ENAMEL
For 2nd gradeDr. Huda Y. K.
LECTURE CONTENTSDefinition of enamel.
Composition of enamel.
Structure of enamel.
Properties of enamel.
Function of the enamel.
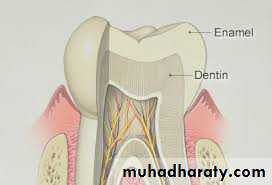
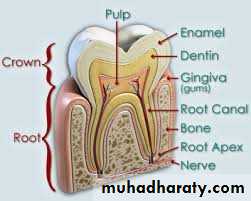
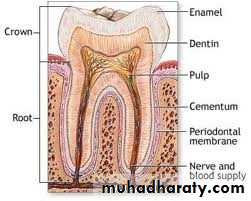
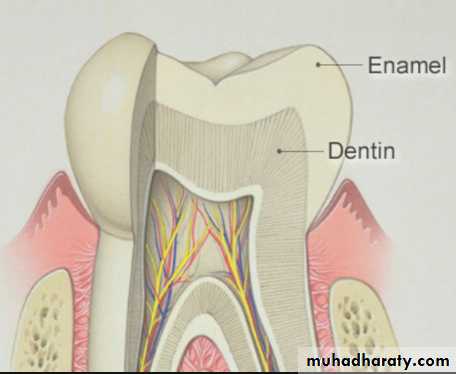
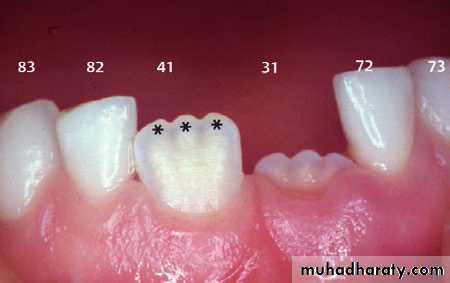
Tooth enamel is the hardest and most highly mineralized substance of the body which covers the crown of the tooth.
Enamel formation, amelogenesis, is accomplished by cells called ameloblasts. These cells originate from the embryonic germ layer known as ectoderm.
One of the main goals in operative dentistry is preservation of enamel.
ENAMEL
Although enamel can serve lifelong, but it is more susceptible to :
CariesAttrition (physical forces)
Fracture
One of the interesting features of enamel is that it cannot repair itself.
So, loss in enamel surface can be compensated only by restorative treatment.Chemical Composition and Structure Of Enamel
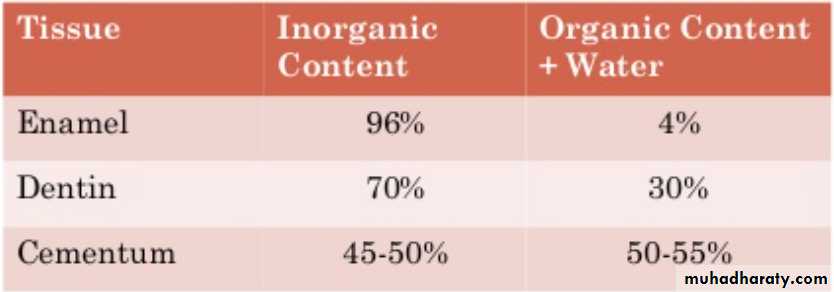
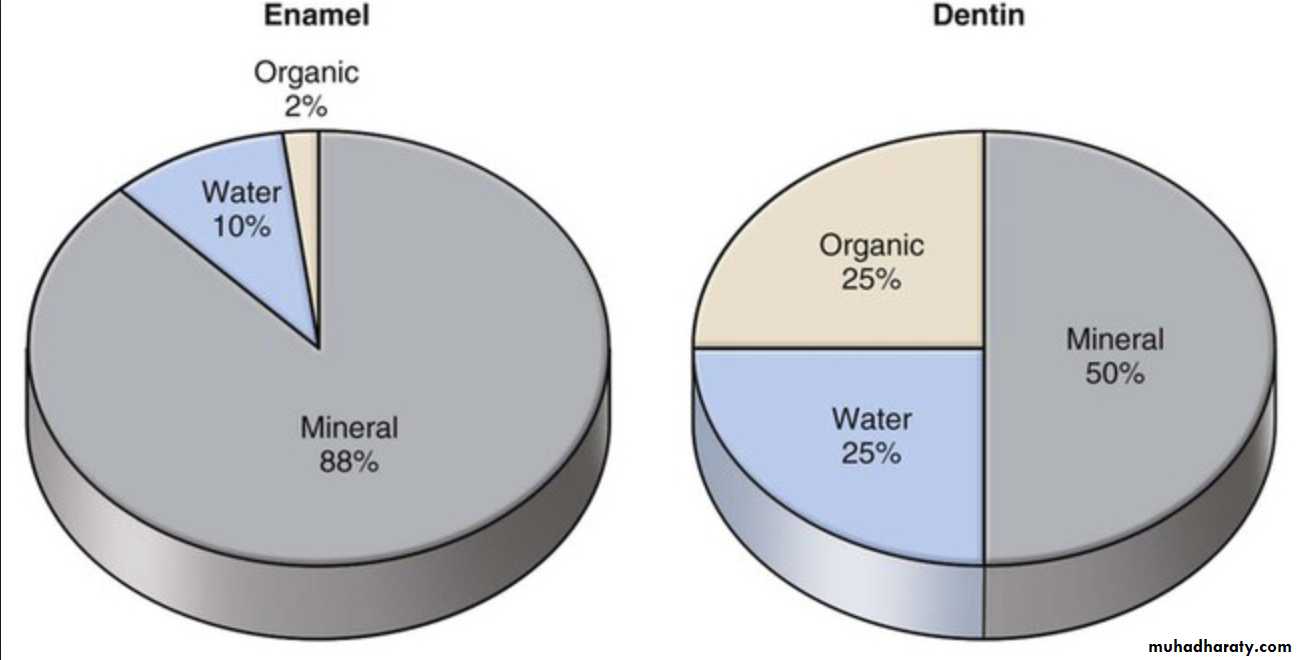
The Inorganic content of mature enamel amounts to 96% by weight; the remainder is organic material (protein, lipid) and water.On the basis of volume around 88% is mineral,10% is water, and 2% is organic material (protein, lipid).
Owing to its hardness, enamel is difficult to cut for histological examinations used to study its structure.
Therefore different approaches have been considered to describe its nature.
One way to do this is at the crystalline level.
In material science, a crystal is a solid substance in which the atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three dimensions.
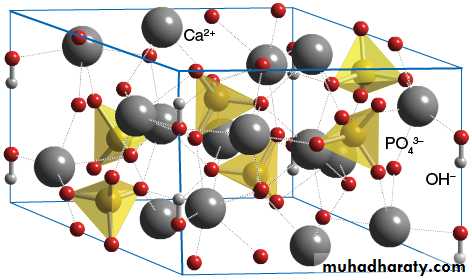
The crystals made by the ameloblasts consist of calcium phosphate
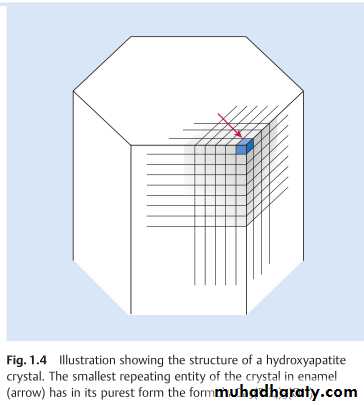
The smallest repeating entity of the crystals in enamel has in its purest form, the formula Ca5(PO4)3(OH) , which is termed hydroxyapatite (HAP).The crystals are approximately hexagonal in cross-section, with a diameter 40nm.
At the chemical level, several substitutions of the ions in HAP can and do occur (resulting in impure forms of HAP) for example:
Substitution with fluoride giving fluoride hydroxyapatite (FHAP);
Substitution with carbonate, carbonate-modified hydroxyapatite (CHAP)
Substitution with magnesium, magnesium-modified hydroxyapatite (MHAP).
More commonly, the OH − ions are only partially replaced by fluoride, and FHAP is formed. These crystals have a lower solubility than HAP, which again has lower solubility than CHAP.
These chemical conditions have great influence on the caries process
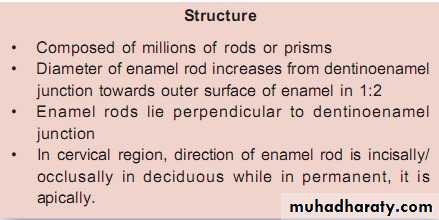
Structure
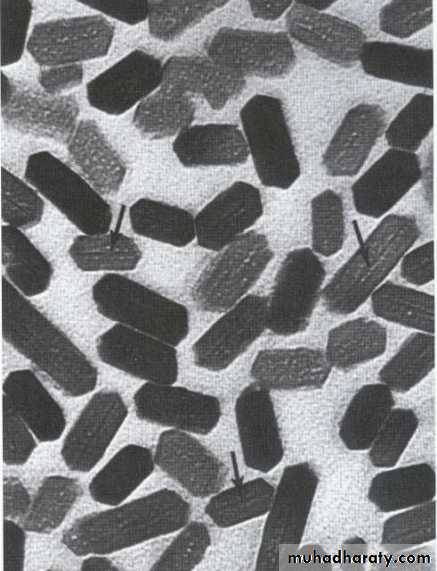
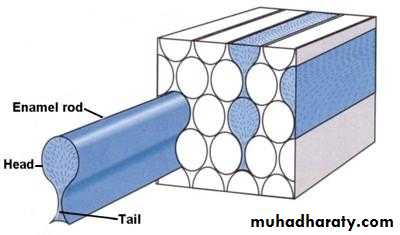
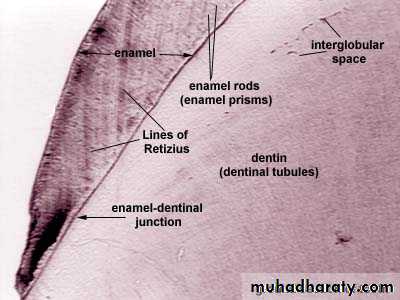
The individual crystals are arranged in rods (or prisms) extending from the enamel–dentin junction to the surface, with an average diameter of about 4–5µm.Each rod formed of about 300 unit crystal length and 40 units wide and 20 unit thick in three dimensional hexagon.
In transverse sections, enamel rods appear as hexagonal and occasionally round or oval. Rods may also resemble fish scales.
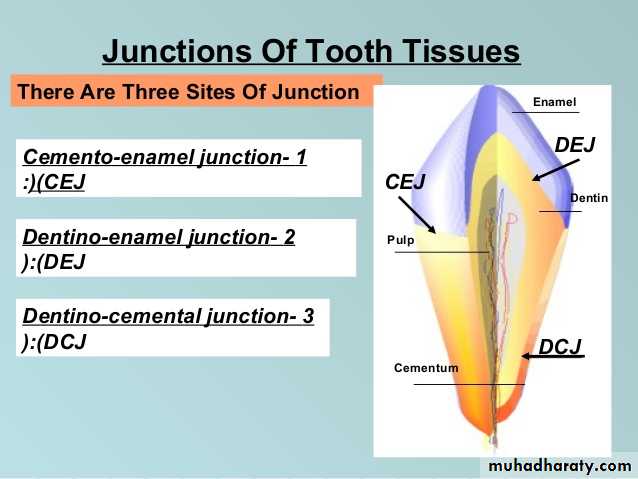
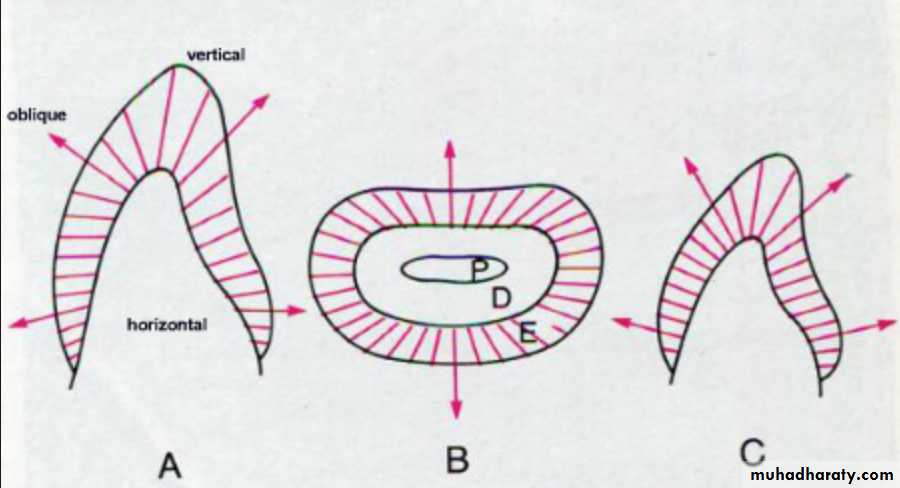
The diameter of rods increases from DEJ towards the outer surface of enamel in a ratio of 1:2.
Enamel rods are arranged in such planes so as to resist the maximum masticatory forces.
Due to this uniform structure of the enamel with tightly packed crystals, light penetrates through the enamel and is reflected or absorbed in the dentin.
Well mineralized, permanent enamel is translucent, and it is the underlying dentin which, eventually, gives the tooth its color.
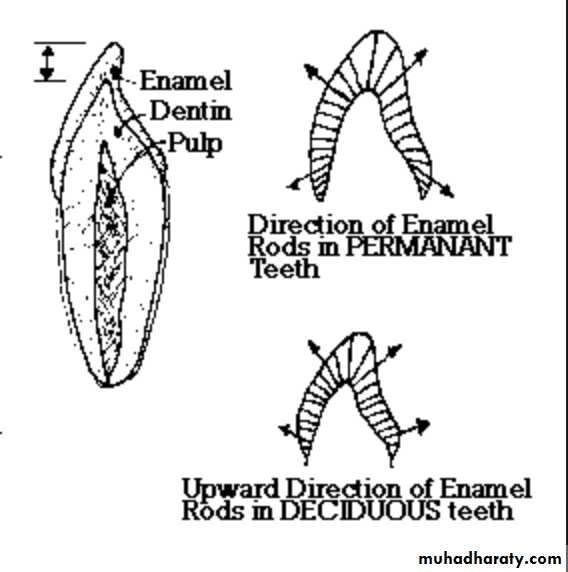
Rods are oriented at perpendicular to the DEJ.
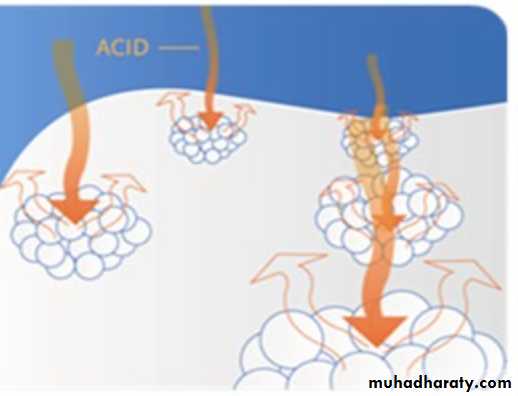
Towards the incisal edge these become increasingly oblique and are almost vertical at the cusp tips.Enamel is soluble when exposed to an acid medium, but the dissolution is not uniform.
Solubility of enamel increases from the enamel surface to the DEJ.When fluorides are present during enamel formation or are topically applied to the enamel surface, the solubility of surface enamel is decreased.
Fluoride concentration decreases toward the DEJ
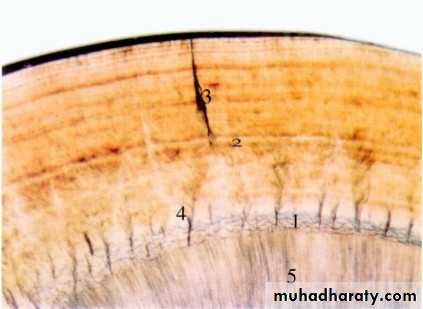
STRUCTURE PRESENT IN ENAMEL
GNARLED ENAMEL.BANDS OF HUNTER-SCHEGER
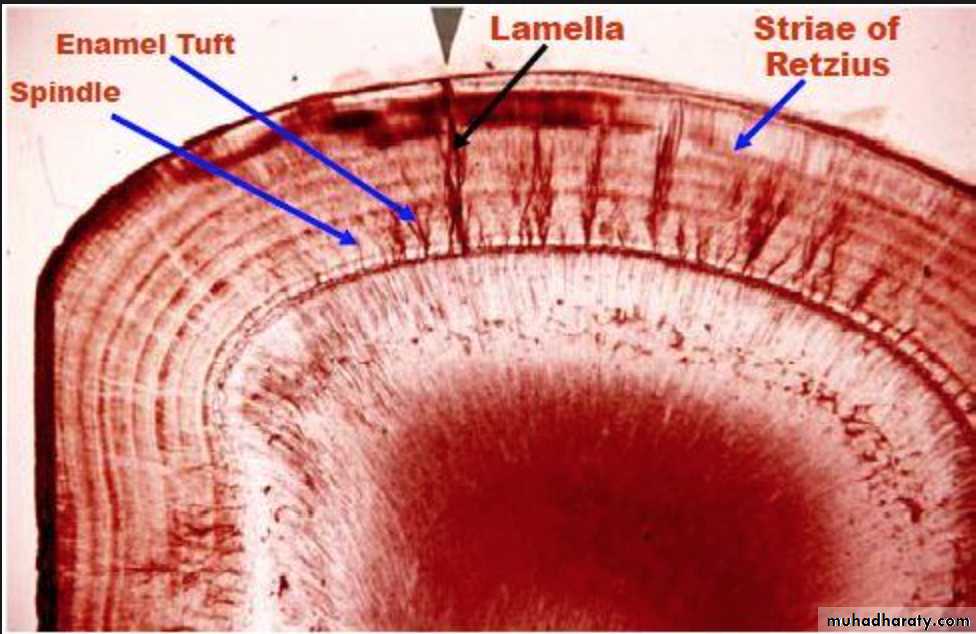
ENAMEL TUFT
ENAMEL LAMELLAE
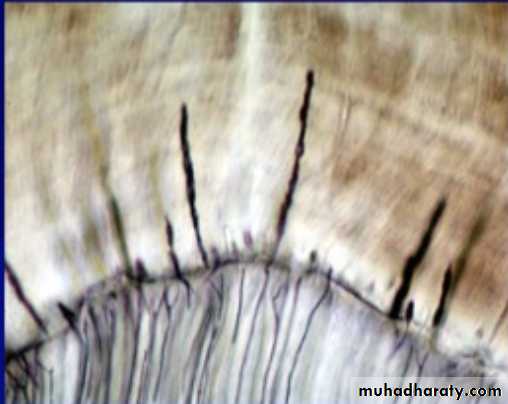
ENAMEL SPINDLE
STRIAE OF RETZIUS
1.Gnarled Enamel
There are group of irregular enamel that is more resistant to cleavage called Gnarled enamel present mostly in cervical, incisal and occlusal portion.This consists of bundles of enamel rods which interwine in an irregular manner with other group of rods, finally taking a twisted and irregular path towards the tooth surface.
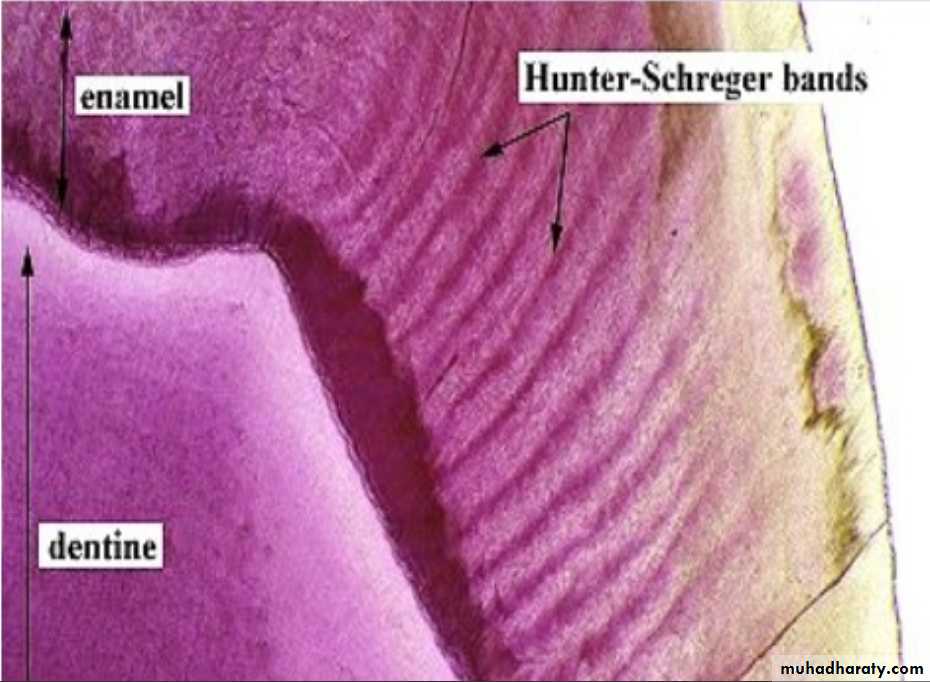
2.Bands of Hunter-Schreger
Hunter-Schreger bands usually occur because of alteration of light reflection(Optical phenomenon) ,due to changes in rod direction.This results in alternating light and dark zones under the microscope.
They are mainly found in the inner surface of tooth. these bands are composed of different contents of organic material and varied permeability.
Significance: They are considered to resist and disperse the strong forces.
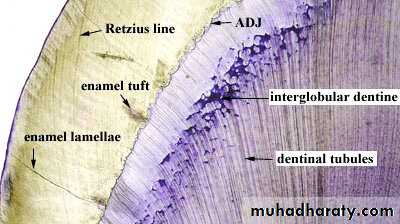
3.Enamel Tufts (Hypomineralized structures)
Enamel tufts are ribbon-like structures which run from dentin to enamel. They are named so because they resemble tufts of grass.They contain greater concentration of enamel proteins.
Significance: Hypomineralized structure in the enamel, thus it plays role in spread of dental caries.4.Enamel Lamellae
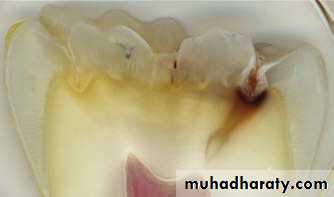
These are leaf like defects present in enamel and may extend to DEJ.They contains organic substances. Lamellae are commonly found at the base of occlusal pits and fissures.
These are caused by “imperfect calcification of enamel tissue”.
Lamella at the base of an occlusal fissure provides an appropriate pathway for bacteria and initiate caries.ENAMEL LAMELLA
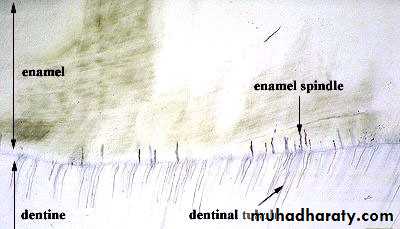
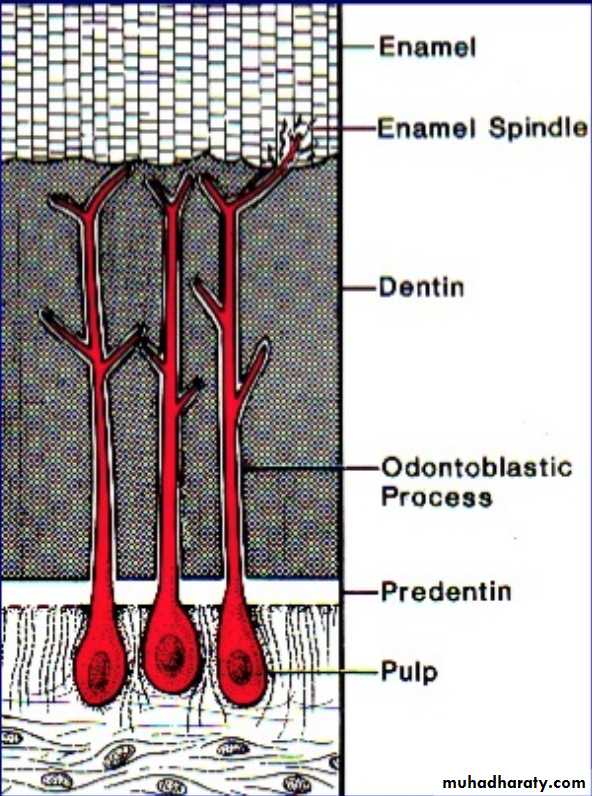
5.Enamel SpindlesOdontoblastic processes sometimes crosses DEJ and their end is thickened.
Spindles serve as pain receptors, that is why, when we cut in the enamel patient complains of pain.
Enamel Spindles
6.Striae of Retzius
They appear as brownish bands in the ground sections and illustrate the incremental pattern of enamel.
These represent the rest periods of ameloblast during enamel formation, therefore also called as growth circles.
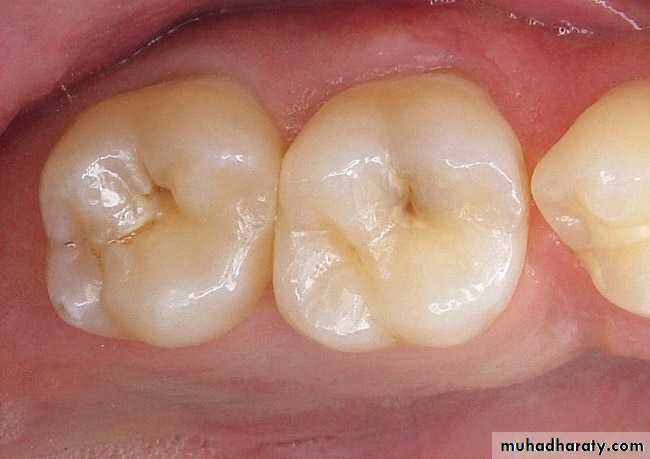
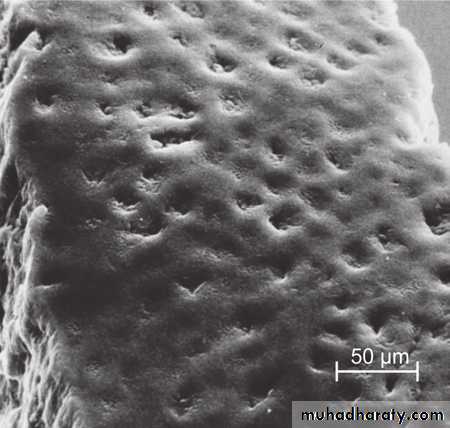
Macroscopically/clinically the enamel generally looks smooth and even.
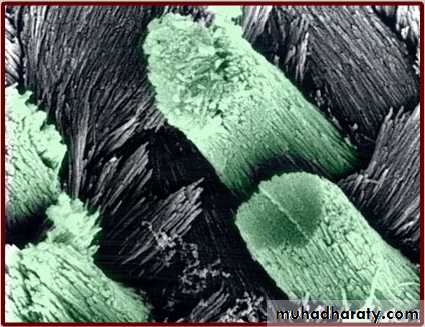
However, at high magnification the surface of enamel is full of developmental defects such as pits, cracks, and fissures as well as normal anatomical features such as Tomes’ process pits corresponding to the head of the ameloblasts.
Thus, there are numbers of surface irregularities on enamel where the microorganism can shelter.
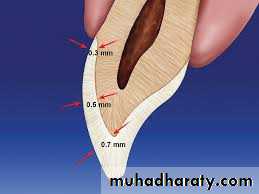
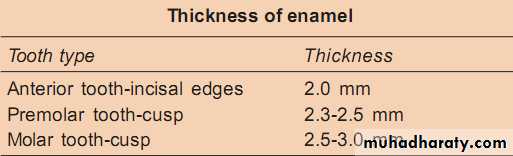
Thickness
The thickness of enamel varies in different areas of the same tooth and from one type of tooth to another type of tooth.The average thickness of enamel at the incisal edges of incisors is 2 mm; at the cusp of premolar and molar from 2.3 to 3.0 mm.
Thickness of enamel decreases gradually from cusps or incisal edges to cementoenamel junction.
PROPERTIES OF ENAMEL
COLOR
STRENGTH
Enamel has a rigid structure. But it is brittle.The hardness also decreases from outer surface of the enamel to its inner surface. Also the density of enamel increases from dentino-enamel junction to the outer surface.
When compared, dentin has high compressive strength than the enamel, this acts as a cushion for enamel when masticatory forces are applied on it.
For this reason during tooth preparation, for maximal strength of underlying remaining tooth structure all enamel rods should be supported by healthy dentin base.