Peripheral neuropathy
Peripheral Neuropathy:
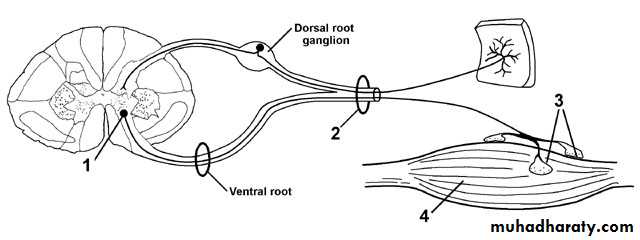
peripheral nerves are composed of sensory, motor, and autonomic elements.Diseases can affect the cell body of a neuron or its peripheral processes, namely the axons or the encasing myelin sheaths.
Most peripheral nerves are mixed and contain sensory and motor as well as autonomic fibers. Thus, peripheral neuropathies can impair sensory, motor, or autonomic function, either singly or in combination.
MONONEURITIS SIMPLEX
This term signifies involvement of a single peripheral nerve.MONONEURITIS MULTIPLEX
Several individual nerves are affected, usually at random and noncontiguous.
POLYNEUROPATHY
The term “polyneuropathy” denotes a disorder in which the function of numerous peripheral nerves is affected at the same time. This leads to a predominantly distal and symmetric deficit, with loss of tendon reflexes except when small fibers are selectively involved.
Approach to Neuropathic Disorders
Is it Motor, sensory, autonomic, or combinations?Is it focal, symmetrical or asymmetrical?
Is it Acute (days to 4 weeks), Subacute (4 to 8 weeks) or Chronic (>8 weeks)?
Is there evidence for a hereditary neuropathy (family history)
Are there any associated medical conditions (DM, cancer, autoimmune, connective tissue)?
Drug history.
Causes of peripheral neuropathy
Idiopathic inflammatory neuropathies• Acute idiopathic polyneuropathy (Guillain-Barré syndrome)
• Chronic inflammatory demyelinating polyneuropathy.(CIDP)
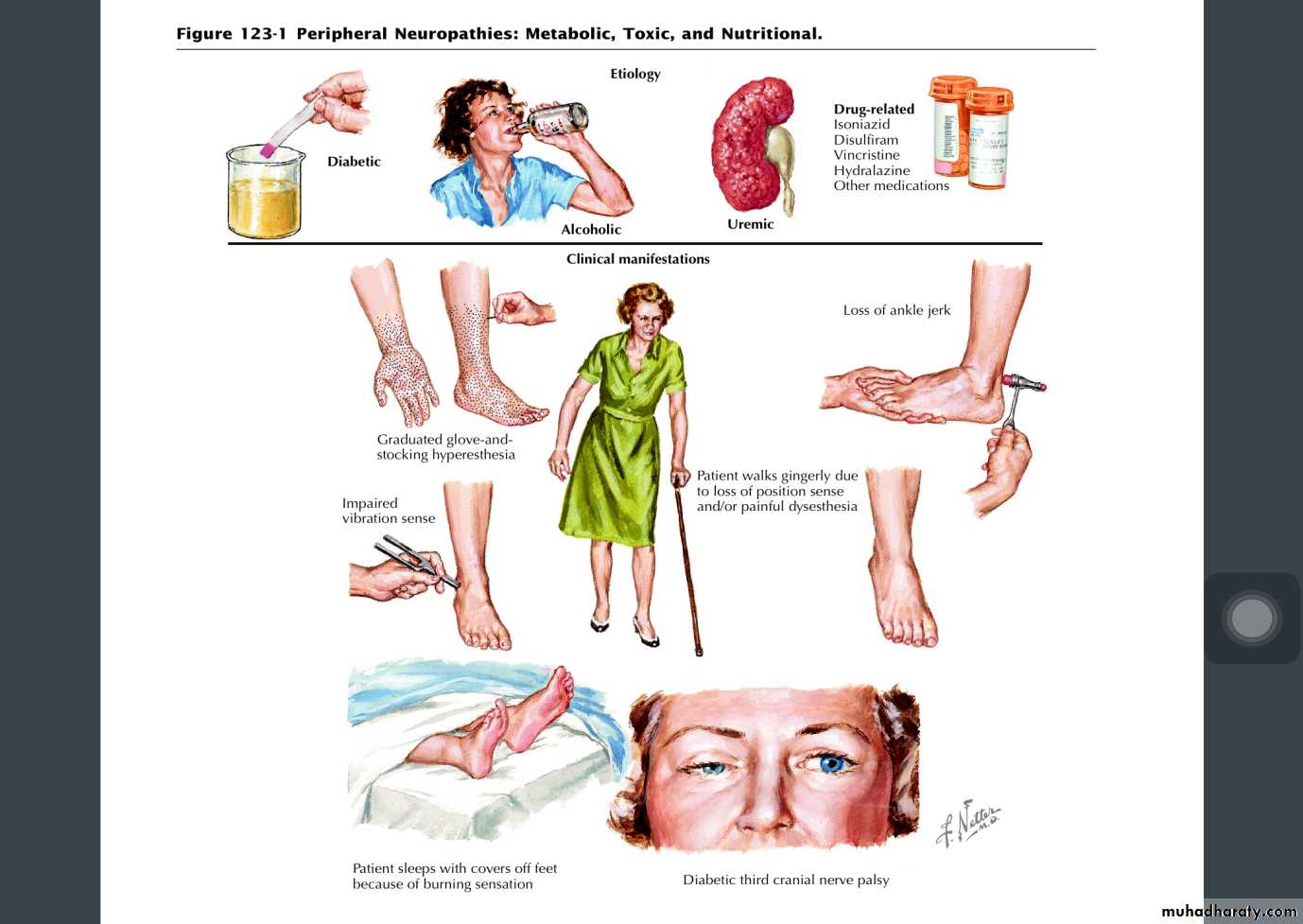
Metabolic and nutritional neuropathies
• Diabetes
• Hypothyroidism
• Vitamin B12 deficiency
Infective and granulomatous neuropathies
AIDS, leprosy, diphtheria, sarcoidosis and sepsis.
Vasculitis neuropathies
• Polyarteritis nodosa
• Rheumatoid arthritis
• SLE
Neoplastic and paraproteinemic neuropathies
• Compression and infiltration by tumor
Paraneoplastic syndromes
Drug ,alcohol and toxin induced neuropathies
Examples of drugs
Pyridoxine
Metronidazole
Dapsone
Isoniazid
Example of toxin:
Organophosphates
Heavy metal
Lead, Thallium, arsenic
Hereditary neuropathies
Friedreich ataxia, CMT (Charcot-Marie-Tooth).
compressive neuropathy:
E.g.: carpel tunnel syndrome
IDIOPATHIC INFLAMMATORY NEUROPATHIES
Acute Idiopathic Polyneuropathy (Guillain-Barré Syndrome)GBS is an acute or subacute polyneuropathy that can follow minor infective illnesses, ,vaccination ,surgical procedures, or may occur without obvious precipitants
Clinical and epidemiologic evidence suggests an association with preceding infection such as Campylobacter jejuni, Cytomegalovirus , Mycoplasma pneumonia, Epstein–Barr virus etc..
Its precise cause is unclear, but it appears to have an immunologic basis. Both demyelinating and axonal forms have been recognized, with distinctive clinical and electrophysiological features, The demyelinated form is more common.
CLINICAL FEATURES
Diagnostic criteria for Guillain-Barré syndrome
• Required for diagnosis
Progressive weakness of more than one limb
Distal areflexia with proximal areflexia or hyporeflexia
• Supportive of diagnosis
Progression for up to 4 weeks
Recovery beginning within 4 weeks after progression stops
Relatively symmetric deficits
Mild sensory involvement
Cranial nerve (especially VII) involvement
Autonomic dysfunction
No fever at onset
Increased CSF protein after 1 weeks
CSF white blood cell count ≤10/mL
Nerve conduction study show slowing or block by several weeks
Against diagnosis
Markedly asymmetric weaknessBowel or bladder dysfunction (at onset or persistent)
CSF white blood cell count >50 .
Well-demarcated sensory level.
Excluding diagnosis
Isolated sensory involvement
Investigative studies.
The cerebrospinal fluid (CSF) often shows a characteristic abnormality, with increased protein concentration but a normal cell count (cytoalbumino dissociation); abnormalities may not be found in the first week.
Electrophysiological studies may reveal marked slowing of motor and sensory conduction velocity.
TREATMENT
Plasmapheresis appears to reduce the time required for recovery and may decrease the likelihood of residual neurologic deficits and need for ventilation a course of plasmapheresis usually consists of 40–50 mL/kg plasma exchange (PE) four to five times.It is best instituted early, and it is indicated especially in patients with a severe or rapidly progressive deficit or respiratory compromise.
Intravenous immunoglobulin (400 mg/kg/d for 5 days) appears to be equally effective and should be used in preference to plasmapheresis in adults with cardiovascular instability and in children;
the two therapies are not additive.
no role for steroid.
In the worsening phase of GBS, most patients require monitoring in a critical care setting, with particular attention to
Vital capacity
Heart rhythm
Blood pressure
Deep vein thrombosis prophylaxis like heparin and compressive stokes
Cardiovascular status monitoring, and chest physiotherapy.
As noted, 30% of patients with GBS require ventilator assistance.
Prognosis and Recovery
Approximately 70-75% of patients recover completely, 25% are left with mild neurologic deficits, and 5% die from respiratory and autonomic dysfunction.Chronic Inflammatory Demyelinating Polyneuropathy
CIDP is distinguished from GBS by its chronic course.
Onset is usually gradual over a few months or longer, but in a few cases the initial attack is indistinguishable from that of GBS.
An acute-onset form of CIDP should be considered when GBS deteriorates >9 weeks after onset or relapses at least three times.
Symptoms are both motor and sensory in most cases, in other respects, this neuropathy shares many features with the common demyelinating form of GBS.
Types of Diabetic Neuropathy
Peripheral Neuropathy.Proximal Neuropathy(diabetic amyotrophy).
Autonomic Neuropathy.
Focal Neuropathy.