Biochemistry 2nd stage
Dr.Ula Abbas ZekiCARBON SKELETON OF AMINO ACID L3
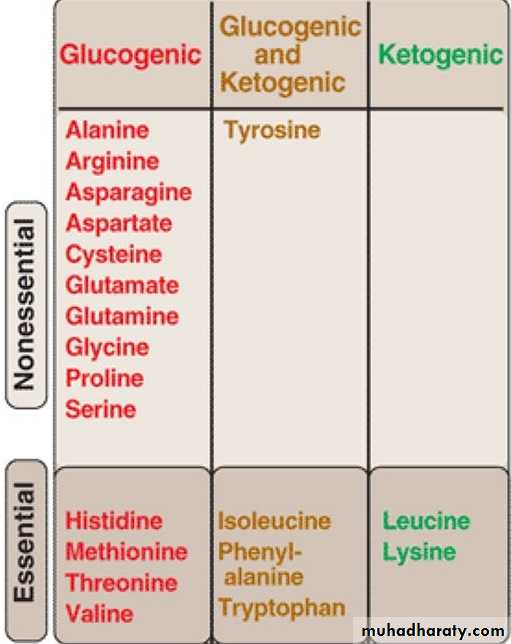
Glucogenic and Ketogenic Amino Acids
Amino acids can be classified as glucogenic, ketogenic, or both based on which of the seven intermediates are produced during their catabolism .A. Glucogenic amino acids
Amino acids whose catabolism yields pyruvate or one of the intermediates of the citric acid cycle are termed glucogenic or glycogenic. These intermediates are substrates for gluconeogenesis and, therefore, can give rise to the net formation of glucose or glycogen in the liver and glycogen in the muscle.B. Ketogenic amino acids
Amino acids whose catabolism yields either acetoacetate or one of its precursors (acetyl CoA or acetoacetyl CoA) are termed ketogenic .C. Glucogenic and ketogenic AAs → both pathways
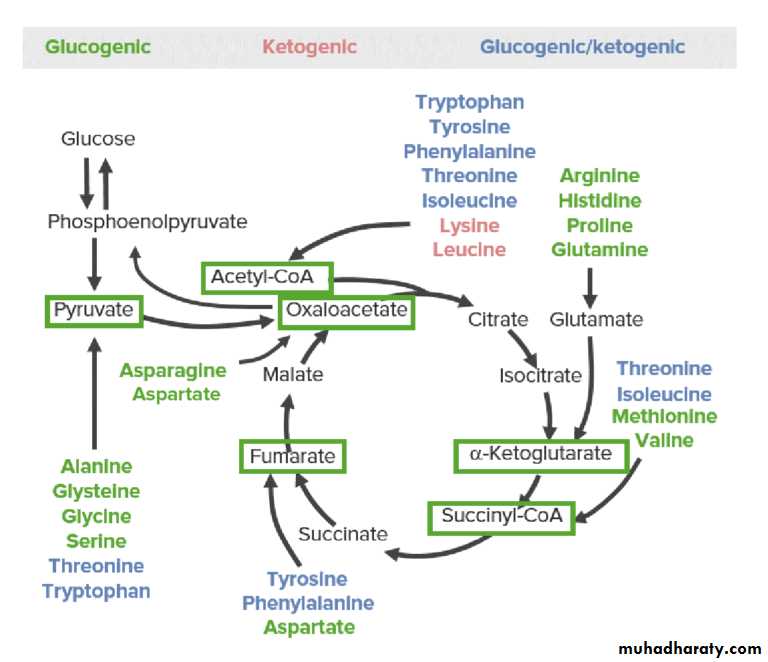
Catabolism of the Carbon Skeletons of Amino AcidsThe pathways by which amino acids are catabolized are conveniently organized according to which one (or more) of the six intermediates : pyruvate, acetyl-CoA, oxaloacetate, alpha-ketoglutarate, succinyl-CoA, and fumarate.
The breakdown of the carbon skeleton of AAs can be classified by the metabolic pathways to which their catabolic products will serve as intermediates.
Glucogenic AAs → gluconeogenesis intermediates
Ketogenic AAs → ketogenesis intermediatesGlucogenic and ketogenic AAs → both pathways
The 3 categories of catabolic products of amino acids: glucogenic (green), ketogenic (red), and both glucogenic and ketogenic (blue). The glucose-pyruvate pathway on the left represents glycolysis and gluconeogenesis. The cyclic pathway on the right represents the citric acid cycle. All amino acids are broken down into 1 of 6 intermediates (green boxes): pyruvate, acetyl-CoA, oxaloacetate, alpha-ketoglutarate, succinyl-CoA, and fumarate.
Amino acids that form succinyl CoA: methionine
Methionine is one of four amino acids that form succinyl CoA. This sulfur-containing amino acid deserves special attention because it is converted to S-adenosylmethionine (SAM), the major methyl-group donor in one-carbon metabolism . Methionine is also the source of homocysteine—a metabolite associated with atherosclerotic vascular disease.
Relationship of homocysteine to vascular disease:
Elevations in plasma homocysteine levels promote oxidative damage, inflammation, and endothelial dysfunction, and are an independent risk factor for occlusive vascular disease Mild elevations are seen in about 7% of the population.Role of Folic Acid in Amino Acid Metabolism
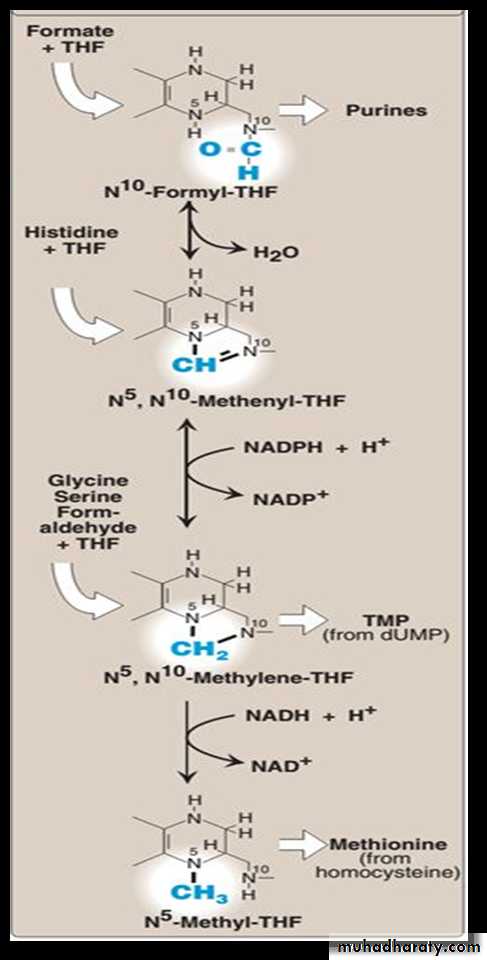
Folic acid: a carrier of one-carbon unitsThe active form of folic acid, tetrahydrofolic acid (THF), is produced from folate by dihydrofolate reductase in a two-step reaction requiring two moles of NADPH. The carbon unit carried by THF is bound to nitrogen N5 or N10, or to both N5 and N10. THF allows one-carbon compounds to be recognized and manipulated by biosynthetic enzymes.
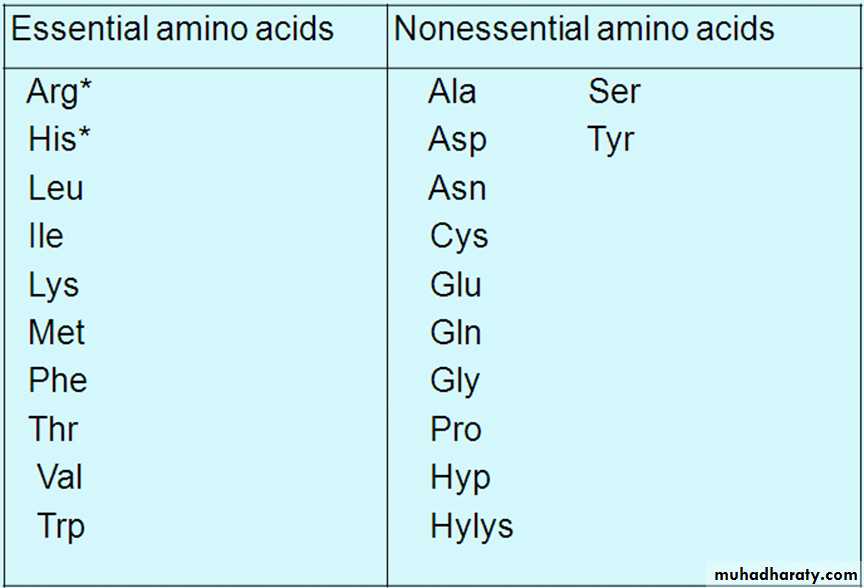
BIOSYNTHESIS OF NONESSENTIAL
AMINO ACIDSNonessential amino acids are synthesized from intermediates of metabolism or,
as in the case of tyrosine and cysteine, from the essential amino acidsphenylalanine and methionine, respectively.
These a.as called nonessential a.as i.e. they can be made available to the cells even though they are not included in the diet.
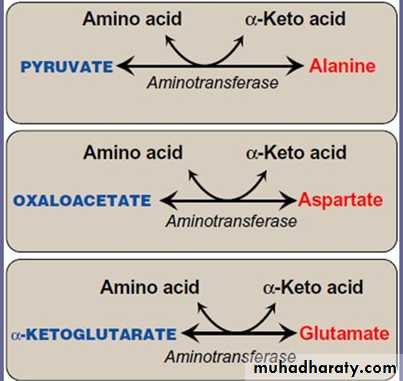
A. Synthesis from α-keto acids
Alanine, aspartate, and glutamate are synthesized by transfer of an aminogroup to the α-keto acids pyruvate, oxaloacetate, and α-ketoglutarate,
respectively. These transamination reactions are the most direct of the biosynthetic pathways.
Glutamate is unusual in that it can also be synthesized by reversal of oxidative deamination, catalyzed by glutamate dehydrogenase, when ammonia levels are high
B. Synthesis by amidation
1. Glutamine: formed from glutamate by glutamine synthetase .In addition to producing glutamine for protein synthesis, the reaction also serves as a major mechanism for the transport of ammonia in a nontoxic form.
glutamate + ATP + NHз →→→→→ glutamine + ADP + Pi
2. Asparagine: is formed from aspartate by asparagine synthetase, using glutamine as the amide donor.aspartate +glutamine + ATP →→→→→ Asparagine + ADP + Pi
C. Proline: from Glutamate by cyclization and reduction reactions.D. Serine, glycine, and cysteine
The pathways of synthesis for these amino acids are interconnected.Serine: arises from 3-phosphoglycerate, a glycolytic intermediate
Glycine: synthesized from serine by removal of a
hydroxymethyl group, also by serine hydroxymethyltransferase.
Cysteine: synthesized by two consecutive reactions in which Hcy combines with serine, forming cystathionine, which, in turn, is hydrolyzed to α-ketobutyrate and cysteine .Hcy is derived from methionine, and because methionine is an essential amino acid, cysteine synthesis requires adequate dietary intake of methionine.
Cystathionine Synthase
homocysteine + serine cystathionineCystathionase
Cystathioninecysteine + α – ketobutyrate
E. Tyrosine
Formed from phenylalanine & oxygen by the NADPH dependent enzyme called phenylalanine hydroxylase which require the coenzyme tetrahydrobiopterinPhenylalanine + NADPH + H⁺ + O tyrosine + NADP⁺ + H2O
Tyrosine and cysteine are formed from an essential amino acid and, is therefore, nonessential only in the presence of adequate dietary phenylalanine and methionine