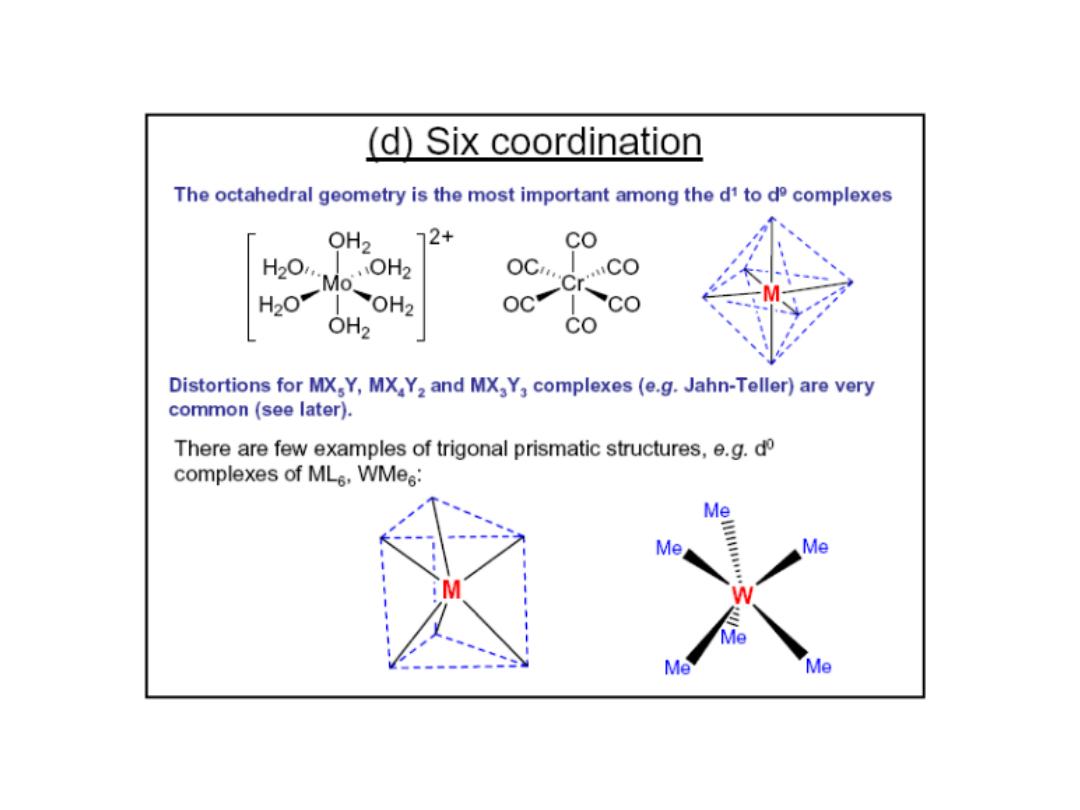
Prof.Dr. Amer A. Taqa
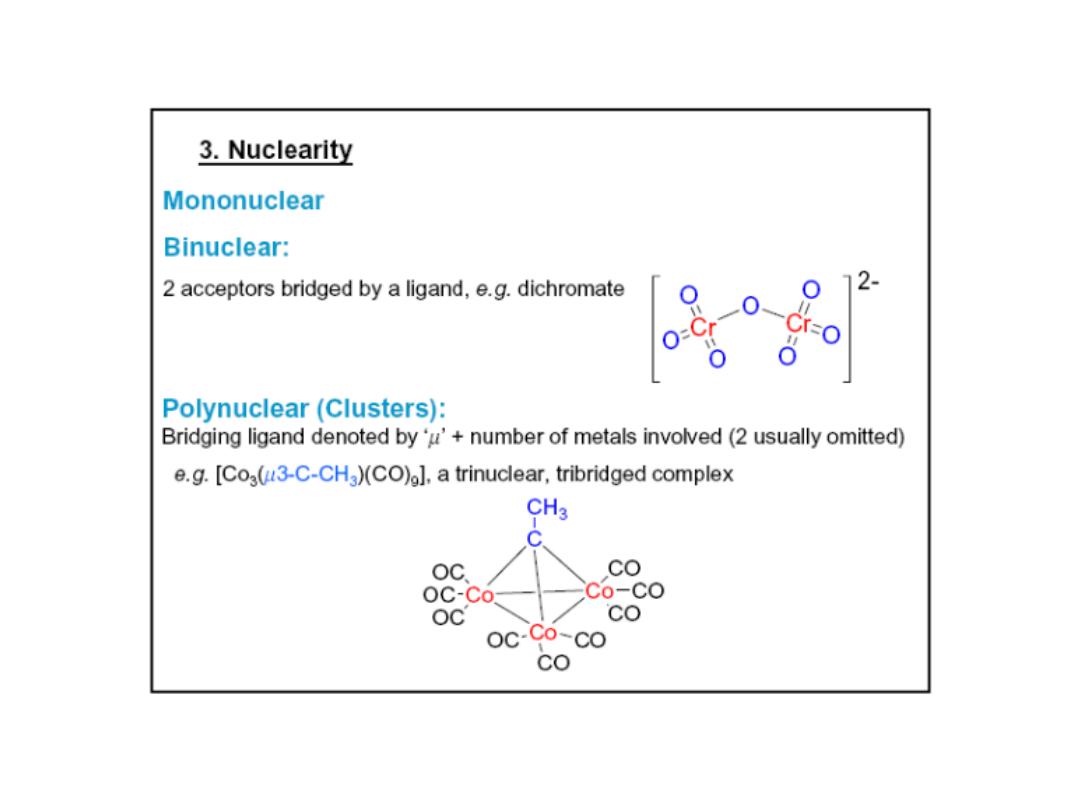
Prof.Dr. Amer A. Taqa
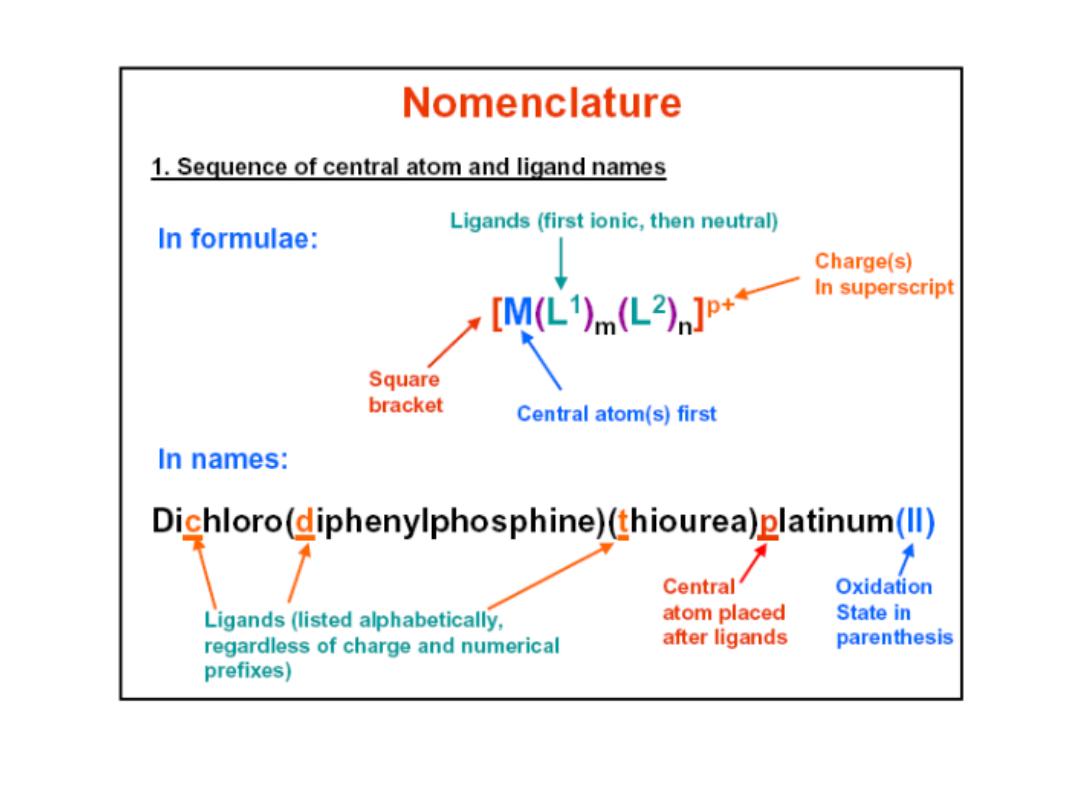
Prof.Dr. Amer A. Taqa
Prof.Dr. Amer A. Taqa

Prof.Dr. Amer A. Taqa
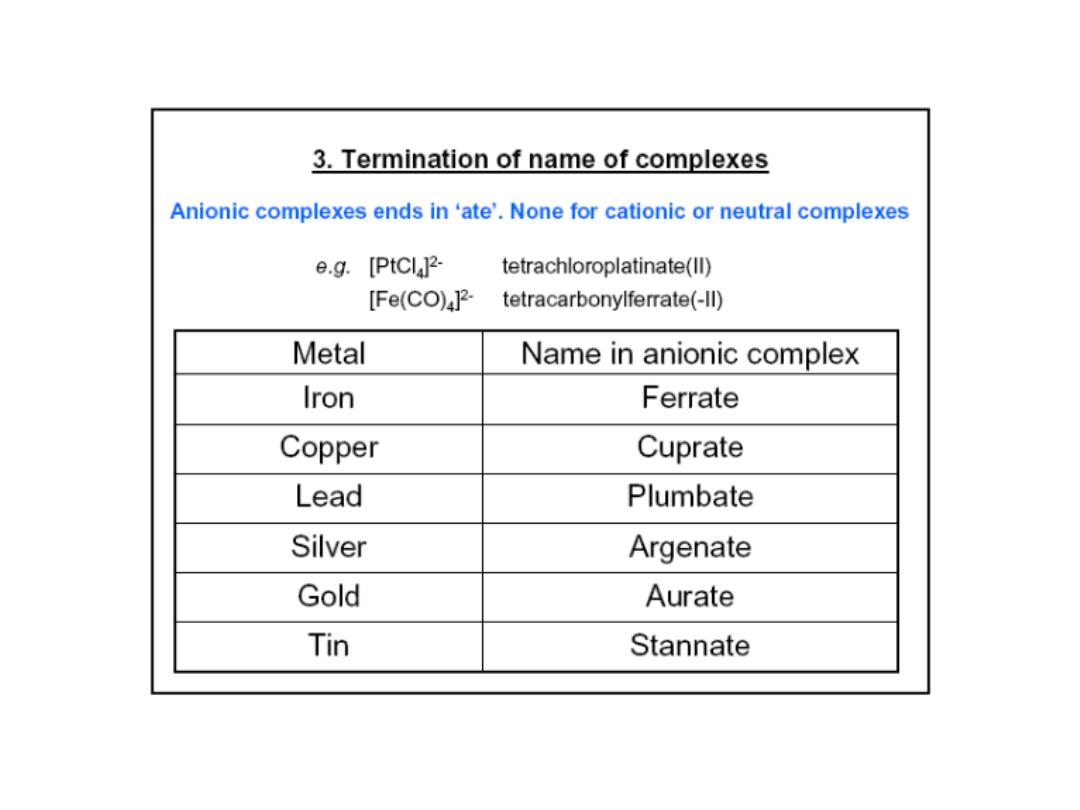
Prof.Dr. Amer A. Taqa

Prof.Dr. Amer A. Taqa

Coordination Compound
• Consist of a
complex ion
and necessary
counter
ions
•
[Co(NH
3
)
5
Cl]Cl
2
•
• Complex ion:
[Co(NH
3
)
5
Cl]
2+
•
Co
3+
+ 5 NH
3
+ Cl
‐
•
=
1(3+) + 5 (0) + 1(1‐)
•
= 2+
• Counter ions:
2 Cl
‐
Prof.Dr. Amer A. Taqa

K
3
[Fe(CN)
6
]
K
2
[PtCl
4
]
Na
2
[Fe(CO)
4
]
[Co(H
2
O)
2
(NH
3
)
4
]Cl
3
[Ni(H
2
O)(NH
3
)
4
]SO
4
Na
2
[OsCl
5
N]
[CoCl(NO
2
)(NH
3
)
4
]Cl
[CoCl(NH
2
)(en)
2
]NO
3
[FeH(CO)
3
(NO)]
[PtCl(NH
2
CH
3
)(NH
3
)]Cl
NOW some for you to try!!!
Potassium hexacyanoferrate(III)
Potassium tetrachloroplatinate(II)
Sodium Tetracarbonylferrate(II)
Tetraammindiaquacobalt(III) chloride
Tetraaminediaquanickel(II) sulfate
Sodium pentachloronitridoosmate(VI)
Tetraaminechloronitritocobalt(III) chloride
Amidochlorobis(ethylenediamine)cobalt(III) chloride
Tricarbonylhydridonitrosyliron(I) ?(II)??
Amminchloro(methylamine) platinum(II) chloride
Prof.Dr. Amer A. Taqa

[V(H
2
O)
6
]
2+
[V(H
2
O)
6
]
3+
[Cr(NH
3
)
6
]
3+
[Cr(NH
3
)
5
Cl]
2+s
Prof.Dr. Amer A. Taqa
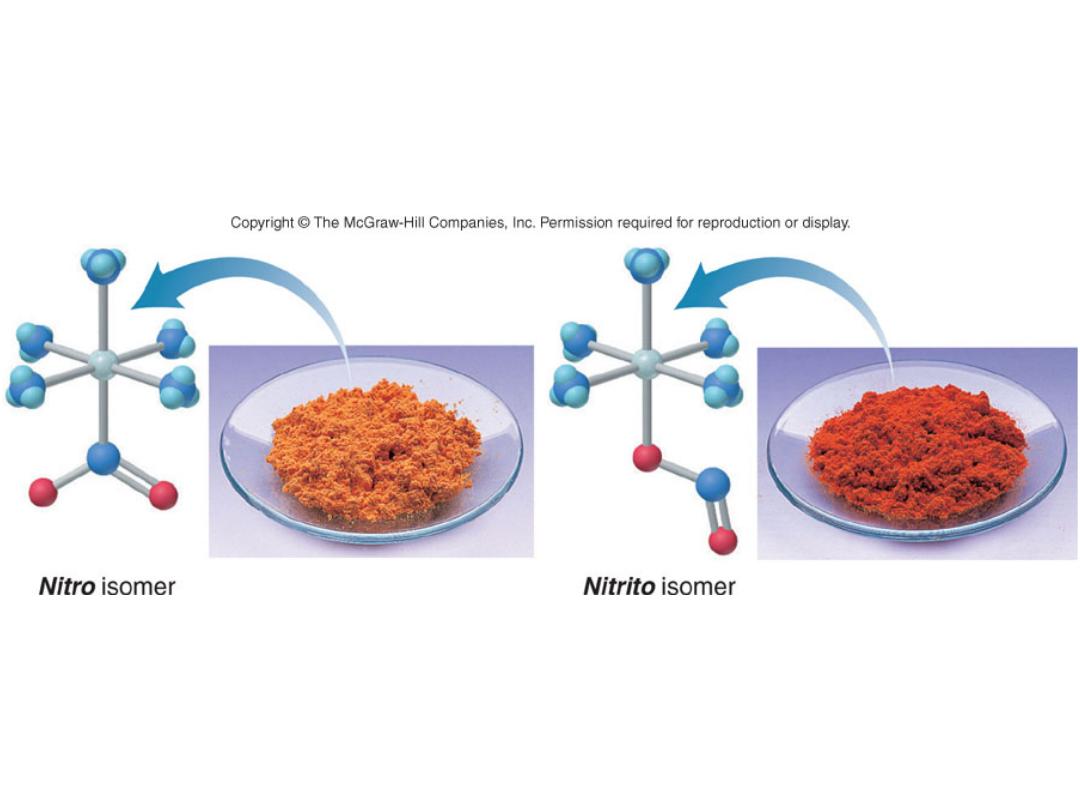
Linkage Isomers
[Co(NH
3
)
5
(NO
2
)]Cl
2
Pentaamminenitrocobalt(III)
chloride
[Co(NH
3
)
5
(ONO)]Cl
2
Pentaamminenitritocobalt(III)
chloride
Prof.Dr. Amer A. Taqa
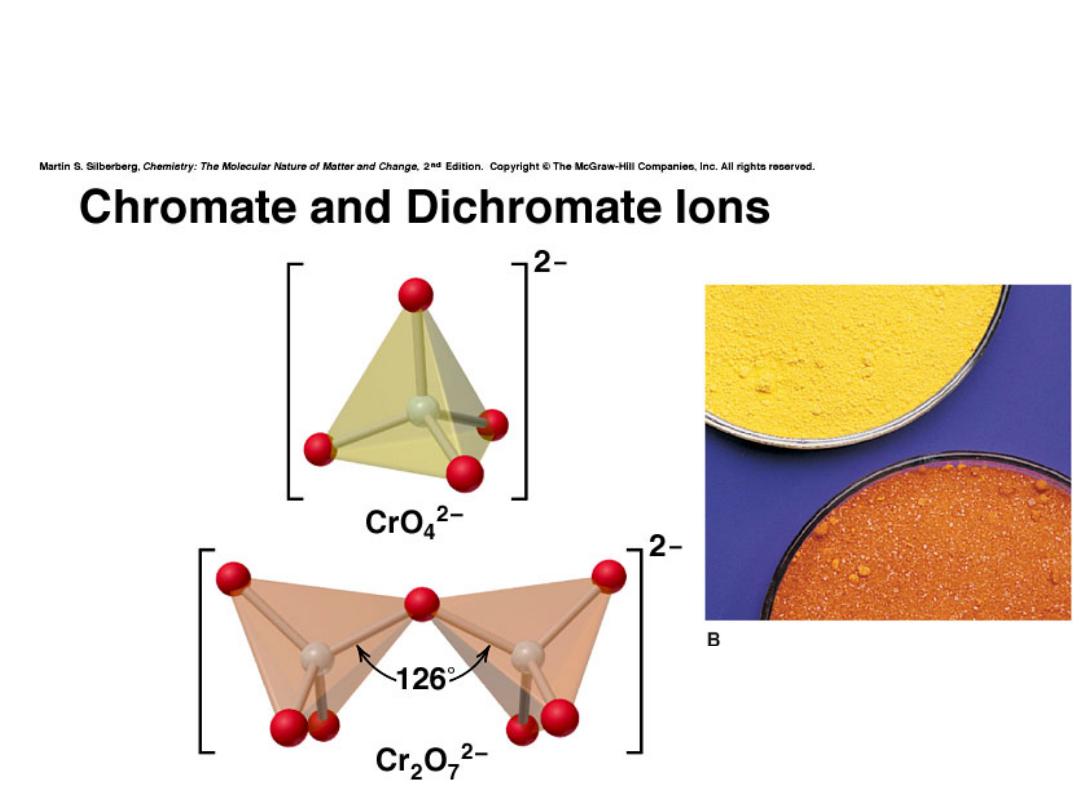
Chromium
Chemical properties reflect oxidation state
Prof.Dr. Amer A. Taqa
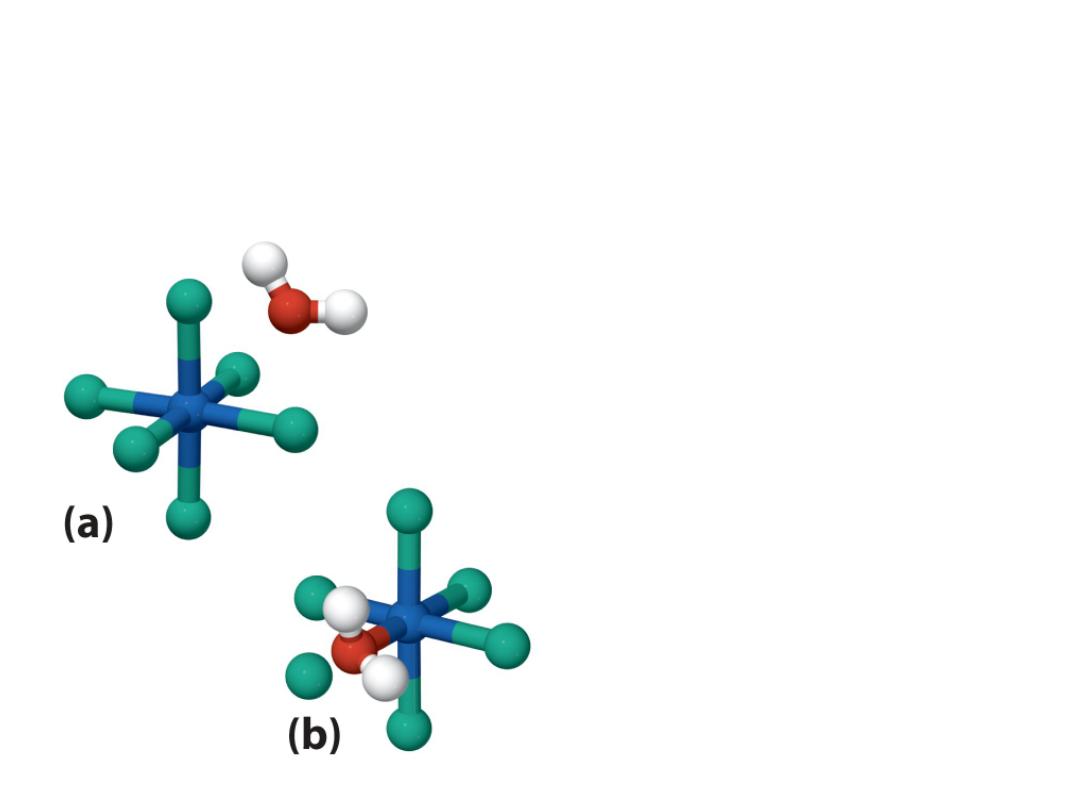
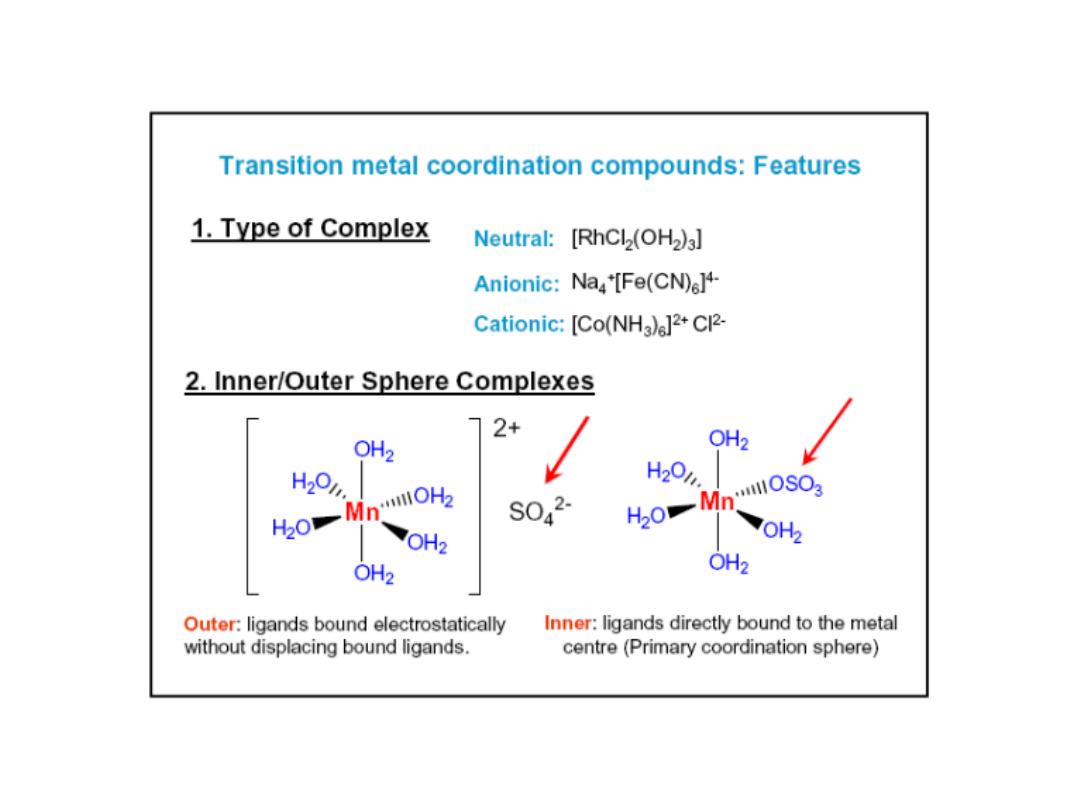
Water in outer sphere (water that is
part of solvent)
Water in the inner sphere
water (water is a ligand in
the coordination sphere of
the metal)
Hydrate isomers:
Prof.Dr. Amer A. Taqa
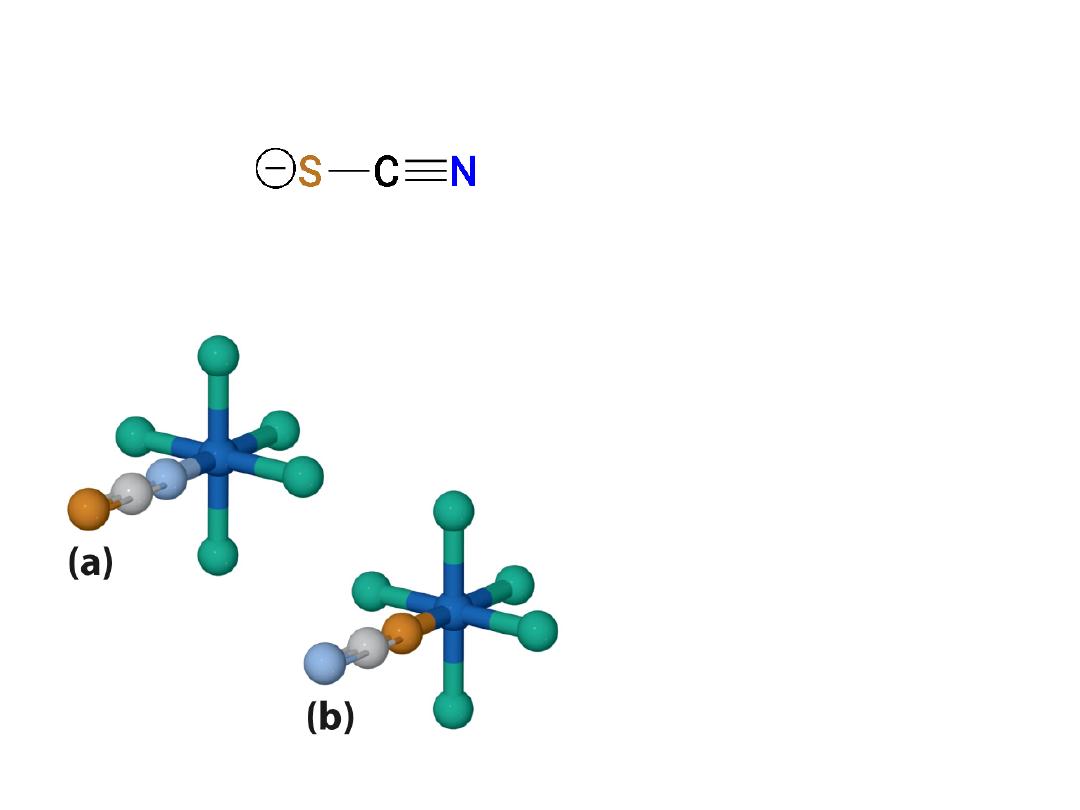
Linkage isomers
Bonding to metal may occur at the
S
or
the
N
atom
Example:
Bonding occurs from
N
atom to metal
Bonding occurs from
S
atom to metal
Prof.Dr. Amer A. Taqa
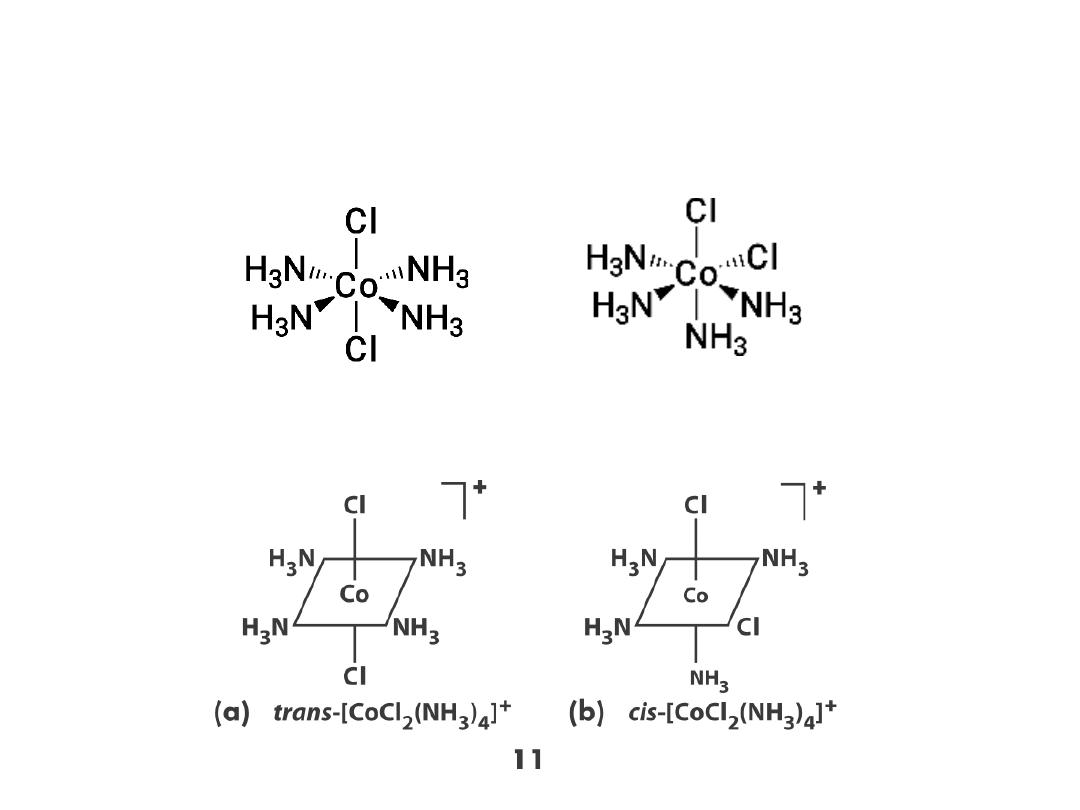
Stereoisomers: geometric isomers (cis and trans)
Cl
-
Cl
‐
Prof.Dr. Amer A. Taqa
