
De t e rminat ion of Ascorbic Acid Cont e nt
of Some Fruit J uice s.

OBJ ECTIVES
•
To measure the concentration of ascorbic acid

Int roduct ion
•
Vitamin C is a water-soluble vitamin that is necessary
for normal growth and development.
•
Water-soluble vitamins dissolve in water. The body
cannot store them. Leftover amounts of the vitamin
leave the body through the urine. That means you
need a continuous supply of such vitamins in your
diet.
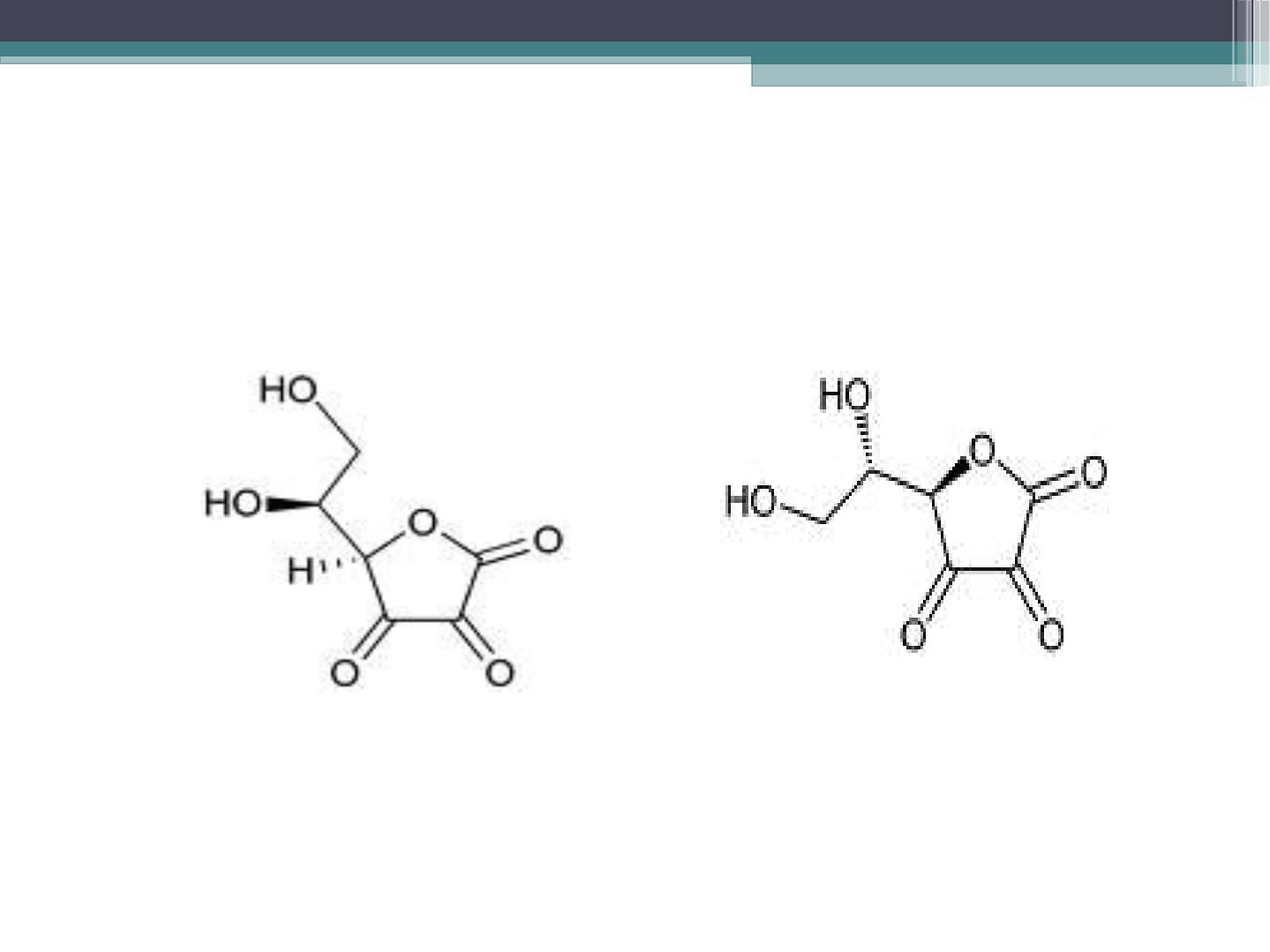
Int roduct ion
Ascorbic acid
Dehydroscorbic acid

Functions of Vitamin C
•
Vitamin C is required for the growth and repair of
tissues in all parts of your body.
•
It is necessary to form
collagen
•
Vitamin C is one of many
antioxidants
in the body
*
The body does not manufacture vitamin C on its own
( Why ? )
De ficie ncy of Ascorbic acid
•
“Scurvy “

Principle
•
Equipment Needed
•
burette and stand
•
100 mL or 200 mL volumetric flask
•
20 mL pipette
•
10 mL and 100 mL measuring cylinders
•
250 mL conical flasks.



Solutions Needed
•
Iodine solution
: (0.005 mol L
−1
). Weigh 2 g of
potassium iodide into a 100 mL beaker. Weigh 1.3 g
of iodine and add it into the same beaker. Add a few
mL of distilled water and swirl for a few minutes
until iodine is dissolved. Transfer iodine solution to a
1 L volumetric flask, making sure to rinse all traces
of solution into the volumetric flask using distilled
water. Make the solution up to the 1 L mark with
distilled water.

Solutions Needed
•
Starch indicator solution: (0.5%). Weigh 0.25 g of
soluble starch and add it to 50 mL of near boiling
water in a 100 mL conical flask. Stir to dissolve and
cool before using

Sample Prepare
•
Strain the juice through cheesecloth(filter paper)
to remove seeds and pulp which may block
pipettes.

Titration
•
Pipette a 20 mL aliquot of the sample solution into a
250 mL conical flask and add about 150 mL of
distilled water and 1 mL of starch indicator solution.
•
Titrate the sample with 0.005 mol L−
1 iodine
solution. The endpoint of the titration is identified as
the first permanent trace of a dark blue-black color
due to the starch-iodine complex.
•
Repeat the titration.
•
Calculate the concentration of ascorbic acid solutions
.
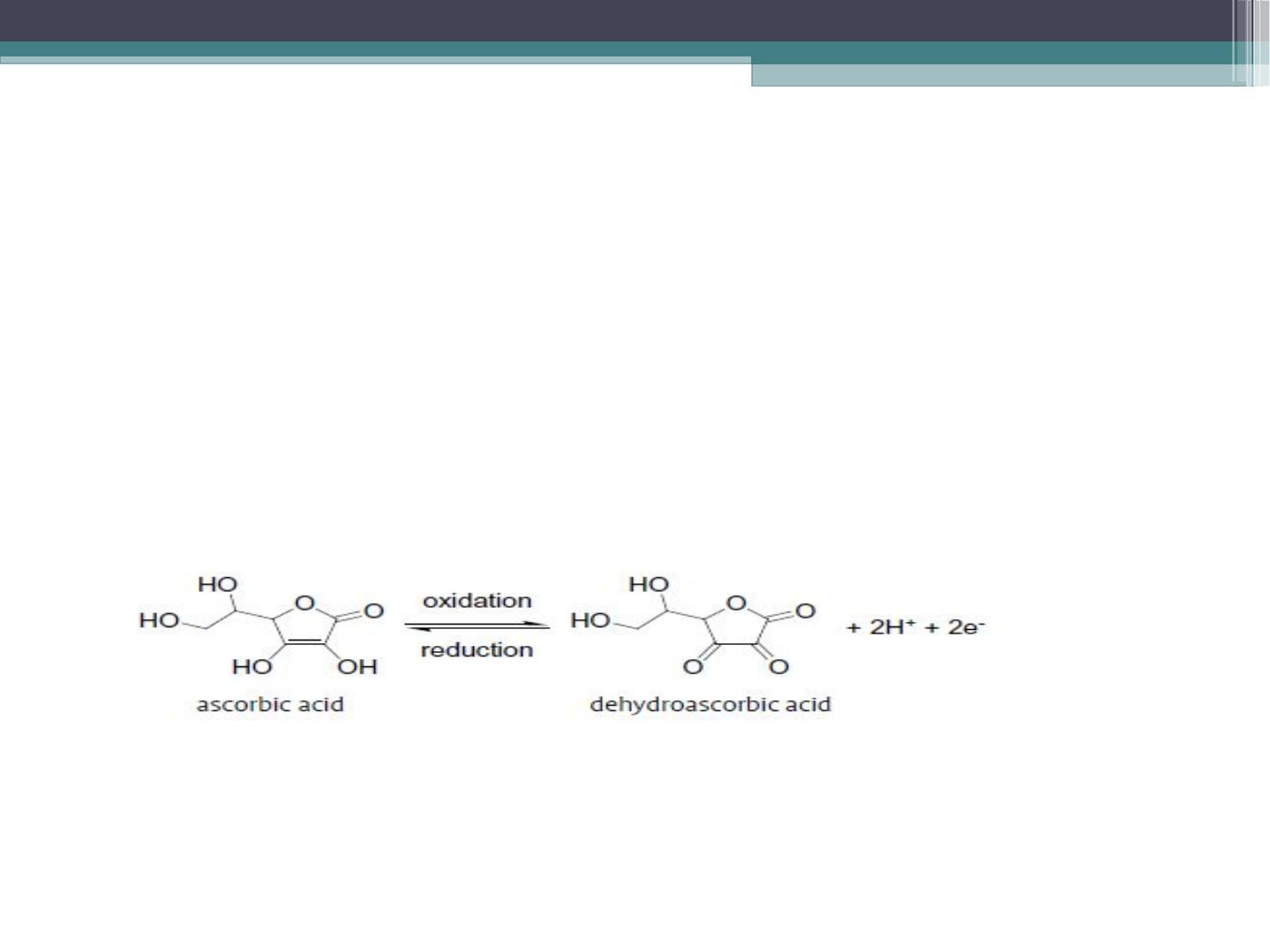
Chemical Equations
•
ascorbic acid + I
2
→ 2 I
−
+ dehydroascorbic acid
