Phagocytosis
By• Dr. Dhafer A. Alghezi
• Ph.D. Clinical immunology/ Cancer research
• College of Medicine
Phagocytosis
“phago”=eat, “cyte”=cell.It is a specific form of endocytosis.
Represents a cellular process used to ingest and eliminate particles larger than 0.5 μm in diameter, including microorganisms, foreign substances, and apoptotic cells.
Cells that perform phagocytosis are called a phagocytes.
Phagocytes
They are protected the body by ingesting harmful foreign particles, bacteria, and dead cells.Phagocytes are called "professional" or "non-professional" depending on how effective they are at phagocytosis.
The professional phagocytes are responsible of removing microorganisms and of presenting antigens to lymphocytes in order to activate an adaptive immune response. They include many types of white blood cells such as neutrophils, monocytes, macrophages, mast cells, and dendritic cells.
Fibroblasts, epithelial cells, and endothelial cells can also accomplish phagocytosis with low-efficiency and are thus described as non-professional phagocytes. These cells cannot ingest microorganisms, but are important in eliminating dead cells and maintaining homeostasis.
Professional Vs non-professional
Professional Vs non-professionalProfessional phagocytes have molecules called receptors on their surfaces that can detect harmful objects, such as bacteria, that are not normally found in the body.
Numerous receptors are involved in phagocytosis such as complement receptors and Fc receptors are particularly important for the recognition and phagocytosis of opsonized microbes and other solid matter.
Other receptors, including opsonin receptors, scavenger receptors, and Toll-like receptors, are also important in the uptake of many pathogenic microorganisms.
Opsonin receptors increase the phagocytosis of bacteria that have been coated with immunoglobulin G (IgG) antibodies or with complement.
Scavenger receptors bind to a large range of molecules on the surface of bacterial cells.
Toll-like receptors—so called because of their similarity to well-studied receptors in fruit flies that are encoded by the Toll gene—bind to more specific molecules.Binding to Toll-like receptors increases phagocytosis and causes the phagocyte to release a group of hormones that cause inflammation.
The general activities of phagocytes are summaries below:
• To survey the tissue compartments and discover microbes, particulate matter (dust, carbon particles, antigen-antibody complexes, and injured or dead cells).• To ingest and eliminate these materials.
• To extract immunogenic information (antigens) from foreign matter.
The three main types of phagocytes are neutrophils, monocytes, and macrophages.
Neutrophil
The neutrophil is the primary phagocyte that arrives early at the site of inflammation, usually within 90 minutes of injury.The neutrophils’ cytoplasmic granules contain enzymes and other antibacterial substances that are used in destroying and degrading the engulfed particles.
The neutrophil count in the blood often increases greatly during the inflammatory process, especially with bacterial infections.
Increased neutrophil count in the blood is called neutrophilia.
Neutrophils are also a primary component of pus.
Eosinophils:
The second major polymorphonuclear granulocyte.They spend short time circulating in the blood stream, then they migrate into tissues.
Eosinophils are attracted to sites of parasitic infections and antigen-antibody reactions, though they play only a minor phagocytic role.
The granules of eosinophils contain a protein that is highly toxic to large parasitic worms that cannot be phagocytized.
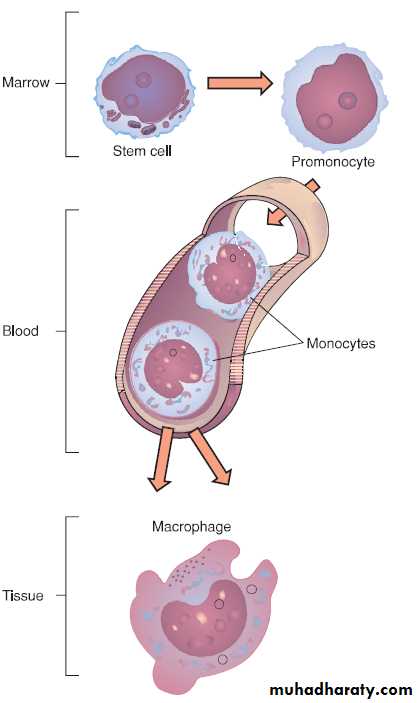
Monocytes
The monocytes are the largest white blood cells.They help to destroy the causative agent, aid in the signaling processes of specific immunity, and serve to resolve the inflammatory process.
The monocytes, which migrate in increased numbers into the tissues in response to inflammatory stimuli, mature into macrophages.
This process is marked by an increase in size and by enhanced development of lysosomes and other organelles.
The developmental stages of monocytes and macrophages. The cells progress through maturational stages in the bone marrow and peripheral blood.
Migrated monocytes are transformed by various inflammatory mediators into macrophages.
This process is marked by an increase in size and by enhanced development of lysosomes and other organelles.
Macrophages, king of phagocytes, can be classified into fixed (adherent to tissue) or wandering macrophage.
The fixed macrophage concentrates in specific areas that are more vulnerable to intruders like the lungs or the intestine, whereas, wandering macrophages travel throughout both blood and lymph streams to perform their job.
Specialized macrophages called histiocytes migrate to a certain tissue and remain there during their lifespan.
Examples are alveolar macrophages (lung), the Kupffer cells in the liver, Langerhans cells in the skin and macrophages in the spleen, lymph nodes, bone marrow, kidney, and brain.
Other macrophages do not reside permanently in a particular tissue and drift nomadically throughout the RES.
Not only are macrophages dynamic scavengers, but they also process foreign substances and prepare them for reactions with B and T lymphocytes.
Liver tissue with Kupffer cells.
micrograph view of a lung with an alveolar macrophage.Langerhans cells deep in the epidermis
Mechanisms of Phagocytic (steps of phagocytosis)
Step 1: Activation of the Phagocyte.Step 2: Chemotaxis of Phagocytes
Step 3: Attachment of the Phagocyte to the microbe or cell.Step 4: Ingestion of the microbe or cell by the Phagocyte
Step 5: Destruction of the microbe or cellStep 1: Activation of the Phagocyte
Resting phagocytes are activated by inflammatory mediators such as bacterial products (bacterial proteins, capsules, LPS, peptidoglycan, teichoic acids, etc.), complement proteins, inflammatory cytokines, and prostaglandins.As a result, the circulating phagocytes produce surface glycoprotein receptors that increase their ability to adhere to the inner surface of capillary walls, enabling them to squeeze out of the capillary and be attracted to the site of infection.
Step 2: Chemotaxis of Phagocytes
Chemotaxis is the movement of phagocytes toward an increasing concentration of some attractant such as bacterial factors (bacterial proteins, capsules, LPS, peptidoglycan, teichoic acids, etc.), complement proteins (C5a), chemokines (chemotactic cytokines such as interleukin-8), fibrin split products, kinins, and phospholipids released by injured host cells.Some microbes, such as the influenza A viruses, Mycobacterium tuberculosis, blood invasive strains of Neisseria gonorrhoeae, and Bordetella pertussis have been shown to block chemotaxis.
Phagocytes contain membranous sacs called lysosomes produced by the Golgi apparatus that contain various digestive enzymes, microbicidal chemicals, and toxic oxygen radicals.
The lysosomes travel along microtubules within the phagocyte and fuse with the phagosomes containing the ingested microbes and the microbes are destroyed
Step 3: Attachment of the Phagocyte to the Microbe or Cell
Attachment of microorganisms is necessary for ingestion. Attachment may be unenhanced or enhanced.Unenhanced attachment: It is the innate recognition of pathogen-associated molecular patterns or PAMPs - components of common molecules such as peptidoglycan, teichoic acids, lipopolysaccharide, mannans, and glucans common in microbial cell walls but not found on human cells - by means of endocytic pattern-recognition receptors, such as scavenger receptors and mannose receptors, on the surface of the phagocytes.
Enhanced attachment: It is the attachment of microbes to phagocytes by way of an antibody molecule called IgG, the complement proteins C3b and C4b produced during the complement pathways and acute phase proteins such as mannose-binding lectin (MBL) and C-reactive protein (CRP).
Molecules such as IgG, C3b, and mannose-binding lectin (MBL) that promote enhanced attachment are called opsonin and the process is also known as opsonization. Enhanced attachment is much more specific and efficient than unenhanced.
Extracellular trapping with neutrophil extracellular traps (NETs): In response to certain pathogen associated molecular patterns such as LPS, and certain cytokines such as IL-8, neutrophils release DNA and antimicrobial granular proteins.
These (NETs) bind to bacteria, prevent them from spreading, and kill them with antimicrobial proteins.
Neutrophil extracellular traps (NETs) are networks of extracellular fibers, primarily composed of DNA from neutrophils, which bind pathogens.
NETs allow neutrophils to kill extracellular pathogens while minimizing damage to the host cells.
Step 4: Ingestion of the Microbe or Cell by the Phagocyte
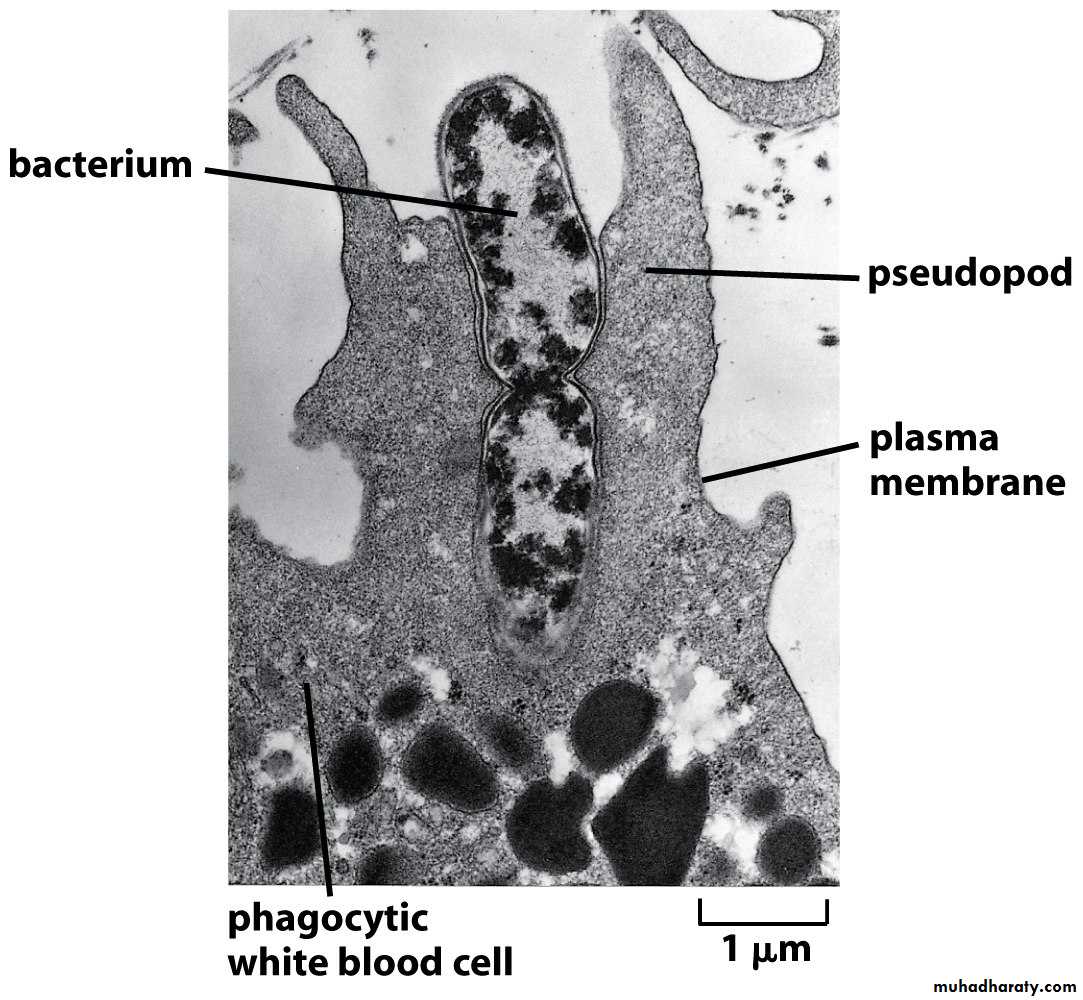
Following attachment, polymerization and then depolymerization of actin filaments send pseudopods out to engulf the microbe and place it in an endocytic vesicle called a phagosome.During this process, an electron pump brings protons (H+) into the phagosome.
This lowers the pH within the phagosome to 3.5 - 4.0 so that when a lysosome fuses with the phagosome, the pH is correct for the acid hydrolases to effectively break down cellular proteins.
The acidification also releases defensins, cathelicidin, and bacterial permeability inducing protein (BPI), peptides and enzymes that can kill microbes, from a matrix and enabling their activation.
Intracellular microbes, such as viruses and bacteria that invade host cells, can also be engulfed once they enter the cytosol of the cell by a process called autophagy.
A membrane-bound compartment called an autophagosome grows around the microbe and the surrounding cytosol and subsequently delivers it to lysosomes for destruction.
Step 5: Destruction of the Microbe or Cell
Phagocytes contain membranous sacs called lysosomes produced by the Golgi apparatus that contain various digestive enzymes, microbicidal chemicals, and toxic oxygen radicals.The lysosomes travel along microtubules within the phagocyte and fuse with the phagosomes containing the ingested microbes and the microbes are destroyed.
There are 2 killing systems in neutrophils and macrophages: the oxygen-dependent system and the oxygen-independent system
1. The oxygen-dependent system: production of reactive oxygen species (ROS)
2. The oxygen-independent system
The oxygen-dependent system: production of reactive oxygen species (ROS)
The cytoplasmic membrane of phagocytes contains the enzyme oxidase which converts oxygen into superoxide anion (O2-).This can combine with water by way of the enzyme dismutase to form hydrogen peroxide (H2O2) and hydroxyl (OH) radicals.
In the case of neutrophils, but not macrophages, the hydrogen peroxide can then combine with chloride (Cl2-) ions by the action of the enzyme myeloperoxidase (MPO) to form hypochlorous acid (HOCL), and singlet oxygen.
In macrophages, nitric oxide (NO) can combine with hydrogen peroxide to form peroxynitrite radicals.
In addition to ROS and NO, macrophages secrete inflammatory cytokines such as TNF-alpha, IL-1, IL-8, and IL-12 to promote an inflammatory response.
These compounds are very microbicidal because they are powerful oxidizing agents which oxidize most of the chemical groups found in proteins, enzymes, carbohydrates, DNA, and lipids.
Lipid oxidation can break down cytoplasmic membranes.
Collectively, these oxidizing free radicals are called reactive oxygen species (ROS).
Oxidase also acts as an electron pump that brings protons (H+) into the phagosome. This lowers the pH within the phagosome so that when lysosomes fuse with the phagosome, the pH is correct for the acid hydrolases, like elastase, to effectively break down cellular proteins.
In addition to phagocytes using this oxygen-dependant system to kill microbes intracellularly, neutrophils also routinely release these oxidizing agents, as well as acid hydrolases, for the purpose of killing microbes extracellularly.
These agents, however, also wind up killing the neutrophils themselves as well as some surrounding body cells and tissues as mentioned above.
The oxygen-independent system
Some lysosomes contain defensins , cationic peptides that alter cytoplasmic membranes.lysozyme, an enzyme that breaks down peptidoglycan.
lactoferrin, a protein that deprives bacteria of needed iron.
cathepsin G, a protease that causes damage to microbial membranes. elastase, a protease that kills many types of bacteria.
cathelicidins, proteins that upon cleavage are directly toxic to a variety of microorganisms; bactericidal permeability inducing protein (BPI ), proteins used by neutrophils to kill certain bacteria by damaging their membranes; collagenase ; and various other digestive enzymes that exhibit antimicrobial activity by breaking down proteins, RNA, phosphate compounds, lipids, and carbohydrates.
• The enzyme NADPH oxidase in the phagosome membrane reduces oxygen by the addition of electrons to form superoxide anion . This can then give rise to hydroxyl radicals (•OH), singlet oxygen (Δg′O2) and H2O2, all of which are potentially toxic. If lysosome fusion occurs, myeloperoxidase or in some cases, catalase from peroxisomes, acts on peroxides in the presence of halides to generate toxic oxidants such as hypohalite. NADPH, nicotinamide adenine dinucleotide phosphate.