Biochemistry
2nd stageDr.Lamees Majid Al-Janabi
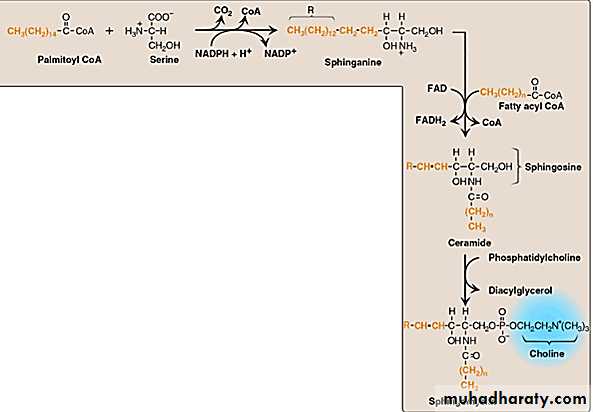
SPHINGOLIPIDS
Sphingomyelin:-
Shingomyelin is one of the principal structural lipids of membranes of nerve tissue. This class of phospholipid has sphingosine rather than glycerol as the alcohol. Shingomyelin of the myelin sheath( a structure that insulate and protects neuronal fibers of the central nervous system) contains predominantly longer chain fatty acids such as lignoceric and nervonic acid, wherease gray matter of the brain has sphingomyelin that contains primarily stearic acid.
Degradation of sphingomyelin:-
Sphingomyelin is degraded by sphingomyelinase, a lysosomal enzyme that hydrolytically removes phosphorylcholine, leaving a ceramide. The ceramide is, in turn, cleaved by ceramidase into sphingosine and a free fatty acid.Glycolipids:-
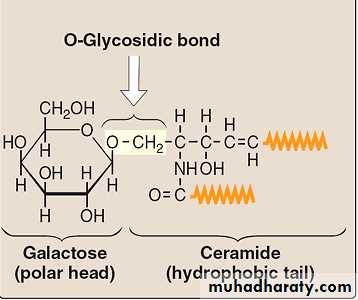
Glycolipids are molecules that contain both carbohydrate and lipid components. Like the phospholipid sphingomyelin, glycolipids are derivatives of ceramides in which a long-chain fatty acid is attached to the amino alcohol sphingosine. They are, therefore, called glycosphingolipids.-Functions:-
They are essential components of all membranes in the body, but they are found in greatest amounts in nerve tissue. They are located in the outer leaflet of the plasma membrane, where they interact with the extracellular environment. As such, they play a role in the regulation of cellular interactions, growth, and development.Glycosphingolipids are antigenic, and they have been identified as a source of blood group antigens, various embryonic antigens specific for particular stages of fetal development, and some tumor antigens.
They also serve as cell surface receptors for cholera and tetanus toxins, as well as for certain viruses and microbes.
-Types of glycolipid:-
A. Neutral glycosphingolipids:-The simplest neutral (uncharged) glycosphingolipids are the cerebrosides. These are ceramidemonosaccharides that contain either a molecule of galactose (galactocerebroside—the most common cerebroside found in membranes) or glucose (glucocerebroside, which serves primarily as an intermediate in the synthesis and degradation of the more complex glycosphingolipids).Cerebrosides are found predominantly in the brain and peripheral nervous tissue, with high concentrations in the myelin sheath.
Galactocerebroside
B. Acidic glycosphingolipids:--Gangliosides: These are the most complex glycosphingolipids, and are found primarily in the ganglion cells of the central nervous system, particularly at the nerve endings. They are derivatives of ceramide oligosaccharides, and contain one or more molecules of NANA(N-acetylneuraminic acid).
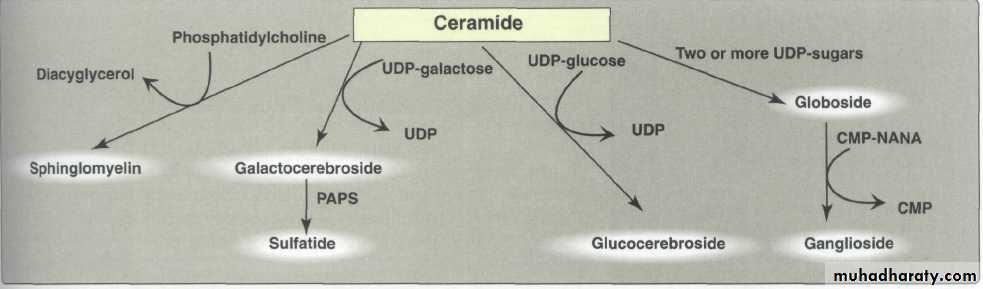
-Synthesis of Glycosphingolipids:-
Synthesis of glycosphingolipids occurs primarily in the Golgi by sequential addition of glycosyl monomers transferred from UDP–sugar donors to the acceptor molecule. The mechanism is similar to that used in glycoprotein synthesis .
The enzymes involved in the synthesis of glycosphingolipids are glycosyltransferases, each specific for a particular sugar nucleotide and acceptor.
UDP-GAL = uridine diphosphate galactose.
UDP-GLU = uridine diphosphate glucose.PAPS = phosphoadenosine phospho sulfate
-Degradation of glycosphingolipids:-
All of the enzymes required for the degradative process are present in lysosomes, which fuse with the endocytotic vesicles. The lysosomal enzymes hydrolytically and irreversibly cleave specific bonds in the glycosphingolipid. As seen with the glycosaminoglycans and glycoproteins, degradation is a sequential process following the rule “last on, first off,” in which the last group added during synthesis is the first group removed in degradation.
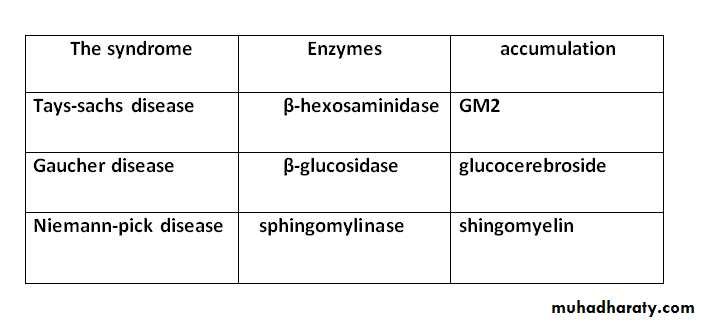
-Sphingolipidoses (lipid storage diseases) :-
are agroup of inherited diseases that are often manifested in childhood. These diseases are part of a larger group of lysosomal disorders and exhibit several constant features:-(1) Complex lipids containing ceramide accumulation cells, particularly neurons, causing neurodegeneration and shortening the life span.
(2) The rate of synthesis of the stored lipid is normal.
(3) The enzymatic defect is in the lysosomal degradation pathway of sphingolipids.
(4) The extent to which the activity of the affected enzyme is decreased is similar in all tissues.
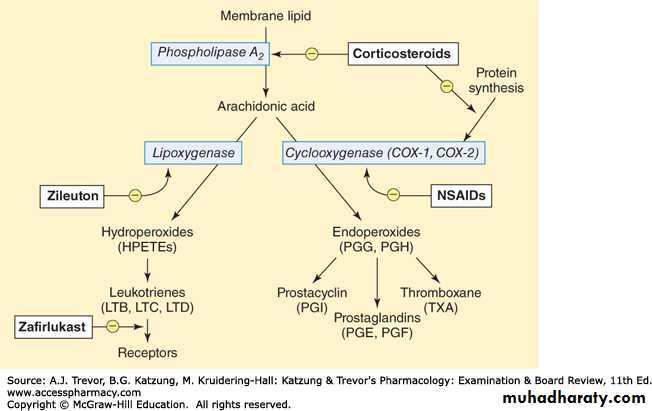
Prostaglandins and Related Compounds
Prostaglandins, and the related compounds thromboxanes and leukotrienes, are collectively known as eicosanoids to reflect their origin from polyunsaturated fatty acids with 20 carbons. They are extremely potent compounds that elicit a wide range of responses, both physiologic and pathologic. These compounds have been very difficult to study because they have an extremely short half-life and are produced in very small amounts.Although they have been compared to hormones in terms of their actions but they differ from the true hormones in that:-
they are produced in very small amounts in almost all tissues rather than in specialized glands.
They also act locally rather than after transport in the blood to distant sites, as occurs with true hormones such as insulin.
They are not stored, and they have an extremely short half-life, being rapidly metabolized to inactive products. Their biologic actions are mediated by plasma membrane G protein–coupled receptors, which are different in different organ systems.
Synthesis of prostaglandins:-
The dietary precursor of the prostaglandins is the essential fatty acid, linoleic acid. It is elongated and desaturated to arachidonic acid, the immediate precursor of the predominant class of prostaglandins (those with two double bonds) in humans.