1
Clostridium Species
The clostridia are large anaerobic, gram-positive, motile rods. Many species
decompose proteins or form toxins, and some do both. Their natural habitat is the soil
or the intestinal tract of animals and humans, where they live as saprophytes. Among
the pathogens are the organisms causing botulism, tetanus, gas gangrene, and
pseudomembranous colitis.
Morphology & Identification
Typical Organisms
Spores of clostridia are usually wider than the diameter of the rods in which they
are formed. In the various species, the spore is placed centrally, subterminally, or
terminally. Most species of clostridia are motile and possess peritrichous flagella.
Culture
Clostridia are anaerobes and grow under anaerobic conditions; a few species are
aero-tolerant and will also grow in ambient air.in general, the clostridia grow well on
the blood-enriched media used to grow anaerobes and on other media used to culture
anaerobes .
Colony Forms
Some clostridia produce large raised colonies (eg, C perfringens); others produce
smaller colonies (eg, C tetani). Some clostridia form colonies that spread on the agar
surface. Many clostridia produce a zone of hemolysis on blood agar. C perfringens
typically produces multiple zones of hemolysis around colonies.
Growth Characteristics
Clostridia can ferment a variety of sugars; many can digest proteins. Milk is turned
acid by some and digested by others and undergoes "stormy fermentation" (ie, clot
torn by gas) with a third group (eg, C perfringens). Various enzymes are produced by
different species .
Antigenic Characteristics
Clostridia share some antigens but also possess specific soluble antigens that permit
grouping by precipitin tests.
Clostridium Botulinum
Clostridium botulinum causes botulism is worldwide in distribution; it is found in
soil and occasionally in animal feces.
2
Types of C botulinum are distinguished by the antigenic type of toxin they produce.
Spores of the organism are highly resistant to heat, withstanding 100 °C for several
hours. Heat resistance is diminished at acid pH or high salt concentration.
Toxin
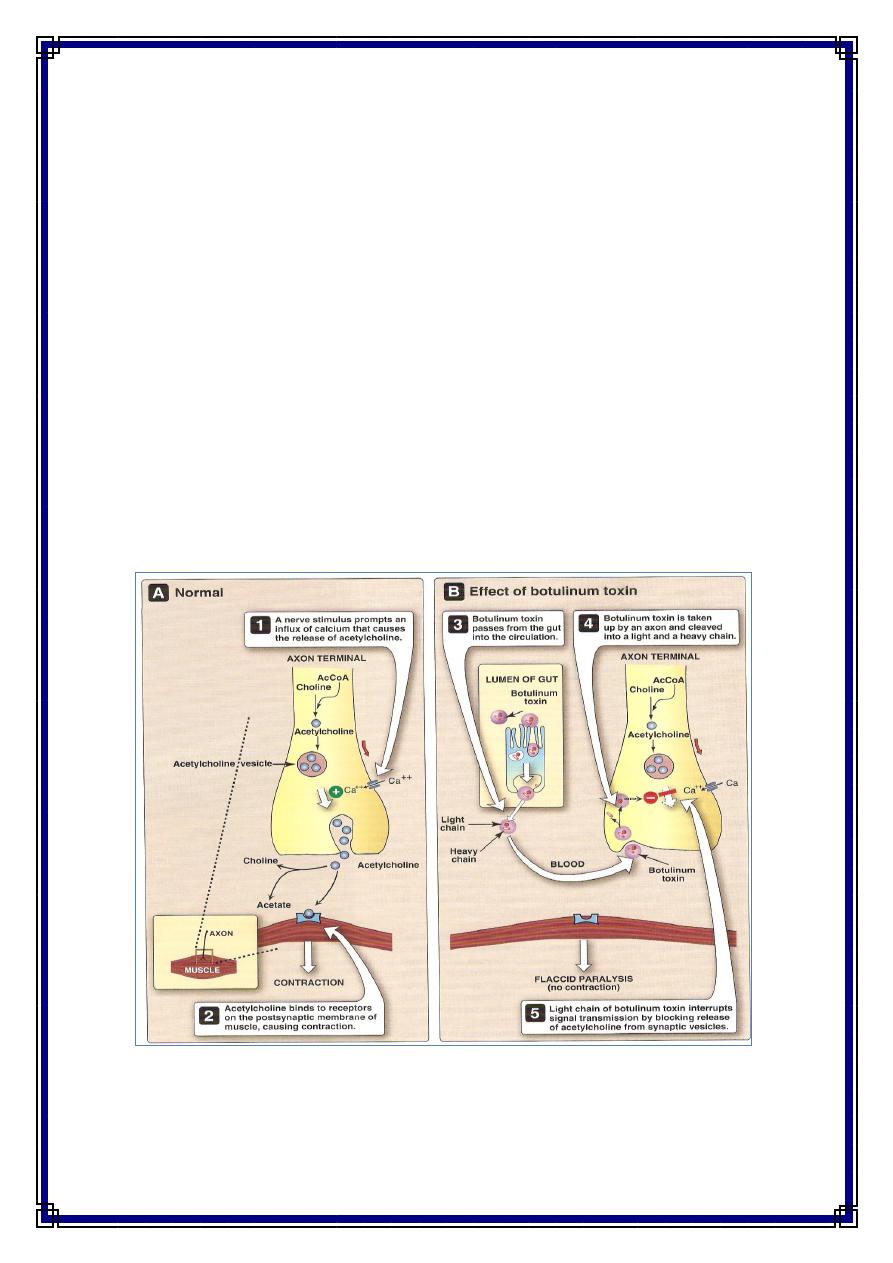
During the growth of C botulinum and during autolysis of the bacteria, toxin is
liberated into the environment. . Types A and B have been associated with a variety of
foods and type E predominantly with fish products. toxin is a 150,000-MW protein
that is cleaved into 100,000-MW and 50,000-MW protein subunits linked by a
disulfide bond. Botulinum toxin is absorbed from the gut and binds to receptors of
presynaptic membranes of motor neurons of the peripheral nervous system and cranial
nerves. Proteolysis—by the light chain of botulinum toxin of the target SNARE
proteins in the neurons inhibits the release of acetylcholine at the synapse, resulting in
lack of muscle contraction and paralysis which called Flaccid paralysis . The SNARE
proteins are synaptobrevin, SNAP 25, and syntaxin. The toxins of C botulinum types
A and E cleave the 25,000-MW SNAP-25. Type B toxin cleaves synaptobrevin. C
botulinum toxins are among the most toxic substances known . The lethal dose for a
human is probably about 1–2 µg. The toxins are destroyed by heating for 20 minutes
at 100 °C.
3
Pathogenesis
Although C botulinum types A and B have been implicated in cases of wound
infection and botulism, most often the illness is not an infection. Rather, it is an
intoxication resulting from the ingestion of food in which C botulinum has grown and
produced toxin. The most common offenders are spiced, smoked, vacuum-packed, or
canned alkaline foods that are eaten without cooking. In such foods, spores of C
botulinum germinate; under anaerobic conditions, vegetative forms grow and produce
toxin.
Clinical Findings
Symptoms begin 18–24 hours after ingestion of the toxic food, with visual
disturbances (incoordination of eye muscles, double vision), inability to swallow, and
speech difficulty; signs of bulbar paralysis are progressive, and death occurs from
respiratory paralysis or cardiac arrest. Gastrointestinal symptoms are not regularly
prominent. There is no fever. The patient remains fully conscious until shortly before
death. The mortality rate is high. Patients who recover do not develop antitoxin in the
blood.
In the United States, infant botulism is as common as or more common than the
classic form of paralytic botulism associated with the ingestion of toxin-contaminated
food. The infants in the first months of life develop poor feeding, weakness, and signs
of paralysis ("floppy baby"). Infant botulism may be one of the causes of sudden
infant death syndrome. C botulinum and botulinum toxin are found in feces but not in
serum. It is assumed that C botulinum spores are in the babies' food, yielding toxin
production in the gut.
Diagnostic Laboratory Tests
Toxin can often be demonstrated in serum from the patient, and toxin may be found
in leftover food. Mice injected intraperitoneally die rapidly. The antigenic type of
toxin is identified by neutralization with specific antitoxin in mice. C botulinum may
be grown from food remains and tested for toxin production, but this is rarely done
and is of questionable significance. In infant botulism, C botulinum and toxin can be
demonstrated in bowel contents but not in serum. Toxin may be demonstrated by
passive hemagglutination or radioimmunoassay.
Treatment
Potent antitoxins to three types of botulinum toxins have been prepared in horses.
Since the type responsible for an individual case is usually not known, trivalent (A, B,
E) antitoxin must be promptly administered intravenously with customary
precautions. Adequate ventilation must be maintained by mechanical respirator, if
necessary. These measures have reduced the mortality rate from 65% to below 25%.
4
Epidemiology, Prevention, & Control
Since spores of C botulinum are widely distributed in soil, they often contaminate
vegetables, fruits, and other materials. A large restaurant-based outbreak was
associated with sautéed onions. When such foods are canned or otherwise preserved,
they either must be sufficiently heated to ensure destruction of spores or must be
boiled for 20 minutes before consumption. Strict regulation of commercial canning
has largely overcome the danger of widespread outbreaks, but commercially prepared
foods have caused deaths. A chief risk factor for botulism lies in home-canned foods,
particularly string beans, corn, peppers, olives, peas, and smoked fish or vacuum-
packed fresh fish in plastic bags. Toxic foods may be spoiled and rancid, and cans
may "swell," or the appearance may be innocuous. The risk from home-canned foods
can be reduced if the food is boiled for more than 20 minutes before consumption.
Toxoids are used for active immunization of cattle in South Africa.
Clostridium Tetani
Clostridium tetani, which causes tetanus, is worldwide in distribution in the soil
and in the feces of horses and other animals. Several types of C tetani can be
distinguished by specific flagellar antigens. All share a common O (somatic) antigen,
which may be masked, and all produce the same antigenic type of neurotoxin,
tetanospasmin.
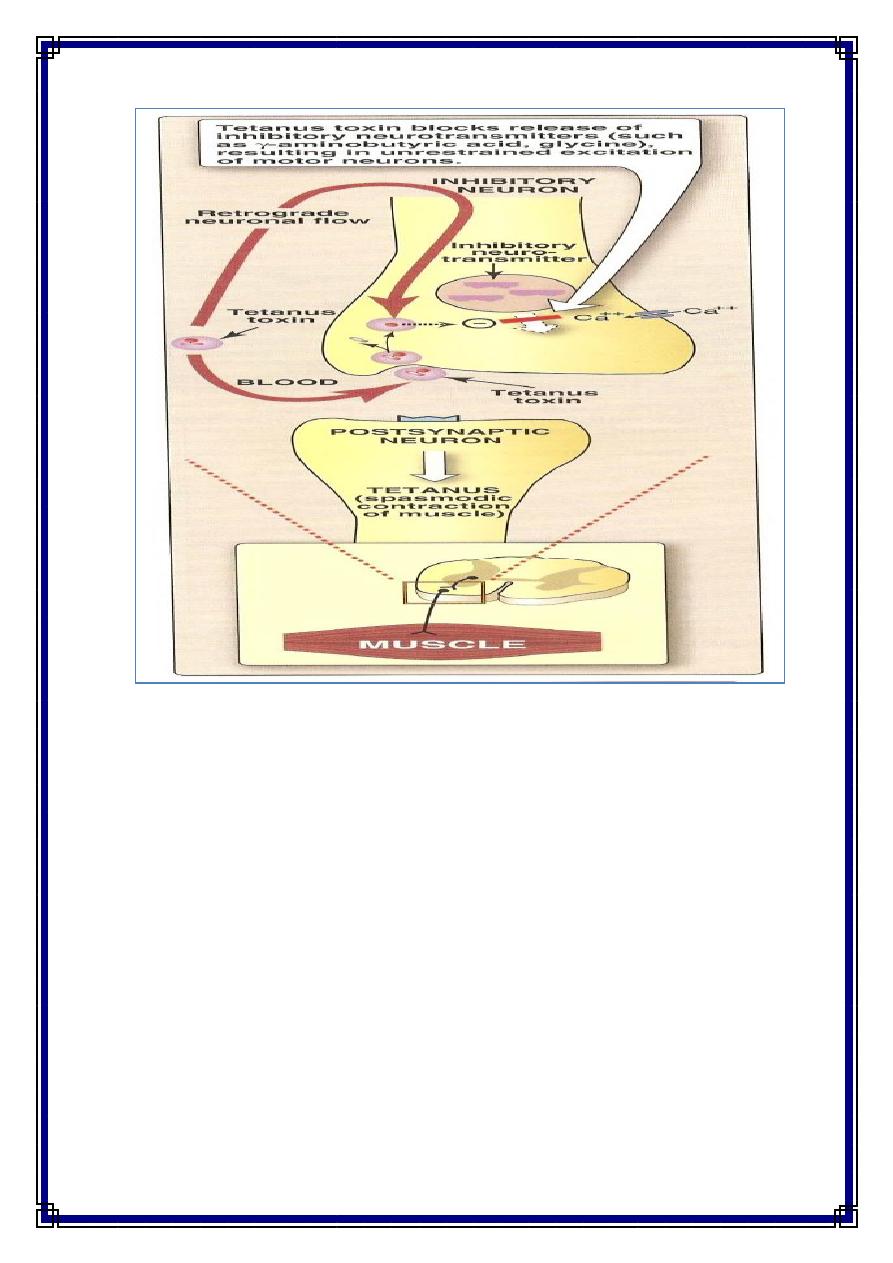
Toxin
The vegetative cells of C tetani produce the toxin tetanospasmin , the toxin initially
binds to receptors on the presynaptic membranes of motor neurons. It then migrates
by the retrograde axonal transport system to the cell bodies of these neurons to the
spinal cord and brain stem. The toxin diffuses to terminals of inhibitory cells,
including both glycinergic interneurons and aminobutyric acid-secreting neurons from
the brain stem. The toxin degrades synaptobrevin, a protein required for docking of
neurotransmitter vesicles on the presynaptic membrane. Release of the inhibitory
glycine and γ-aminobutyric acid is blocked, and the motor neurons are not inhibited.
Hyperreflexia, muscle spasms, and spastic paralysis result. Extremely small amounts
of toxin can be lethal for humans.
5
Pathogenesis
C tetani is not an invasive organism. The infection remains strictly localized in the
area of devitalized tissue (wound, burn, injury, umbilical stump, surgical suture) into
which the spores have been introduced. The volume of infected tissue is small, and
the disease is almost entirely a toxemia. Germination of the spore and development of
vegetative organisms that produce toxin are aided by (1) necrotic tissue, (2) calcium
salts, and (3) associated pyogenic infections, all of which aid establishment of low
oxidation-reduction potential.
The toxin released from vegetative cells reaches the central nervous system and
rapidly becomes fixed to receptors in the spinal cord and brain stem and exerts the
actions described above.
Clinical Findings
The incubation period may range from 4–5 days to as many weeks. The disease is
characterized by tonic contraction of voluntary muscles. Muscular spasms often
involve first the area of injury and infection and then the muscles of the jaw (trismus,
lockjaw), which contract so that the mouth cannot be opened. Gradually, other
voluntary muscles become involved, resulting in tonic spasms. Any external stimulus
6
may precipitate a tetanic generalized muscle spasm. The patient is fully conscious,
and pain may be intense. Death usually results from interference with the mechanics
of respiration. The mortality rate in generalized tetanus is very high.
Diagnosis
The diagnosis rests on the clinical picture and a history of injury, although only
50% of patients with tetanus have an injury for which they seek medical attention.
The primary differential diagnosis of tetanus is strychnine poisoning. Anaerobic
culture of tissues from contaminated wounds may yield C tetani, but neither
preventive nor therapeutic use of antitoxin should ever be withheld pending such
demonstration. Proof of isolation of C tetani must rest on production of toxin and its
neutralization by specific antitoxin.
Prevention & Treatment
The results of treatment of tetanus are not satisfactory. Therefore, prevention is all-
important. Prevention of tetanus depends upon (1) active immunization with toxoids;
(2) proper care of wounds contaminated with soil, etc; (3) prophylactic use of
antitoxin; and (4) administration of penicillin. The intramuscular administration of
250–500 units of human antitoxin (tetanus immune globulin) gives adequate systemic
protection (0.01 unit or more per milliliter of serum) for 2–4 weeks. It neutralizes the
toxin that has not been fixed to nervous tissue. Active immunization with tetanus
toxoid should accompany antitoxin prophylaxis.
Patients who develop symptoms of tetanus should receive muscle relaxants,
sedation, and assisted ventilation. Sometimes they are given very large doses of
antitoxin (3000–10,000 units of tetanus immune globulin) intravenously to neutralize
toxin that has not yet been bound to nervous tissue. Surgical debridement is vitally
important because it removes the necrotic tissue that is essential for proliferation of
the organisms. Hyperbaric oxygen has no proved effect.
Penicillin strongly inhibits the growth of C tetani and stops further toxin
production. Antibiotics may also control associated pyogenic infection. When a
previously immunized individual sustains a potentially dangerous wound, an
additional dose of toxoid should be injected to restimulate antitoxin production.
Control
Tetanus is a totally preventable disease. Universal active immunization with tetanus
toxoid should be mandatory. Three injections comprise the initial course of
immunization, followed by another dose about 1 year later. Initial immunization
should be carried out in all children during the first year of life. A "booster" injection
of toxoid is given upon entry into school. Thereafter, "boosters" can be spaced 10
years apart to maintain serum levels of more than 0.01 unit antitoxin per milliliter. In
young children, tetanus toxoid is often combined with diphtheria toxoid and pertussis
vaccine.
7
Clostridia that Produce Invasive Infections
Many different toxin-producing clostridia (Clostridium perfringens and related
clostridia) can produce invasive infection (including myonecrosis and gas gangrene)
if introduced into damaged tissue. About 30 species of clostridia may produce such an
effect, but the most common in invasive disease is Clostridium perfringens (90%).
Toxins
The invasive clostridia produce a large variety of toxins and enzymes that result in
a spreading infection. Many of these toxins have lethal, necrotizing, and hemolytic
properties. The alpha toxin of C perfringens type A is a lecithinase, and its lethal
action is proportionate to the rate at which it splits lecithin (an important constituent
of cell membranes) to phosphorylcholine and diglyceride. The theta toxin has similar
hemolytic and necrotizing effects but is not a lecithinase. DNase and hyaluronidase,
and collagenase that digests collagen of subcutaneous tissue and muscle, are also
produced.
Some strains of C perfringens produce a powerful enterotoxin, especially when
grown in meat dishes. When more than 10
8
vegetative cells are ingest and sporulate in
the gut, enterotoxin is formed. The enterotoxin is a protein (MW 35,000) that may be
a nonessential component of the spore coat; it is distinct from other clostridial toxins.
It induces intense diarrhea in 6–18 hours. The action of C perfringens enterotoxin
involves marked hyper-secretion in the jejunum and ileum, with loss of fluids and
electrolytes in diarrhea. Much less frequent symptoms include nausea, vomiting, and
fever. This illness is similar to that produced by B cereus and tends to be self-limited.
Pathogenesis
In invasive clostridial infections, spores reach tissue either by contamination of
traumatized areas (soil, feces) or from the intestinal tract. The spores germinate at low
oxidation-reduction potential; vegetative cells multiply, ferment carbohydrates present
in tissue, and produce gas. The distention of tissue and interference with blood supply,
together with the secretion of necrotizing toxin and hyaluronidase, favor the spread of
infection. Tissue necrosis extends, providing an opportunity for increased bacterial
growth, hemolytic anemia, and, ultimately, severe toxemia and death.
In gas gangrene (clostridial myonecrosis), a mixed infection is the rule. In addition
to the toxigenic clostridia, proteolytic clostridia and various cocci and gram-negative
organisms are also usually present. C perfringens occurs in the genital tract of 5% of
women. Clostridial bacteremia is a frequent occurrence in patients with neoplasms.
Also, C perfringens type C produces a necrotizing enteritis (pigbel) that can be highly
fatal in children. Immunization with type C toxoid appears to have preventive value.
8
Clinical Findings
From a contaminated wound (eg, a compound fracture, postpartum uterus), the
infection spreads in 1–3 days to produce crepitation in the subcutaneous tissue and
muscle, foul-smelling discharge, rapidly progressing necrosis, fever, hemolysis,
toxemia, shock, and death. Treatment is with early surgery (amputation) and antibiotic
administration. Until the advent of specific therapy, early amputation was the only
treatment.
Diagnostic Laboratory Tests
Specimens consist of material from wounds, pus, tissue. The presence of large
gram-positive rods in Gram-stained smears suggests gas gangrene clostridia; spores
are not regularly present.
Material is inoculated into chopped meat-glucose medium and thioglycolate
medium and onto blood agar plates incubated anaerobically. The growth from one of
the media is transferred into milk. A clot torn by gas in 24 hours is suggestive of C
perfringens. Once pure cultures have been obtained by selecting colonies from
anaerobically incubated blood plates, they are identified by biochemical reactions
(various sugars in thioglycolate, action on milk), hemolysis, and colony form.
Lecithinase activity is evaluated by the precipitate formed around colonies on egg
yolk media. Final identification rests on toxin production and neutralization by
specific antitoxin. C perfringens rarely produces spores when cultured on agar in the
laboratory.
Treatment
The most important aspect of treatment is prompt and extensive surgical
debridement of the involved area and excision of all devitalized tissue, in which the
organisms are prone to grow. Administration of antimicrobial drugs, particularly
penicillin, is begun at the same time. Hyperbaric oxygen may be of help in the
medical management of clostridial tissue infections. It is said to "detoxify" patients
rapidly.
Antitoxins are available against the toxins of C perfringens, Clostridium novyi,
Clostridium histolyticum, and Clostridium septicum, usually in the form of
concentrated immune globulins. Polyvalent antitoxin (containing antibodies to several
toxins) has been used. Although such antitoxin is sometimes administered to
individuals with contaminated wounds containing much devitalized tissue, there is no
evidence for its efficacy. Food poisoning due to C perfringens enterotoxin usually
requires only symptomatic care.
Prevention & Control
Early and adequate cleansing of contaminated wounds and surgical debridement,
together with the administration of antimicrobial drugs directed against clostridia (eg,
penicillin), are the best available preventive measures. Antitoxins should not be relied
on. Although toxoids for active immunization have been prepared, they have not
9
come into practical use.
Clostridium Difficile & Diarrheal Disease
Pseudomembranous Colitis
Pseudomembranous colitis is diagnosed by detection of one or both C difficile
toxins in stool and by endoscopic observation of pseudomembranes or microabscesses
in patients who have diarrhea and have been given antibiotics. Plaques and
microabscesses may be localized to one area of the bowel. The diarrhea may be
watery or bloody, and the patient frequently has associated abdominal cramps,
leukocytosis, and fever. Although many antibiotics have been associated with
pseudomembranous colitis, the most common are ampicillin and clindamycin. The
disease is treated by discontinuing administration of the offending antibiotic and
orally giving either metronidazole or vancomycin.
Administration of antibiotics results in proliferation of drug-resistant C difficile that
produces two toxins. Toxin A, a potent enterotoxin that also has some cytotoxic
activity, binds to the brush border membranes of the gut at receptor sites. Toxin B is a
potent cytotoxin. Both toxins are found in the stools of patients with
pseudomembranous colitis. Not all strains of C difficile produce the toxins, and the
tox genes apparently are not carried on plasmids or phage.
Antibiotic-Associated Diarrhea
The administration of antibiotics frequently leads to a mild to moderate form of
diarrhea, termed antibiotic-associated diarrhea. This disease is generally less severe
than the classic form of pseudomembranous colitis. As many as 25% of cases of
antibiotic-associated diarrhea may be associated with C difficile.
