By
Dr Dhafer A. Alghezi
PhD cancer research/ UK
Presented by
Dr. Minen Al-KafajyAntigen recognition molecules
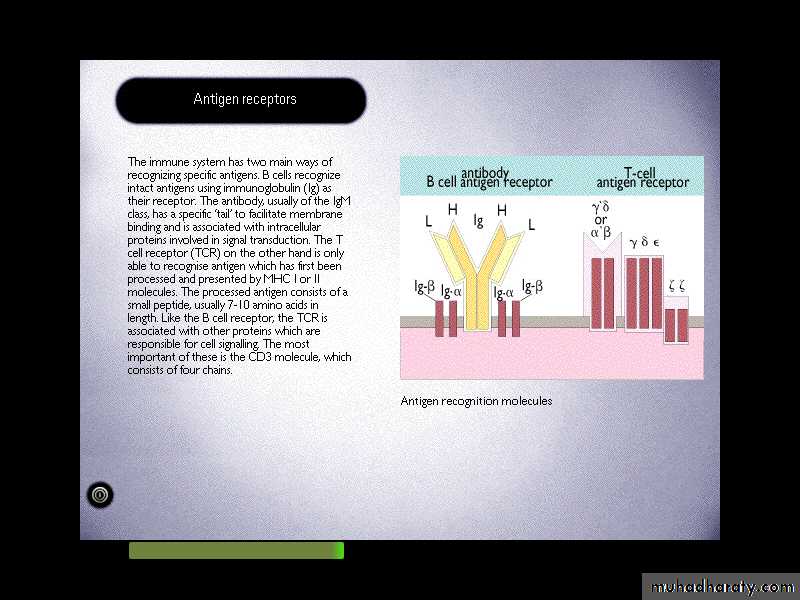
Two distinct types of molecules are involved in these processes, including immunoglobulins (Igs) and T-cell Ag-receptor (TCR).Diversity and heterogeneity are characteristic features of these molecules.
In both cases, there is evidence of extensive gene-rearrangements which generate Igs or TCR capable of recognizing many different Antigens.
Immunoglobulins (Antibodies)
They are group of glycoproteins present in the serum or tissue fluids of all mammals, including human.They are produced by B-lymphocytes (plasma cells).
They act as a critical part of the immune response by specifically recognizing and binding to particular antigens, such as bacteria or viruses, and aiding in their destruction.
Structure of Igs
IgG, IgM, IgA, IgD and IgE.
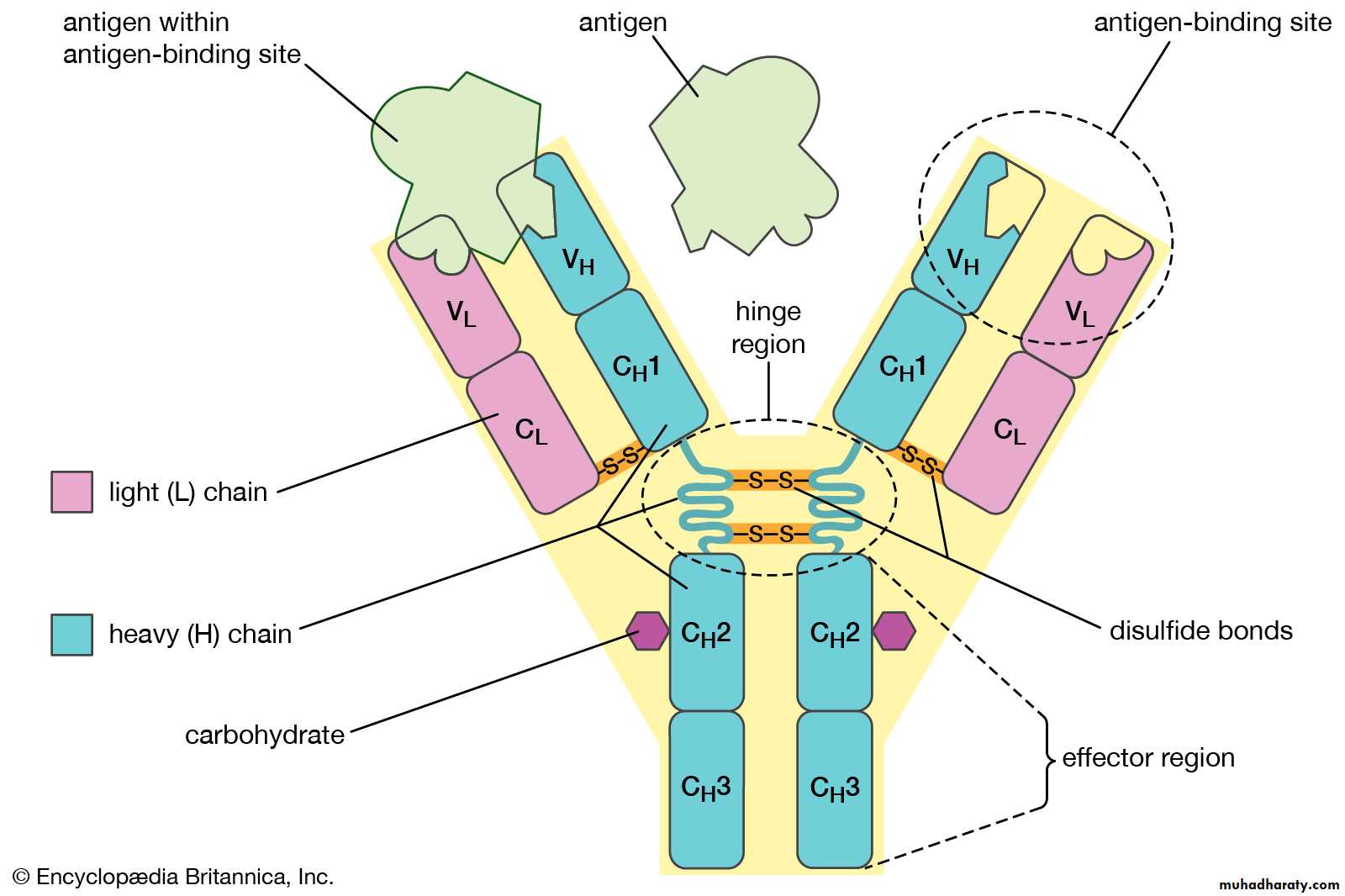
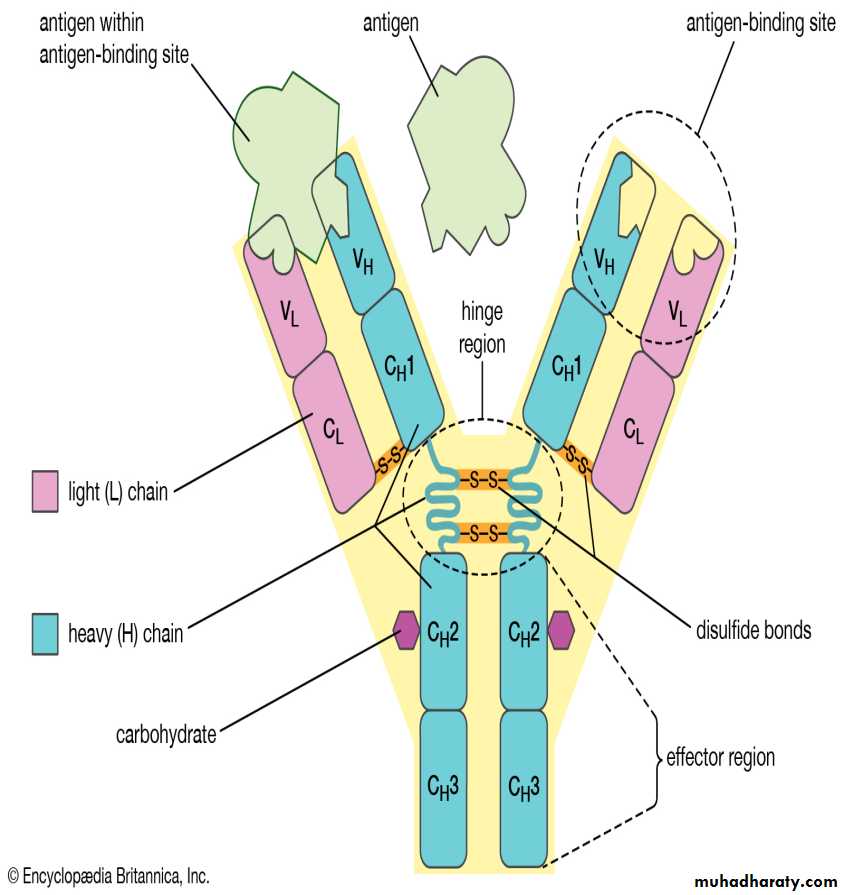
Each has two functional regions: Fab and Fc regions.
The basic Ig unit (monomer) consists of 4 polypeptide chains. Two identical heavy chains and two light chains held together by disulphate bonds.
Light chains: there are two antigenic types called kappa (Ƙ) and lambda (ƛ) . only one type of light chain is found in any individual molecules.
Heavy chain: there are five main types: Gamma (γ) in IgG, alpha (α) in IgA, Mu (μ) in IgM, delta (δ) in IgD, and epsilon (ε) in IgE.
Each chain consist of two parts, the N-terminal of the chain shows much sequence variability and known as variable region (V) and c-terminal of the chain which is constant and called constant region (C).
The constant portion of the heavy chain is further divided into three structurally discrete regions:CH1, CH2, CH3….., these globular regions stabilized by intrachain disulphide bound and are referred to as “Domains”.
The hinge region is a segment of heavy chain between the CH1 and CH2-domains, flexibility in this area permits the two Ag-binding sites to operate independently.
IgA and IgM have additional peptide chain, a J (joining) chain, thought to assist the processes of polymerization.
• Properties
• IgG• IgM
• IgA
• IgE
• IgD
• %in serum
• 75
• 9
• 15
• 0.004
• 0.2
• M.W (X1000)
• 160
• 900
• 170-400
• 190
• 180
• Half life
• 23
• 5
• 6
• 2.5
• 3
• Molecular form
• Monomer
• Pentamer
• Monomer or dimer
• Monomer
• Monomer
• Subclasses
• 4
• 2
• 2
• 1
• 1
• H chain
• γ
• μ
• α
• ε
• δ
• J chain
• -
• +
• +
• -
• -
• Secretory piece
• -
• -
• +
• -
• -
• Complement fixation
• +
• ++
• -
• -
• -
• Crosses placenta
• +
• -
• -
• -
• -
• Mediates allergy
• -
• -
• -
• +
• -
• Opsonization
• +
• _
• -
• -
• -
• Binds to Fc receptor in NK cells or phagocytes (ADCC)
• +
• -
• -
• -
• -
• Ag receptor on B cells
• -
• +
• -
• -
• ±
Interaction of Abs with Ags
The binding of Ag to Ab involves the formation of multiple non-covalent bounds between the Ag and amino acids of the binding site.
The attractive forces (hydrogen and electrostatic bounds, Van der Waals and hydrophobic forces) are weak by comparison with covalent bounds.
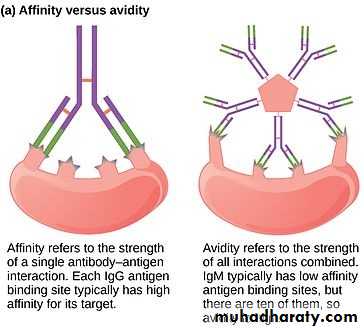
The strength of the single bond between Ag and Ab is known as the Ab-affinity, it is the sum of the attractive and repulsive forces, whereas the overall strength of interaction between the Ab and Ag indicates Ab-avidity.
When some of the epitopes of an Ag are shared by another Ag then a proportion of the Abs directed to that Ag and this phenomenon is termed cross-reactivity.
Function of the antibodies:
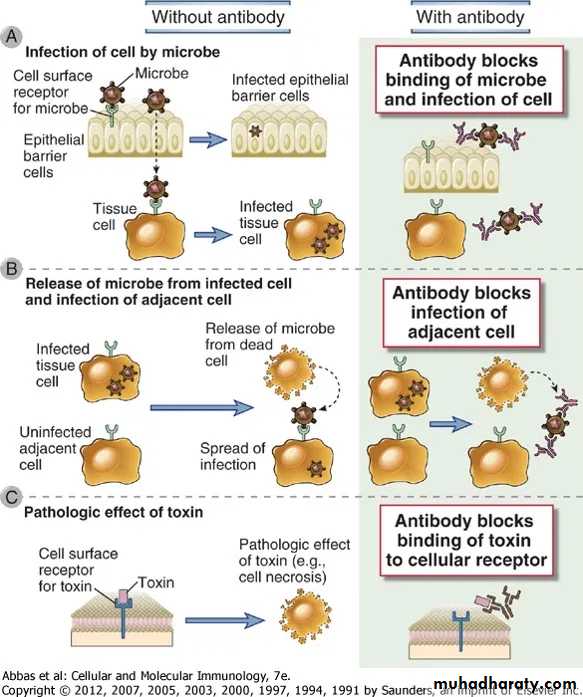
1. Neutralization of infectivity:Neutralization is the ability of antibody to bind to and inactivate virus infectivity.
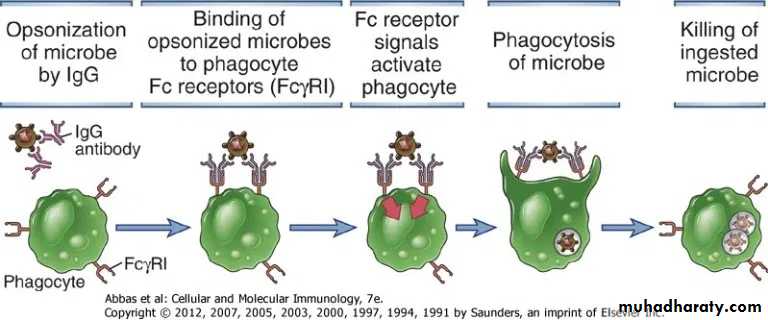
2. Phagocytosis: Antibodies facilitate phagocytosis of foreign substances by a process called opsonization.
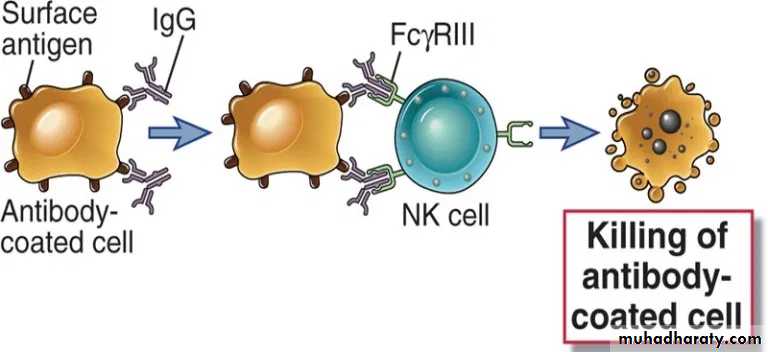
3. Antibody-dependent cellular cytotoxicity (ADCC)
ADCC occurs when antibody forms a bridge between an infected target cells (virus infected cells of the host) and an FcR-bearing effector cell, particularly natural killer (NK) cells. The result of this three-way interaction is the death of the target cell, either by lysis or apoptosis.
Complement-mediated lysis of pathogens or of infected cells: Antibodies activate the complement system to destroy bacterial cells by lysis
Antibodies (IgM and most IgG subclasses) activate the complement system which can result in the lysis of organisms or infected cells.
An important byproduct of the complement cascade is C3b, which is a protein fragment that can bind nonspecifically to cell and Ag-Ab complexes.
Binding of Ag-Ab complexes by the C3b receptors of an RBC allows it to deliver the complexes to liver or spleen where resident macrophages remove them without destroying red blood cell.
In addition, organisms or Ag-Ab complexes bound by complement can be internalized by phagocytic cells, with the resultant clearance. Internalization through complement receptors on antigen-presenting cells (APCs) can also result in the processing of antigen for presentation to T lymphocytes.
5. Transcytosis, mucosal immunity & neonatal immunity
Some antibodies can move across epithelial layers (depends on the property of the constant region of that antibody molecule) via a process called transcytosis.IgA is the major immunoglobulin that undergoes transcytosis and is available in secretory form (sIgA) in the mucosal surfaces of respiratory, gastrointestinal and urogenital tracts.
Most subclasses of IgG can cross the placental barrier (since maternal and fetal circulatory system are separate) thus conferring sample of mother’s repertoire of antibody to the developing fetus as protective endowment against pathogens. This passive immunization of developing fetus occurs during the third trimester of gestation.
Generation of Ab-diversity:
Multiple V-region genes in the germ line; i.e many gene coding for the variable (V) region.Somatic recombination between elements forming a V-region gene. i.e a number of gene segments could recombine to give a complete V-gene.
Gene conversion; a panel of pseudo genes can also be used to alter the sequences within the variable region.
Nucleotide addition; during cutting and joining of the DNA extra nucleotides may be inserted.
Somatic mutation; so the relatively few genes give rise to many mutated genes during the lifetime of the individual.
Mammals may use all five mechanisms to generate diversity and another possible mechanism like recombinational inaccuracies and assorted heavy and light chain.
In humans the genes encoding kappa and lambda light chains are found on chromosomes 2 and 22 respectively whereas, the heavy chain gene locus is found on chromosome 14.
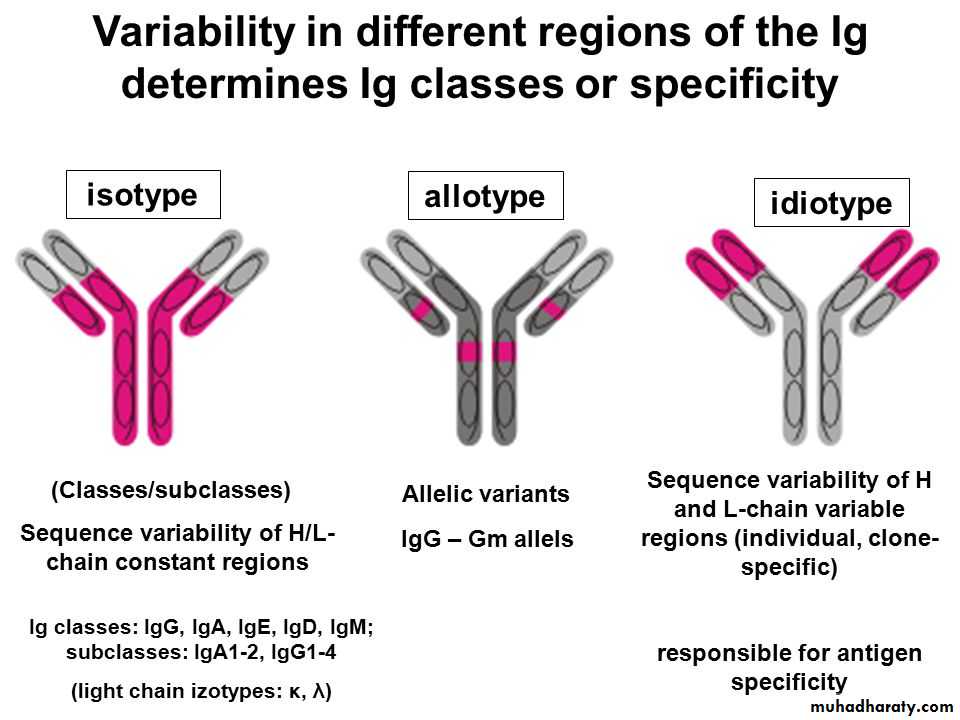
The genes for isotypic variants are gamma 1,2,3&4 , mu, alpha1&2, delta, epsilon, kabba and lambda-chain genes, all present in human geneome (Isotypic varation). Thus, isotypic variation is present in the germ line of all members of a species, producing the heavy and light chains and the V-region framework (Subgroups).
Allotypic variation is interspecies allelic variability. Allotypes occur mostly as variants of heavy chain constant regions. It is not found in all people and is therefore an allotype.
The idiotypic variation refers to the diversity at the Ag-binding site (paratope) and in particular relates to the hyper variable segments. These determine the binding specificity of the Ag-binding site. The hyper variable regions are sometimes referred to as complementarity determining region (CDRs).
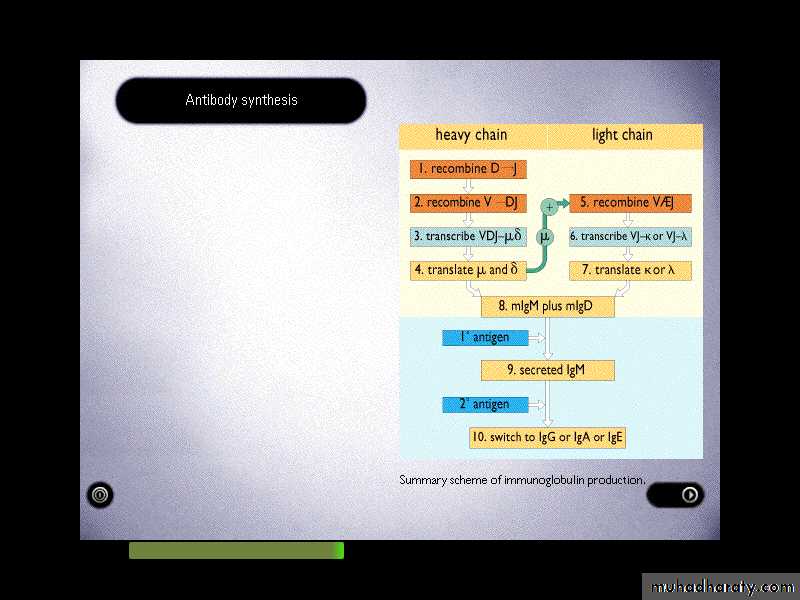
Immunoglobulin gene recombination:
Light chain genes recombine V and J segments to make a gene for the VL domain. Whereas, heavy chain genes recombine V, D and J gene segments to make a gene for the VH domain.Several Vk-genes on the germ line DNA (V1-Vn) and Jk segment (Jk1-Jk5) and only one constant-region (k-gene). But in the lambda-gene a set of V-genes (v1-Vn), just one J-gene and seven C-genes. Rearrangement of the V and J genes recombined with –region to form light chain.
Also in the heavy chain region the variable region encoded by V and J segments genes and a third gene segment, the D (diversity)-segment gene. The D is highly variable both in number and sequence of base paired.
VH (V1-Vn), DH (D1-D30), J (J1-J6). More than one D-segment may join to form an enlarged D-region. Eighty seven VH segments are found on chromosome 14, of which at least 32 are pseudo genes. The recombination of V,D,J segments in the heavy chain is largely responsible for the variability of CDRs. The place at which V & J segment gene join may vary slightly and these slight variations in the position of recombination generate additional diversity.