
D R . M O H A N N E D H U S S A M A L K U M A I T
A S S I S T A N T P R O F E S S O R O F U R O L O G Y
T I K R I T C O L L E G E O F M E D I C I N E
5
T H
Y E A R
Testicular cancer

Incidence and mortality
Primary testicular cancer (TC) is the most common
solid cancer in men aged 20 to45; rare below 15 years
and above 60 years. Constituting 1 to 2% of all male
cancers, the lifetime risk of developing testicular
cancer is 1 in 500. It is also considered the most
curable cancer

Epidemiology and aetiology
Age
: the most common affected age group is 20 to 45 years, with
germ cell tumours; teratomas are more common at ages 20 to 35;
while seminoma is more common at ages 35 to 45 years. Rarely,
infants and boys below 10 years develop yolk sac tumours and 50%
men >60 years with TC have lymphoma.
Race
: white people are three times more likely to develop TC than
black people in the USA.
Cryptorchidism:
10% of TC occur in undescended testes: the
risk increases by 3 to 14 times compared to men with normally
descended testes. Ultrastructural changes are present in these testes
by age 3 years, although earlier orchidopexy does not completely
eliminate the risk of developing TC. 5 to 10% of patients with a
cryptorchid testis develop malignancy in the normally descended
contralateral testis

Intratubular germ cell neoplasia (IGCN):
synonymous with carcinoma in situ, although the disease arises
from malignant change in spermatogonia. 50% of cases develop
invasive germ cell TC within 5 years. The population incidence is
0.8%. Risk factors include cryptorchidism, extra-gonadal germ cell
tumour, previous or contralateral TC (5%), atrophic contralateral
testis, 45XO karyotype, and infertility.
Human immunodeficiency virus (HIV):
patients
infected with the HIV virus are developing seminoma more
frequently than expected.
Genetic factors
appear to play a role, given that first-degree
relatives are at higher risk, but a defined familial inheritance
pattern is not apparent.
Maternal oestrogen
ingestion during pregnancy increases
the risk of cryptorchidism and TC in the male offspring.

Testicular cancer: clinical presentation
Symptoms
Most patients present with a scrotal lump, usually painless
or slightly aching. Delay in presentation is not uncommon,
particularly those with metastatic disease. This may be due
to patient factors (fear, self-neglect, ignorance, denial) or
earlier misdiagnosis. Occasionally (5%) acute scrotal pain
may occur, due to intra-tumoural haemorrhage, causing
diagnostic confusion. The lump may have been noted by
the patient, sometimes after minor trauma, or by his
partner. In 10%, symptoms suggestive of advanced disease
include weight loss, lumps in the neck, chest symptoms,
and bone pain.

Signs
Examination of the genitalia should be carried out in a warm
room with the patient relaxed. Observation may reveal
asymmetry or slight scrotal skin discolouration.
Using careful bimanual palpation, the normal side is first
examined, followed by the abnormal side. This will reveal a
hard, non-tender, irregular, non-transilluminable mass in the
testis, or replacing the testis. Care should be taken to assess
the epididymis, spermatic cord, and overlying scrotal wall,
which may be normal or involved in 10 to 15% of cases.
Rarely, a secondary hydrocoele may be present if the tunica
albuginea has been breached. General examination may reveal
cachexia, supraclavicular lymphadenopathy, chest signs,
hepatomegaly, lower limb oedema, or abdominal mass ”all
suggestive of metastatic disease. Gynaecomastia is seen in
~5% of patients with TC, due to endocrine manifestations of
some tumours.

Differential diagnosis
Testicular torsion, epididymo-orchitis, hydrocoele,
epidiymal cyst, hernia, haematoma, or syphilitic
gumma (rare).
The majority of scrotal lumps are harmless lesions,
but no risks should be taken. Every patient who is
concerned should be seen, examined, and if any doubt
persists, should be investigated further.

Investigations
Ultrasound
is an extension of the physical
examination and will confirm that the palpable lesion is
within the testis, distorting its normally regular outline
and internal echo pattern. Any hypoechoic area within
the tunica albuginea should be regarded with suspicion.
It may distinguish a primary from a secondary
hydrocoele. Ultrasound may also be used to identify
impalpable lesions as small as 1 to 2mm
occult primary
tumour in a patient presenting with systemic symptoms
and signs or an incidental finding.
Abdominal and chest CT
scans are usually
obtained for staging purposes if the diagnosis of TC is
confirmed or considered likely.

Testicular cancer: serum markers
Onco-fetal proteins
Alpha-fetoprotein (AFP)
is expressed by trophoblastic
elements within 50 to 70% of teratomas and yolk sac tumours. With
respect to seminoma, the presence of elevated serum AFP strongly
suggests a non-seminomatous element. Serum half-life is 3 to 5 days;
normal <10ng/ml.
Human chorionic gonadotrophin (hCG)
is expressed
syncytiotrophoblastic elements of choriocarcinomas (100%),
teratomas (40%), and seminomas (10%). Serum half-life is 24 to 36h.
Assays measure the beta-subunit; normal <5mIU/ml.
When used together, 90% of patients with advanced disease have
elevation of one or both markers; less among patients with low-stage
tumours.

Cellular enzymes
Lactate dehydrogenase (LDH)
is an
enzyme, elevated in serum for various causes,
therefore less specific. It is elevated in 10 to 20% of
seminomas, correlating with tumour burden, and is
most useful in monitoring treatment response in
advanced seminoma.
Placental alkaline phosphatase (PLAP)
is a fetal
isoenzyme, elevated in up to 40% of patients with
advanced germ cell tumours. It is not widely used as it
is non-specific. May be elevated in smokers.

Clinical use
These markers are measured at presentation, 1 to 2
weeks after radical orchidectomy, and during follow-
up to assess response to treatment and residual
disease.
Normal markers prior to orchidectomy do not exclude
metastatic disease; normalization of markers post
orchidectomy cannot be equated with absence of
disease; and persistent elevations of markers post-
orchidectomy may occur with liver dysfunction and
hypogonadotrophism, but usually indicate metastatic
disease.

WHO histopathological classification of
testicular tumours
Germ cell tumours (90%)
Seminoma (48%)
Spermatocytic, classical, and anaplastic subtypes
Non-seminomatous GCT (42%)
Teratoma:
Differentiated/mature
Intermediate/immature
Undifferentiated/malignant
Yolk sac tumour
Choriocarcinoma
Mixed NSGCT
Mixed GCT (10%)
Other tumours (7%)
Epidermoid cyst (benign)
Adenomatoid tumour
Adenocarcinoma of the rete testis
Carcinoid
Lymphoma (5%)
Metastatic, from another site (1%)
Sex cord stromal tumours (3%) (10% malignant)
Leydig cell
Sertoli cell
Mixed

90% of testicular tumours are malignant germ cell
tumours (GCT), split into seminomatous and non-
seminomatous (NS) GCTs for clinical purposes.
Seminoma, the most common germ cell tumour,
appears pale and homogeneous. NSGCTs are
heterogeneous and sometimes contain bizarre tissues
such as cartilage or hair. Metastases to the testis are
rare, notably from the prostate (35%), lung (19%),
colon (9%), and kidney (7%).

The right testis is affected slightly more commonly
than the left; synchronous bilateral TC occurs in 2% of
cases. TC spreads by local extension into the
epididymis, spermatic cord, and, rarely, the scrotal
wall.
Lymphatic spread occurs via the testicular vessels,
initially to the para-aortic nodes. Involvement of the
epididymis, spermatic cord, or scrotum may lead to
pelvic and inguinal node metastasis. Blood-borne
metastasis to the lungs, liver, and bones is more likely
once the disease has breached the tunica albuginea.

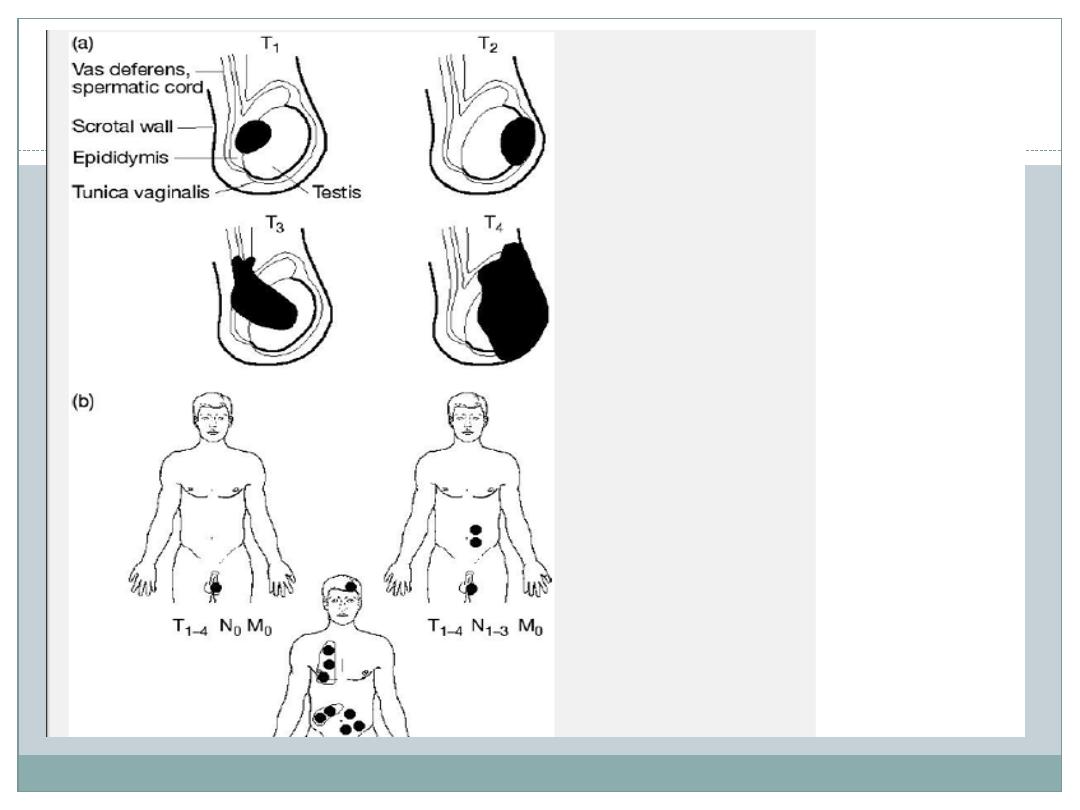
TNM staging of testicular germ cell tumours
Tx The primary tumour has not been assessed (no radical orchidectomy)
T0 No evidence of primary tumour
Tis Intratubular germ cell neoplasia (carcinoma in situ)
T1 Tumour limited to testis and epididymis without vascular invasion; may invade tunica
albuginea but not tunica vaginalis
T2 Tumour limited to testis and epididymis with vascular/lymphatic invasion, or tumour involving
tumica vaginalis
T3 Tumour invades spermatic cord with or without vascular invasion
T4 Tumour invades scrotum with or without vascular invasion
Nx Regional lymph nodes cannot be assessed
N0 No regional lymph node metastasis
N1 Metastasis with a lymph node less than 2cm or multiple lymph nodes, none >2cm
N2 Metastasis with a lymph node size 2 to 5cm or multiple lymph nodes, collected size 2 to 5cm
N3 Metastasis with a lymph node mass >5cm
Mx Distant metastasis cannot be assessed
M0 No distant metastasis
M1a Non-regional lymph node or pulmonary metastasis
M1b Distant metastasis other than to non-regional lymph node or lungs

Treatment
Radical orchidectomy
The final investigation and the primary treatment for all
testicular tumours, unless tissue diagnosis has been
made from a metastasis. This involves excision of the
testis, epididymis, and cord, with their coverings,
through a groin incision. The cord is clamped, transfixed,
and divided near the internal inguinal ring before the
testis is manipulated into the wound, preventing
inadvertent metastasis. A silicone prosthesis may be
inserted at the time or at a later date. This treatment is
curative in ~80% of patients. Fertility prophylaxis by
freezing sperm should be offered to patients without a
normal contralateral testis. Contralateral testis biopsy
should be considered in patients at high risk for IGCN

Testicular cancer: management of non-
seminomatous germ cell tumours (NSGCT)
Following radical orchidectomy and formal staging,
the patient is normally managed by the oncologist,
though the urologist may be asked to perform
retroperitoneal lymph node dissection (RPLND) in
selected cases. In the presence of elevated AFP, a
seminoma would be managed as for teratoma.
Combination chemotherapy, introduced in the
1980s, revolutionized the treatment of metastatic
testicular teratoma, which was hitherto virtually
untreatable.

Non-metastatic disease
T 1 to 4N0M0S0: surveillance or chemotherapy
(bleomycin, low-dose etoposide, cisplatin) depending
on risk factors for relapse (lymphatic or vascular
invasion, (T2 to 4); surveillance in presence of risk
factors results in 25% relapse rate, most <1 year post
orchidectomy.

Metastatic disease
Good prognosis
: chemotherapy (bleomycin
etoposide and cisplatin 1 to 3 cycles),residual or
recurrent mass; salvage chemotherapy if histology
confirms tumour.
Intermediate and poor prognosis
:
chemotherapy (bleomycin,etoposide,cisplatin 1 to 4
cycles); RPLND for residual or recurrent mass; salvage
chemotherapy if histology confirms tumour.

Surveillance and follow-up after treatment
Surveillance requires the following:
Year 1: monthly clinic visit with serum markers and
chest X-ray, abdominal CT months 3,6,9, and 12
months
Year 2: 2-monthly clinic visit with serum markers and
chest X-ray, abdominal CT month 24
Years 3,4, and 5: 3-monthly clinic visit with serum
markers and chest X-ray
Annual clinic visit with serum markers and chest X-
ray, thereafter to 10 years

Testicular cancer: management of seminoma,
IGCN, and lymphoma
Of all seminomas, 75% are confined to the testis at
presentation and are cured by radical orchidectomy;
10 to 15% of patients harbour regional node
metastasis; and 5 to 10% have more advanced disease.
Following radical orchidectomy and formal staging,
the patient is managed by the oncologist. Treatment
and follow-up depends largely on disease stage
according to presence of metastases and size of nodal
disease, as follows

Non-metastatic disease
T1N0M0S0 1: risk of subsequent para-aortic node
relapse is 20%. Adjuvant radiotherapy (RT) 20Gy in 10
fractions reduces risk to 1%. RT includes para-aortic
nodes.
Spermatocytic subtype usually warrants surveillance

Metastatic disease
T1to3 N1 M0S0 : RT
T1to3 N2 M0 S0 : RT; chemotherapy if nodes near
kidneys.
T1to4 N3 M0 S0 : chemotherapy (either bleomycin,
etoposide, and cisplatin or etoposide and cisplatin); if
residual node mass >3cm (rare), retroperitoneal lymph
node dissection (RPLND) considered; if histology reveals
tumour (30%), salvage chemotherapy.
T1to4N0 M1: chemotherapy; if residual node mass (rare),
RPLND considered; if histology reveals tumour (30%),
salvage chemotherapy.
