
CHO Metabolism
Dr. Wajdy J. Majid
Assit.Prof. in clinical biochemistry
College of Medicine
University of Thi-Qar

Digestion of dietary CHO
The principal source of carbohydrate in the diet include the
polysaccharide starch and glycogen, which are based on glucose units
linked by
α-glucosidic links.
The first step in metabolism of digestible CHO is the conversion of higher
polymers to simpler , soluble forms that can be transported across the
intestinal wall and delivered to the tissue.
The breakdown of polymeric sugar begin in the mouth , saliva has a
slightly acidic PH of 6.8 and contain lingual amylase that begins the
digestion of CHO the action of salivary amylase is limited to the area of
the mouth and esophagus , it is inactivated by stronger acid PH of the
stomach once the food has arrived in the stomach , acid hydrolysis
contributes to its degradation , specific gastric protease and lipase aid this
process for protein and fat , respectively the mixture of gastric secretion,
saliva and food known collectively as chyme which move to the small
intestine.

The main polymeric CHO digesting enzyme of S.I is α-amylase this enzyme
secreted by pancreas and has the same activity as salivary amylase
producing disaccharide and trisaccharide , the later are converted to
monosaccharide by intestinal disaccharidase including maltase that
hydrolyse di and trisaccharide , and more specific disaccharidase sucrase,
lactase and trehalase, the net result is conversion of digestible CHO to
constituent monosaccharides.
The resultant glucose and other simple CHO are absorbed by S.I. and
transport to the liver and other tissue .they are converted to fatty acid ,
amino acid and glycogen, or else oxidized by various catabolic pathways of
cells . the oxidation of glucose called glycolysis, glucose is oxidized either to
pyruvate or lactate .

Under aerobic condition the dominant product in most tissue is pyruvate
and the pathway is called
aerobic glycolysis,
when oxygen is depleted ex:
during prolong vigorous exercise the dominant product of glycolysis in
many tissue is lactate and the process is called
anaerobic glycolysis
.
Under aerobic condition the dominant product in most tissue is pyruvate and
the pathway is called
aerobic glycolysis
,
when oxygen is depleted ex: during
prolong vigorous exercise the dominant product of glycolysis in many tissue is
lactate and the process is called
anaerobic glycolysis
.

Site of Glycolysis
Enzymes of glycolysis are present in the cytosol of most of the cells present
in the body .
Source of Glucose
Dietary glucose formed from the digestion of dietary carbohydrates enter
liver through portal venous system after its absorption from the intestine.
Liver distributes glucose to all other organs (cells) of the body.
Entry of the Glucose in to the Cells
Entry of the Glucose in to the Cells
Glucose enters cells by facilitated transport.
1.
Liver Glucose
enters liver cells by facilitated diffusion. It is an insulin-
independent transport mechanism for the transport of glucose across liver
cells.
2-
Extra hepatic tissues
Glucose enters adipocytes, erythrocytes, brain and
skeletal muscle by facilitated transport involving carrier molecule.
The transport of glucose across the membranes of adipose tissue and
skeletal muscle by carrier is dependent on insulin.
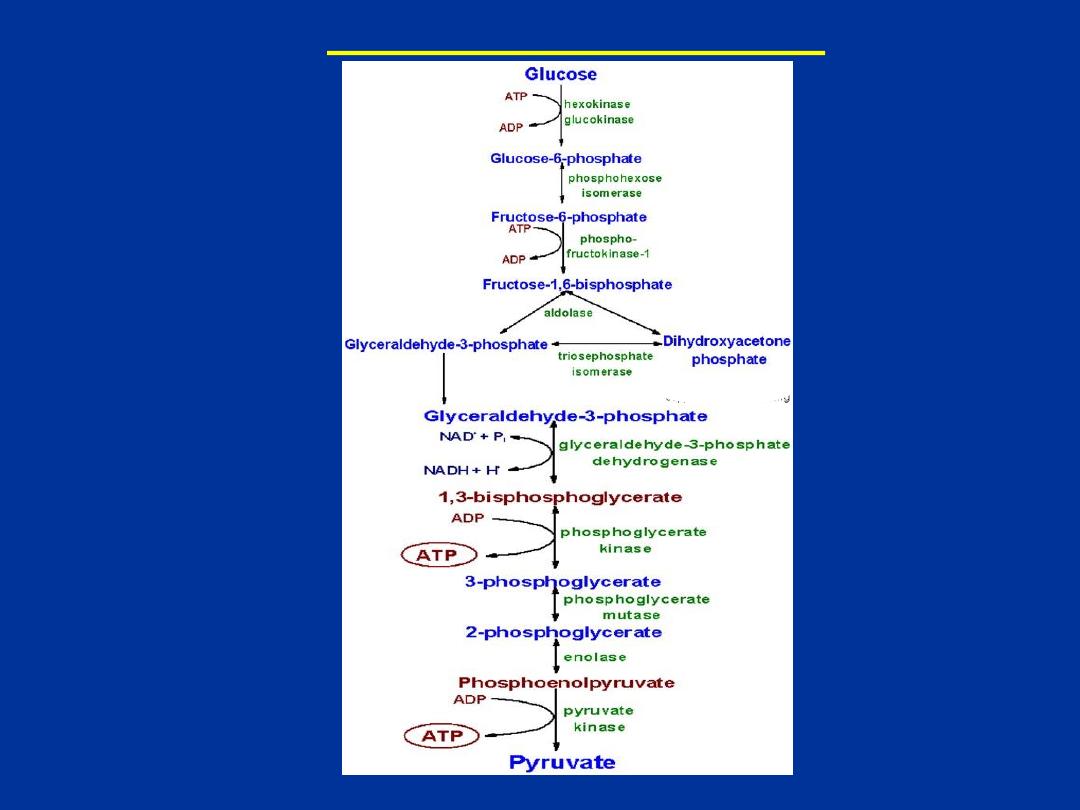
Reactions of Glycolysis

Initial reaction of glycolysis is catalyzed by
hexokinase.
It is widely
distributed. It phosphorylates glucose at 6 carbon in presence of Mg2+ and
ATP.
Hexokinase is an
allosteric enzyme
. The reaction catalyzed by this
enzyme is irreversible under normal physiological conditions .
One high energy bond of ATP is used in this reaction to generate glucose-
6-phosphate.
Liver contains glucokinase,
which phosporylates only
glucose. It is an inducible enzyme.
Its Km for glucose is high compared to
Km of hexokinase
.
Hence,
it phosphorylates glucose only when blood
glucose concentration is high
. Liver hexokinase phosphorylates glucose
even blood glucose is low.

Energetics of Glycolysis
Degradation of glucose to two molecules of pyruvate or lactate by sequence
of enzyme catalyzed reactions constitutes the process of
glycolysis
. It is a
catabolic pathway. If glucose is degraded to pyruvate then it is called as
aerobic glycolysis
.
Usually it occurs in presence of oxygen. If glucose is
degraded to lactate then it is
anaerobic glycolysis
, usually it occurs in the
absence of oxygen.
Generation and consumption of ATP in anaerobic and aerobic glycolysis is
given below :
In aerobic glycolysis:
1. Number of ATPs generated by phosphoglycerate kinase
2
2. Number of ATPs generated by Pyruvate kinase 2
3. Number of ATPs generated by respiratory chain oxidation of
2 NADH produced in reaction 6
4. Number of ATPs consumed in reaction 1 and 3
–2
Net result = 8

In anaerobic glycolysis :
2 NADH produced in reaction 6 are used to convert pyruvate to lactate. Hence, ATP
is not generated.
Therefore, the net ATP production in anaerobic glycolysis is only 2
(8 – 6 = 2).
Thus, oxidation of glucose to pyruvate
(aerobic glycolysis) generates 8 ATP
molecules
whereas oxidation of glucose to lactate
(anaerobic glycolysis) generates 2
ATP molecules
.

Anaerobic glycolysis :
Under aerobic condition pyruvate in most cells is further metabolized via
the TCA cycle . under anaerobic condition and in erythrocyte under
aerobic condition , puruvate is converted to lactate by the enzyme lactate
dehydrogenase ( LDH ) and the lactate is transported out of the cells
into the circulation .
The conversion of pyruvate to lactate under anaerobic condition provides
the cell with a mechanism for oxidation of NADH ( producing during
G3PDH reaction) to NAD which occur during the LDH catalyzed reaction ,
this reduction is required since NAD is a necessary substrate for G3PDH
without which glycolysis will cease.

Aerobic glycolysis generate substantially more ATP per mole of glucose oxidized
than does anaerobic glycolysis.
The utility of anaerobic glycolysis to muscle cell when it needs large amount of
energy during muscle contraction since that the rate of ATP production from
anaerobic glycolysis is approximately 100 faster than from oxidative
phosphorylation, during excersion muscle cells do not need to energize anabolic
reaction pathway,
the requirement is to generate the maximum amount of ATP
for muscle contraction in the shortest time frame,
this is why muscle cells derive
almost all of ATP consumed during exertion from anaerobic glycolysis.

Usually metabolic pathways are regulated by altering activities of few
enzymes of that pathway. Glycolysis is under allosteric and hormonal control.
Hexokinase , phosphofructo kinase
and
pyruvate kinase
are regulatory
enzymes of glycolysis. Their activities are allosterically controlled. Further
glucokinase,
phosphofructokinase-1
and
pyruvate
kinase
are
under
hormonal control also.
-Allosteric regulation of glycolysis
Phosphofructokinase-1
is
the
major
regulatory
enzymes
of
glycolysis
. It is an
allosteric enzyme
and catalyzes rate limiting reaction of
glycolysis. It is inhibited by ATP and citrate. AMP and fructose-6-phosphate
are activators of this enzyme .
Pyruvate kinase is the second regulatory
enzyme
.
It is inhibited by ATP and phosphoenolpyrvate.
Regulation of glycolysis
:

Glucose 6-phosphate inhibits activity of hexokinase. So, when ATP
(energy) concentration is high glycolysis is inhibited and decrease in
ATP level increases rate of glycolysis
.
Hormonal regulation of glycolysis
Insulin increases rate of glycolysis
by increasing concentration of
glucokinase, phosphofructokinase-1 and pyruvate kinase.
Metabolic fates of pyruvate :
- Pyruvate is the branch point molecule of glycolysis .
-The fate of pyruvate depends on the oxidation state of the cell,
-The fate of pyruvate during anaerobic glycolysis is reduction to lactate,
or pyruvate enter the TCA in the form of acetyl-CoA by pyruvate
dehydrogenase reaction with generation of NADH molecule to complete
aerobic oxidation of pyruvate
.

The citric acid cycle ( catabolism of acetyl-CoA) :
The citric acid cycle (Krebs cycle, tricarboxylic acid cycle) is a series
of reactions in the mitochondria that oxidize acetyl CoA to formation
of ATP .
The TCA cycle is the final common pathway for aerobic oxidation of CHO ,
lipid and protein
because glucose , fatty acid and most amino acid are
metabolized to acetyl CoA or intermediate of the cycle.

Acetyl CoA react with oxaloacetate to form citrate by a series of
dehydrogenation and decarboxylation , citrate is degraded ,
releasing reduced co-enzyme and CO2 and regenerating
oxaloacetate .
The reduced co-enzyme are oxidized by the respiratory chain
linked to formation of ATP . thus the cycle is the major route for
generation of ATP and is located in the matrix of mitochondria
adjacent to the enzyme of respiratory chain and oxidative
phosphorylation.
TCA cycle has also a central role in gluconeogensis, fatty acid
synthesis and interconversion of amino acid.
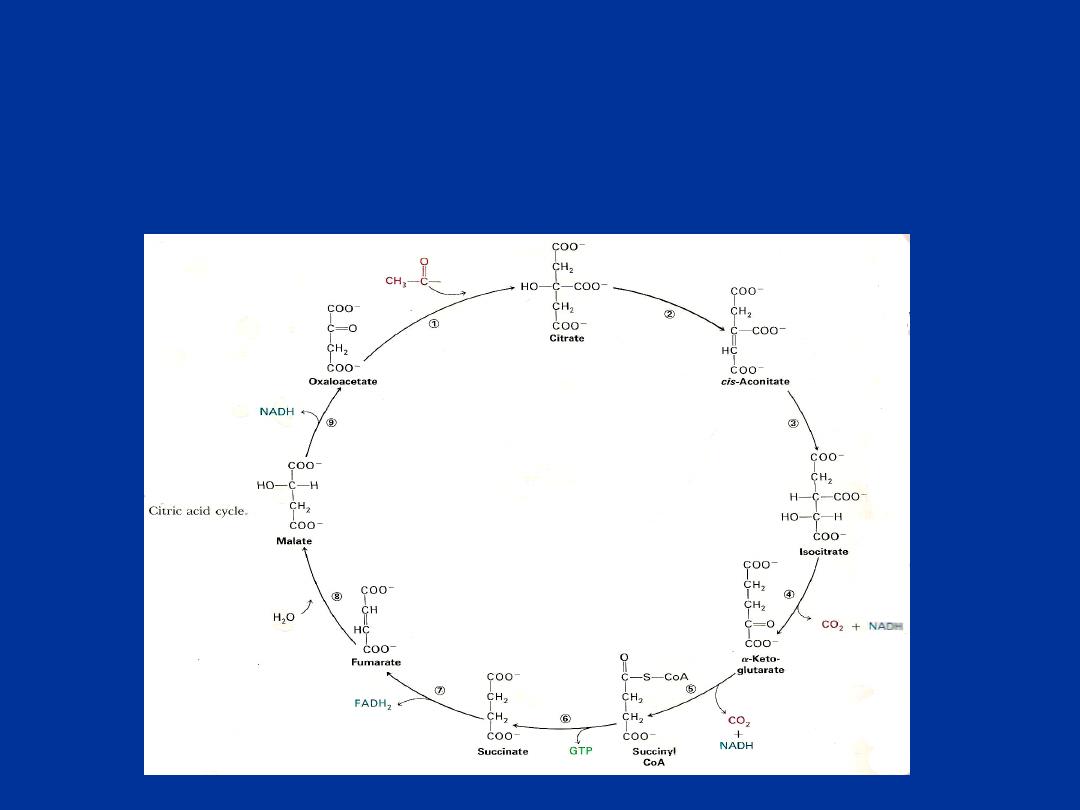
Generation of ATP in Citric Acid Cycle
1. Number of ATP generated by oxidation of 3 NADH 9
2. Number of ATP generated by oxidation of FADH2 2
3. Number of ATP generated from GTP 1
Total 12

Regulation of Citric Acid Cycle
Enzymes of citric acid cycle are under
allosteric control. Citrate synthase,
isocitrate dehydrogenase and α-ketoglutarate dehydrogenase
are involved in the
regulation of citric acid cycle and their activities are allosterically
regulated.
Citrate synthase activity is inhibited by ATP and long chain acyl-
CoA. Isocitrate dehydrogenase is inhibited by ATP and NADH and
activated by ADP. Succinyl-CoA and NADH are allosteric inhibitors
of third regulatory enzyme α-ketoglutarate dehydrogenase.
So the
rate of citric acid cycle increases in the absence of ATP
and decreases in the presence of ATP and NADH
. The energy
demand of cell determines the rate of citric acid cycle

ATP Generation during Oxidation of Glucose :
Process
Number of ATP/ mol of glucose
1. Glycolysis 8
2. Pyruvate dehydrogenase 6
3. Citric acid cycle 24
Total 38
Thus, aerobic oxidation of glucose generates total ( 38 ATP molecules ) .

Glycogen metabolism :
Glycogen
is a highly branched polysaccharide composed of D-glucose unit joined
to each other by glycosidic bond, the major linkage are α-1,4 glycosidic bond , at
interval of about ten units , there are branches in the chain involving α-1,6 linkage ,
each branch then continue with α-1,4 linkage .
The glycogen is the store of excess
glucose to supply the tissue with an oxidizable energy source are found principally
in the liver as glycogen .
The second major source of stored glucose is the glycogen of skeletal muscles.
Muscle glycogen is not generally available to other tissue because
muscle lack the
enzyme glucose-6-phosphatase
. The major site of daily glucose consumption ( 75%
) is the brain via aerobic pathway , most of remainder of it is utilized by erythrocyte
, skeletal muscle and heart muscle.

The body obtained glucose either directly from diet or from amino
acid and lactate via gluconeogensis
.
Glucose obtained from these two primary sources either remains
soluble in the body fluids or stored as glycogen .
Glycogen is considered the principal storage form of glucose and
found mainly in the liver and muscle , with kidney and intestine
adding minor storage sites.
Stores of glycogen in the liver are consider the main buffer of
blood glucose levels.

GLYCOGENESIS
Glycogenesis is the synthesis of glycogen from glucose
.
Site :
glycogen it chiefly occurs in liver and skeletal muscle. In the muscle, about 245
gms of glycogen and in the liver about 72 gms of glycogen is stored under well fed
condition. Even though-energy, rich fat is abundant in the body skeletal muscle prefers
to store glucose (energy) as glycogen because :
1. Fat can not be oxidized under anaerobic condition .
2. Acetyl-CoA of fat oxidation can not be converted to glucose.
3. Skeletal muscle is unable to mobilize fat rapidly.
The rate of( glycogensis) may be increased by insulin which is secreted by β-cells of the
pancreas in response to systemic hyperglycemia and stored as glycogen in the liver and
also the excess glucose enter the muscle under influence of insulin and stored as
glycogen
.

Glycogenolysis :
Degredation of stored glycogen ( glycogenolysis) occur by the action of glycogen
phosphorylase , the phosphorylase is remove single glucose residue from α(1,4)-
linkage within the glycogen molecules.
The product of this reaction is glucose -1-phosphate , which converted to G6P by
phosphoglucomutase
the conversion of G6P to glucose which occur in the liver ,
kidney and intestine by the action of enzyme
glucose-6-phosphatase
which does
not occur in skeletal muscle as these cells because lack this enzyme .
Therefore any glucose released from glycogen stores of muscle will be oxidized in
the glycolytic pathway.
In the liver the action of G-6-phosphatase allow
glycogenolysis to generate free glucose for maintaining blood glucose levels.

Glycogen phosphorylase
can not remove glucose residue from the branch
points (α-1,6 linkage) in glycogen . the activity of phosphorylase cease 4
glucose residue from the branch point .
The removal of these branch point glucose residue require the action of
debranching enzyme
( also called glucan transferase) which contain 2 activities
:
glucotransferase and glucosidase
, the transferase activity remove the terminal 3-
glucose residue of one branch and attaches them to a free C-4 end of a second
branch.
The glucose in α-(1,6)-linkage at the branch is then removed by action of
glucosidase
.

Hormonal Regulation of Glycogen Metabolism
Epinephrine and glucagon
increases cAMP
mediated phosphorylation,
which in turn converts
inactive glycogen phosphorylase B to active phosphorylase
A
, as a result glycogenolysis is enhanced. At the same time cAMP mediated
phosphorylation converts active glycogen synthase A to inactive glycogen synthase
B which results in decreased glycogenesis.
Insulin:
decreases glycogenolysis by decreasing cAMP mediated
phosphorylation
. At the same time insulin favours dephosphorylation of glycogen
synthase B , which results in formation of more glycogen synthase A and increased
glycogenesis.

Medical Importance of Hormonal Regulation of Glycogen
Metabolism
In between meals
hypoglycemia induces glucagon production ,
Glucagon causes breakdown of glycogen in liver
to maintain supply of glucose to
brain and cardiac muscle.
Epinephrine causes breakdown of glycogen in skeletal muscle
to maintain fuel supply
for muscle contraction. After a meal,
hyperglycemia induces insulin secretion.
Insulin causes inactivation of enzymes of glycogenolysis and activation of glycogen
forming enzymes
. As a result glycogenesis occurs in liver and muscle.

Glycogen Storage Diseases :
These are group of inherited (genetic) diseases of glycogen
metabolism. In these diseases, there is an
abnormal
accumulation of large amount of glycogen or its metabolites in
the tissues due to deficiency or absence of enzymes of glycogen
metabolism. Some of them are not serious mild disorders but
few of them are fatal.

a ) Von Geirke’s disease (Type-1 glycogen storage disease) :
It is due to the
deficiency of glucose-6-phosphatase
in liver, kidney and intestine.
The incidence of this disease is 1 in 2,00,000. it is lead to accumulation of glycogen
in liver and kidney and enlargement of liver occurs. Hypoglycemia is common
symptom other symptoms are hyperuricemia, hyperlipemia and ketosis.
b) Pompe’s disease (Type-II)
It is due to deficiency of
lysosomal α-glucosidase
. Accumulation of glycogen occurs
in all tissues. Accumulation of glycogen in heart leads to cardiomegaly. It is a fatal
disorder and death occurs before second year of life due to cardio respiratory failure
.

C) Cori’s disease (Type-III) :
It is due to
deficiency of debranching enzyme
. Limit dextrin a metabolite of
glycogenolysis accumulates in liver. Hence, this condition is also called as limit
dextrnosis
.
d ) Anderson’s disease (Type-IV):
It is a fatal disease. It is due to
absence of branching enzyme
. Amylopectin an
intermediate of glycogenesis accumulatres in liver, spleen and heart. Hence, this
condition is called as amylopectinosis
.
e) Mc Ardle’s syndrome (Type-V):
It is due to the
absence of muscle phosphorylase
. Glycogen accumulates in muscle
and lactic acid production in muscle is not increased after exercise. Affected person
suffer from painful muscle cramps and diminished tolerance to exercise.
f) Her’s disease (Type-VI):
It is due to the
absence of liver phosphorylase
. Glycogenolysis is defective and
glycogen accumulates in liver.

Gluconeogensis :
It is the biosynthesis of new glucose ( not glucose from glycogen)
,
the production of glucose from other metabolite is necessary for use as fuel source by
the brain , testis, erythrocyte and kidney medulla since glucose is the sole energy source
for these organs , it is
the process that converts non-carbohydrate substance to
glucose
.
Glucose is synthesized from pyruvate, which is derived from glucogenic amino acids,
intermediates of TCA cycle and glycerol. Since pyruvate can be formed from lactate by
the reversal of lactate dehydrogenase reaction synthesis of glucose occurs from lactate
also. Gluconeogenesis is an energy-consuming process .
- Gluconeogenesis occurs mainly in the liver and kidney.
-Enzymes of gluconeogenesis are present in mitochondria and cytosol .
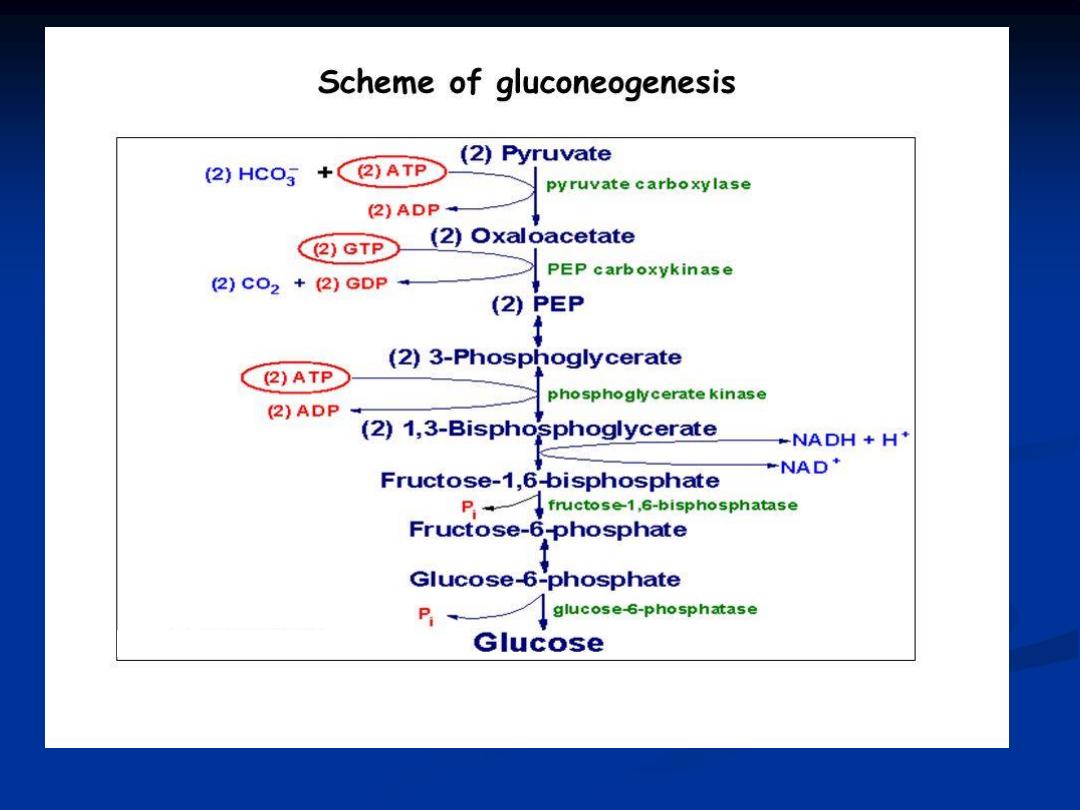

Substrate for gluconeogensis :
lactate :
is a predominant source for glucose synthesis by gluconeogensis .
during anaerobic glycolysis in skeletal muscle , pyruvate is reduced to
lactate by lactate dehydrogenase (LDH) this reduction serves two critical
function during anaerobic glycolysis first , in the direction of lactate
formation the LDH reaction require NADH and yield NAD which then
available for use by glyceraldehydes -3- phosphate dehydrogenase
reaction of glycolysis .secondly , the lactate produced by the LDH
reaction is released to blood stream and transport to the liver where it
is converted to glucose , the glucose then returned to the blood for use
by muscle as an energy source and to replenish glycogen stores.
This
cycle is termed the Cori cycle.

pyruvate :
pyruvate generated in muscle and other peripheral tissue can transaminated
to alanine which is returned to the liver for gluconeogensis. Within the liver alanine
is converted back to pyruvate and used as substrate for gluconeogensis (if that is
hepatic requirement ), or oxidized in TCA cycle.
Amino acids :
All 20 of the amino acid except leucine and lysine
can degraded to TCA cycle
intermediate.
This allow the amino acid to be converted to those in oxaloacetate and then into
pyruvate , the pyruvate can be utilized by gluconeogensis .
When glycogen store are depleted in muscle during exertion and in liver during
fasting , so catabolism of muscle protein to amino acid contribute the major source
of carbon for maintenance of blood glucose levels .

glycerol :
In the liver, glycerol is converted to dihydroxyacetone phosphate which enters
pathway of gluconeogenesis , the glycerol backbone of lipid can be used for
gluconeogensis, this require phosphorylation to glycerol 3-phosphate by glycerol
kinase
and dehydrogenation to (DHAP) by glyceraldehydes-3-phosphate
dehydrogenase (G3PDH) . so the
triacylglycerol which stored in adipose tissue
can be used its glycerol as substrate for gluconeogensis
.
propionate :
Oxidation of fatty acid with an odd number of carbon atoms and oxidation of some
amino acid generate as the terminal oxidation product (propionyl-CoA) ,propionyl –
CoA is converted to the TCA intermediate , (succinyl-CoA) this conversion is carried
out by the ATP-requiring enzyme, propionyl-CoA carboxylase
.

Medical and Biological Importance of gluconeogenesis :
1.
Gluconeogenesis
meets the glucose requirement of body when carbohydrate is
in short supply i.e., during fasting and starvation.
2.Tissues like brain, skeletal muscle, erythrocytes and testis
are completely
depend on glucose for energy and hence decrease in glucose supply cause brain
dysfunction. Body glycogen can meet glucose requirement for only 24 hours so,
beyond that period gluconeogenesis ensures glucose supply to these organs.
3. Gluconeogenesis
clears metabolic products of other tissues from blood , for
example, lactate produced by erythrocytes, skeletal muscle, glycerol produced by
breakdown of adipose tissue TG and a . a. produced by muscle protein breakdown.
4. Gluconeogenesis
converts excess of dietary glucogenic amino acids into glucose.
5. Lactic acidosis
occurs in fructose-1, 6-bis phosphatase deficiency.
6. Gluconeogenesis
is impaired in alcoholics .

Regulation of Gluconeogenesis
Enzymes of gluconeogenesis are subjected to allosteric regulation and hormone
regulation. Pyruvte carboxylase and fructose-1, 6-bisphosphatase regulates
gluconeogenesis
.
Allosteric regulation
Pyruvate carboxylase is an allosteric enzyme. Acetyl-CoA is its activator. When
glucose is in short supply fatty acid oxidation generates acetyl-CoA this in turn
activates gluconeogenesis. Fructose-1, 6-bisphosphatase is another allosteric enzyme.
AMP is its allosteric inhibitor. So when there is energy crisis gluconeogenesis is
inhibited
.
Hormonal regulation
Insulin decreases the synthesis of key enzymes of gluconeogenesis thus inhibit
gluconeogenesis
.

Glucose-alanine Cycle :
In the skeletal muscle pyruvate is converted to alanine by
transamination. Through the circulation alanine reaches liver. In the
liver pyruvate regenerated from alanine by transamination is used for
glucose synthesis. This process is called as glucose-alanine cycle.
This
cycle operates during starvation when muscle proteins are degraded
. This cycle
is meant for the transport of amino group nitrogen from muscle to
liver .

Regulation of blood glucose level :
Because of the demands of the brain for oxidizable glucose, that the human
body regulate the level of glucose circulating in the blood , this level maintained in
the range of 5 mm .
Nearly all CHO ingested in the diet are converted to glucose following
transport to the liver , catabolism of dietary or cellular protein can be utilized for
glucose synthesis via gluconeogensis, additionally other tissue besides the liver that
incompletely oxidize glucose ( predominantly skeletal muscle and erythrocyte)
provide lactate that can be converted to glucose via gluconeogensis.
Maintenance of blood glucose homeostasis is very important for survival of human
organism.
The hormones concerned with glucose homeostasis are :

Insulin :
Is the most important hormone controlling the plasma glucose concentration , it
is secreted by β-cell of pancreas , these cells produce proinsulin, which consists of the
51-amino-acid polypeptide insulin and a linking peptide ( C-peptide) , then released
into plasma mainly in response to rising plasma glucose level. Insulin bind to specific
receptors on the surface of insulin-sensitive cells of adipose tissue and muscles, the
most important effect is
stimulation of glucose entry into these cells with
resultant decrease in plasma level
, also insulin
promote glycogen synthesis in
the liver and in the muscle
, also it
stimulate fat synthesis in adipose tissue and
protein synthesis in the muscle
, but
it
inhibit gluconeogensis , lipolysis and
proteolysis.
The normal response to hyperglycemia there for depend on :
1- adequate insulin secretions.
2- Adequate insulin receptors.
3- Normal intracellular response to receptors binding of insulin ( post receptors
events) .

Glucagon :
Glucagon is a single-chain polypeptide , synthesized by α-cell of pancreas and
it secretion stimulated by hypoglycemia and fasting , glucagons stimulate hepatic
glycogenolysis by activating glycogen phosphorylase and stimulate gluconeogensis.
Growth hormone :
Released from anterior pituitary gland and act to increase blood glucose by
inhibiting uptake of glucose by extrahepatic tissue specially in muscle
, and
increase lipolysis
, but
it increase synthesis of muscle protein
, it is secretion
stimulated by hypoglycemia, stress and sleep.

Glucocorticoid :
It act to increase blood glucose level by
inhibiting glucose uptake in the muscles
, cortisol the major glucocorticoid released from adrenal cortex, is secreted in response to
the increase in circulating ACTH ,
glucocorticoid increase gluconeogensis
,
increase
protein breakdown in the muscles and increase lipolysis in adipose tissue
. It is
secretion stimulated by hypoglycemia and stress.
Adrenalin :
It is secreted from adrenal medulla it stimulate production of glucose by
activating
glycogenolysis in the liver and muscle by activating phosporylase enzyme
in
response to stressful stimuli , adrenalin also
stimulate lipolysis
.

Pentose phosphate pathway :
The pentose phosphate pathway is primarily an anabolic pathway that utilize the
6 carbons of glucose to generate 5 carbon sugars and reducing equivalents.
Primary function of this pathway are :
1-To generate reducing equivalents in the form of NADPH for reductive
biosynthesis reaction within the cells.
2-To provide the cell with ribose-5-phosphate (R5P) for the synthesis of
nucleotides and nucleic acids.
3-It can operate to metabolize dietary pentose sugars derived from the
digestion of nucleic acids as well as to rearrange the carbon skeletons of
dietary CHO into glycolytic and gluconeogenic intermediates.
Site :
Enzymes of this pathway are present in cytosol of liver, adipose tissue,
erythrocytes, adrenal cortex, thyroid, testis, ovaries and lactating mammary gland.
In the skeletal muscle the pathway is less active.
The reaction of F.A and steroid biosynthesis utilize large amount of NADPH ,
erythrocyte utilize the reaction of PPP to generate large amount of NADPH .

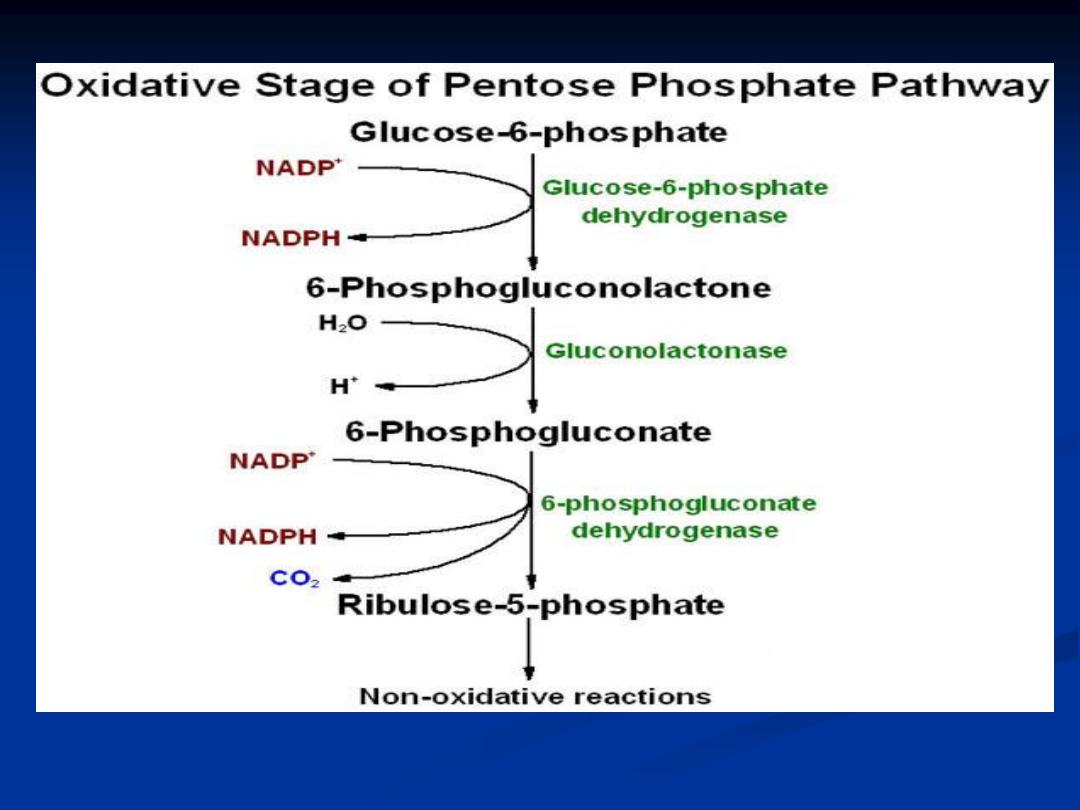
Reactions of hexose monophosphate shunt :
The reaction of PPP take place in the cytoplasm
, the PPP has both
oxidative and non oxidative arm
.
The oxidation steps , utilizing glucose-6-phosphate (G6P) as the substrate occur at
the beginning of pathway and the reaction that generate NADPH. the reaction
catalyzed
by
glucose-6-phosphate
dehydrogenase
and
6-phosphogluconate
dehydrogenase generate one mole of NADPH each for every mole of glucose-6-
phosphate that enter the PPP.
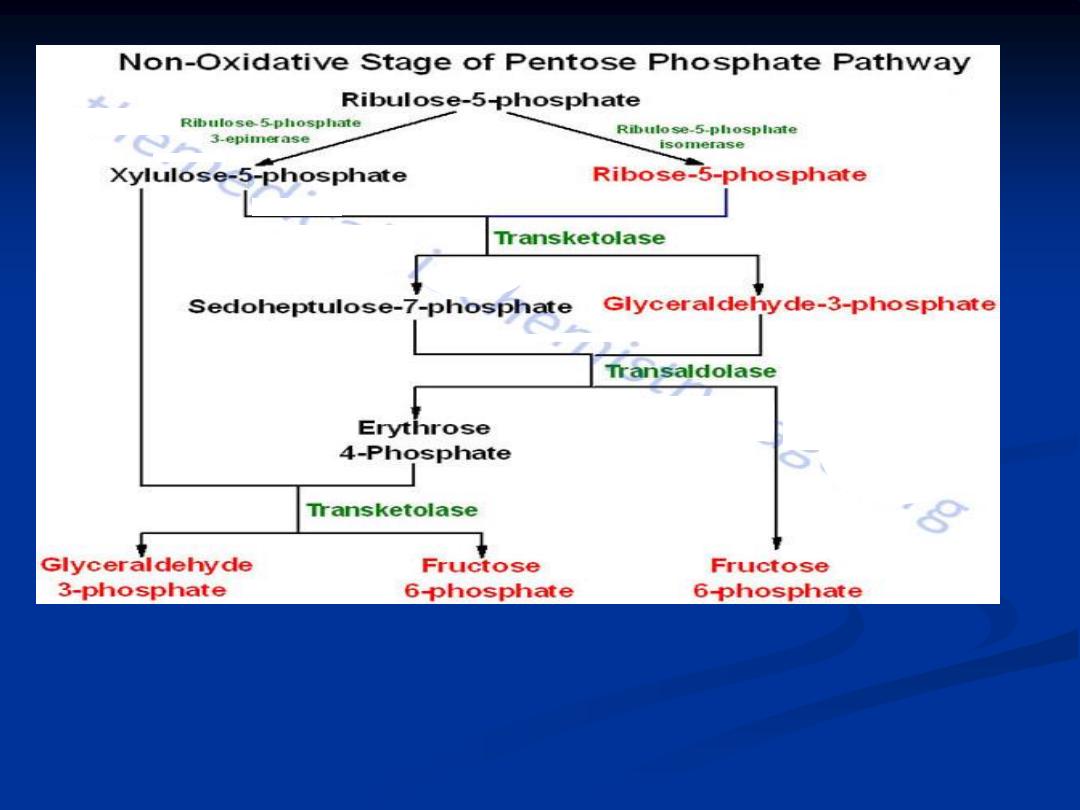
The non oxidative reactions of PPP are primarily designed to generate R-5-P.
Also the important reaction of PPP are to convert dietary 5 carbon sugars into
both (fructose-6-phosphate) and (glceraldehyde-3-phosphate) which can then be
utilized by pathway of glycolysis.

Medical Importance of Pentose Phosphate Pathway:
Glucose-6-Phosphate Dehydrogenase Deficiency ( G6PDD ) :
The PPP supplies the RBC with NADPH to maintain the reduced state of glutathione.
In some individual carry defective gene which less active glucose-6-phosphate
dehydrogenase and becomes inactive in presence of certain drugs. So, the affected
individuals are normal until they are exposed to those drugs.
Glucose-6-phosphate dehydrogenese deficiency
occurs when drugs like aspirin,
antibiotics
:
ciprofloxacin , nitofurantoin , sulphonamide
also
anti-malarial
drug
and
sulfonamide
are
administered to these individuals. Since NADPH production is
blocked in these individuals due to the deficiency of G6PD the susceptibility of RBC
to hemolysis is increased. Therefore, the affected individuals develop hemolytic anemia
on exposure to these drugs. Consumption of fava beans also causes G6PD deficiency
in the susceptible individuals. Favism is the name given to this type of G6PD
deficiency.

The inability to maintain reduced glutathione in the RBC lead to increase
accumulation of peroxides H2O2 , predominantly H2O2 that result in a weakening
of the cell wall and concomitant haemolysis , accumulation of H2O2 also lead to
increase rate of oxidation of hemoglobin to methemoglobin that also lead to
weakening of the cell wall.
Glutathione remove peroxide via the action of glutathione peroxidase, the PPP in
erythrocyte is the only pathway for these cells to produce NADPH , so any defect in
production of NADPH lead to effect on RBC survival .
So deficiency of G6PD may cause haemolytic anemia
this enzyme catalyze the
first step in P.P.P pathway and need for formation of NADPH which is essential for
maintenance of intact red cell mem. and for protection RBC against oxidative stress.
The G6PD is X-linked recessive disorder affecting mainly the male.

The role of liver in the buffering blood glucose :
The blood glucose level in atypical person after an overnight fast is 80 mg/dl (4.4
mmol/l) , the blood glucose level during the day normally range from about 80 mg/dl
before meal to about 120 mg/dl after meal ,
the blood glucose level is controlled
primarily by the action of the liver , which can take up or release large amounts of
glucose in response to hormonal signals and the level of glucose itself
. After the CHO
containing meal , the liver can store some of excess glucose as glycogen , the rate
of glycogen synthesis (glycogensis) from (G6P) may be increased by insulin which is
secreted by the β-cells of pancreas in response to systemic hyperglycemia .
The liver can convert some of excess glucose to fatty acid which are ultimately
transported as triglyceride in VLDL and store in adipose tissue Under aerobic
condition the liver can synthesize glucose (by gluconeogensis) using the metabolic
products from other tissue , such as glycerol , lactate or carbon chains resulting from
deamination of most amino acid (mainly alanine)

The liver contain enzyme( G6Pase)
which can release free glucose from (G6P) ,
the (G6P) result from glycogen breakdown (glycogenolysis) or by gluconeogensis
the releasing of free glucose from (G6P) help to maintain extracellular fasting level.
Hepatic glycogenolysis is stimulated by hormone glucagone secreted by α-cells of
pancreas , and by catecholamine such as adrenalin or nor adrenalin .
During fasting the liver can convert fatty acid , released from adipose tissue as a
consequence of low insulin activity to ketones , these can be used by other tissue ,
including the brain as an energy source when glucose is in short supply.

Other organs
The renal cortex is the only other tissue capable of gluconeogenesis
, and of
converting G6P to glucose.
The gluconeogenic capacity of the kidney is particularly important in
hydrogen ion homeostasis and
during prolonged fasting.
Other tissues, such as muscle, can store glycogen but, because they do
not contain glucose-6-phosphatase, they cannot release glucose from
cells and so can only use it locally; this glycogen plays no part in
maintaining the plasma glucose concentration
.
