
0
Mustafa Hatim Kadhim
Baghdad University
Al-kindy college of medicine
Third Stage
2013 - 2014
1
List of contents
Lecture
number
Lecture name
Doctor name
Page
number
Unit 1: Introduction
2 - 4
1+2
Introduction to parasitology
دكتورة سوسن
3 - 4
Unit 2: Protozoa
5 - 48
1+2+3+4+5
Subphylum Sarcodina (Amoeba)
دكتورة سوسن
6 - 13
6
Subphylum Mastigophora (The flagellate of digestive
tract & urogenital systems)
14 - 16
7
Phylum ciliophora
17
8+9+10+11
Subphylum Mastigophora (Flagellates of blood and
tissues "Hemoflagellates")
دكتور حيدر
18 - 29
12+13+14
Suborder: Haemosporina (Genus Plasmodium)
30 - 39
15+16
Suborder Eimeriina
40 - 48
Unit 3: Helminthes
49 - 93
Introduction to Helminthes
50 - 52
1
Introduction
دكتورة سوسن
51 - 52
Cestodes
53 - 66
1
Introduction
دكتورة سوسن
54 - 55
2
Order Pseudophyllidea
56
3+4+5+6+7+8
Order Cyclophyllidea
57 - 66
Nematodes
67 - 82
1
Introduction
دكتور حيدر
68
2+3+4+5
Intestinal nematodes
69 - 78
6
Blood and tissue nematodes
79 - 82
Trematodes
83 - 93
1
Introduction
دكتورة سوسن
84
2
Hepatic Flukes
85 - 87
3
Intestinal Flukes
88
4
Pulmonary fluke
89
5+6+7
Blood Fluke
90 - 93
Unit 4: Medical Entomology
94 - 101
1+2+3
دكتورة صديقة
95 - 101

2

Unit 1 - Introduction
3
Lecture 1+2 – Introduction to helminthes
Parasitology
: Is the science that deals with parasites
& their pathogenic effects.
Terms & Terminology of parasitology
Parasite:
Is an organism adapted to live on or in other
organism (host).
Symbiosis
: The relationship between two dissimilar
organisms adapted to living together & lost the ability to
live alone.
Symbiont:
The association may be beneficial or harmful
to either of the associates.
Mutualism
: The relationship which is benefit for both
associates.
Commensalisms
: The relationship between the parasite
& the host, when one of the associated is benefited & the
other (host) neither benefited nor harmed. e.g. E. coli
Bacteria live in human colon but doesn’t cause trouble to
human.
Parasitism
: Where the relationship is harmful to the
host. e.g. E. histolytica.
Ectoparasites
: The organisms (parasite) that live on or
in the skin of their hosts. This relationship called
“infestation”, e.g. Arthropods.
Endoparasites
: The parasites which live inside the
body of the host (in the digestive tract, extraintestinal
organs & tissues, intracellular. This relationship called
“infection”
The parasite have achieved secure ecologic niche
within the host, lost some of its morphological features
and develope d physiological & biochemical adaptati on
needed for its new life.
Obligate parasites
: Parasites that are entirely
dependant on their hosts & cannot live outside the host.
Facultative parasites
: Parasites that are capable of
living either free or in or on a host.
Parasites can be classified by the duration of
their association with their hosts:
Temporary parasite
: Visits a host for a short period.
Permanent parasite
: Leads a parasitic life all through
its life.
Wandering or Aberrant parasite
: Parasite reaches a
place where it cannot live.
Pseudoparasite
: Is an object that resembles a parasite
or the egg of a parasite but it is not a parasite (called
artifact) this is include yeast, hairs, spores……..
Coprozoic or Spurious parasite
: These are the eggs
of some helminthes that are accidental ingested in food &
pass through the intestine & are found in faecal without
causing infection to the host.
Types of hosts:
Is the human & organism which harbors the parasite.
Definitive host:
Is the organism in which the adult or
final stage of the parasite develops or where sexual
reproduction of the parasite occurs. e.g. Schistosoma spp.
Intermediate host:
The organism (host) which harbors
the larval (a sexual stage) develops. Some have two
intermediate hosts.
Paratenic host:
Is the host in which the parasite is
transported & neither gains nor loses infectivity for its
definitive host. Or A carrier or transport host where the
parasite remains viable without further development
Reservoir host
: Is an animal species on which the
parasite depends for its survival in nature & acts as a
source of infection for other hosts including man. e.g.
cutaneous Leishmania & its reservoir host is the dog.
Vector:
Is the transmitter of parasites from host to host .
(Agents of transmission).
It is essential for the parasite life cycle called
“biological vector” e.g. Anopheles female which is
the vector of the plasmodium parasite (so it is a
definitive host & biological vector) e.g. Sand fly.
When the vector is not essential for the parasite life
cycle, It is called “Mechanical vector” e.g. E.
histolytica transmitted by the house fly which is a
vector of it but it may be transmitted by other hosts.
Zoonosis
: It is a term applies to the disease which
transmitted from animal to human either incidentally or
commonly.
Classification of animal parasites & vector:
Four groups are of major importance in medical
parasitology:
1) Protozoa 2) Helminths 3) Molluscs 4) Arthropods
According to the zoological nomenclature within the
animal Kingdom we have:
Phylum, Subphylum,…………….larger division
Classes, Orders, Families, Genus & Species ……
Lesser division
All these names must be of Greek or Latin origin.
Species: Designates a population having the same genetic
characters & are capable of continued reproduction of
their kind & can not interbreed with other species.

Unit 1 - Introduction
4
Genus: Is a group of closely related species.
The scientific name consist of generic name with initial
capital letter & specific name with initial small letter.
Pathogenesis and symptomatology:
Parasites that injury the host are Pathogens.
The development of the damage called Pathogenesis.
Degree of injury depends on several factors:
1) Potential virulence of the agent (i.e. its intrinsic
pathogenecity).
2) A mount of inoculum & rapidity of multiplication.
3) The site of inculation.
4) Exposure is single or repeated.
5) Resistance or tolerance of the host to particular strain
of agent.
6) General resistance of the host.
7) Type of damage caused by agent, which either:
Mechanical, Lytic, Toxic & Allergic
Symptoms
:
Are the manifestations of pathological
process result from the effect of the agent.
Host response
: The reaction of the host has distinct
bearing on immediate or sub sequent effect of
pathogens.
In some cases the host unresponsive, but in other may
produce Ab. to counteract the agent Ag produced by
pathogens or may wall of the invader or its products
by cellular infiltration or proliferation.
The host response may be local at the site of injury or
systemic including cellular or humeral changes.
Diagnosis:
Diagnosis of parasitic diseases depends on 2 parts:
1) 1
st.
Clinical features: like fever, pain
2) 2
nd.
Laborator y diagnosis to:
a) Detect the presence of eggs, larvae, cysts (in stool,
urine, blood sputum).
b) Distinguished these stages from each other.
c) Find out whether it is the causative agent or
coincidental.
Epidemiology:
Is the science concerned with factors which determine the
prevalence of infection & incidence of disease.
It is the history of the disease including not only infection
in man but also in animals, and agents that serve as
reservoir & vectors.
Prevalence:
Is define as the number of infected
individuals at a given time in a designated area.
Incidence:
Is the rate or frequency with which a disease
(new infection) occurs.
Infections maintained at a more or less stable rate of
prevalence within the human population of an area are
said to be Endemic, but in high prevalence called hyper
endemic. If it appears irregularly in scattered individuals
it Sporadic. If it appears in high prevalence in unusual
transmission it is Epidemic.
All these terms are special for human. For animals called
(Enzootic, hyper enzootic, Epizootic & Sporadic).
Parasitic disease may be grouped epidemiologically
as follows:
1) Filth-borne or contaminati ve :
Like intestinal protozoa, helminthes, louse infestation.
2) Contracted from soil or water:
Like eggs of Ascaris &Trichuris, or through the skin as
with the infective larvae of hook worms or blood flukes.
3) Food-bor ne infections:
Like Taenia spp. Eating raw or under cooked meat
containing the larval stage of the parasite.
Also ingestion of encysted larvae on aquatic plants.
4) Arthropods-bor ne infection:
Like Malaria- Leishmania
Arthropods essential intermediate host & vector.
5) Infestation by arthropods:
Like the Lice.
6) Arthropod envenomation:
Like the bite of Scorpion.
Mode of infection:
The means by which the different infecting agents are
transmitted from the host to another. e.g. The
Plasmodium reach human through injection by the
Anopheles female.
The avenues where they enter the human body called
(portal of entery).
Control & Prevention:
Parasitic diseases involve the individual & community in
which he lives, therefore measures should be taken for
these entities.
The individual: In the parasitized individual….
Chemotherapy should be used not only to relieve suffering but
also to prevent transmission to new host in the community.
Medical education in methods of personal hygiene &
provision of means for taking precautions against exposure.

5

Unit 2: Protozoa
6
Lecture 1+2+3+4+5 –
Subphylum Sarcodina (Amoeba)
Classification
Subkingdom: Protozoa
Phylum: Sarcomastigophora
a. Subphylum: Sarcodina
b. Subphylum: Mastigophora
Phylum: Ciliophora
Phylum: Apicomplexa
Amoeba
Phylum: Sarcomastigophora
Subphylum: Sarcodina
Superclass: Rhizopoda
Class: Lobosea
General characters of Amoeba
All the members move by pseudopodium.
Having Trophozoite stage.
Multiplication by binary division.
Some of them parasitic, other free living.
Generic classification depends on structures of nuclear
contents.
Commonly parasitizing the large intestine of man, except
Entamoeba gingivalis, which parasitized the oral cavity.
Members of this family are:
1) Entamoeba histolytica
2) Entamoeba hartmani
3) Entamoeba coli
4) Entamoeba gingivalis
5) Endolimax nana
6) Iodamoeba buetschlii
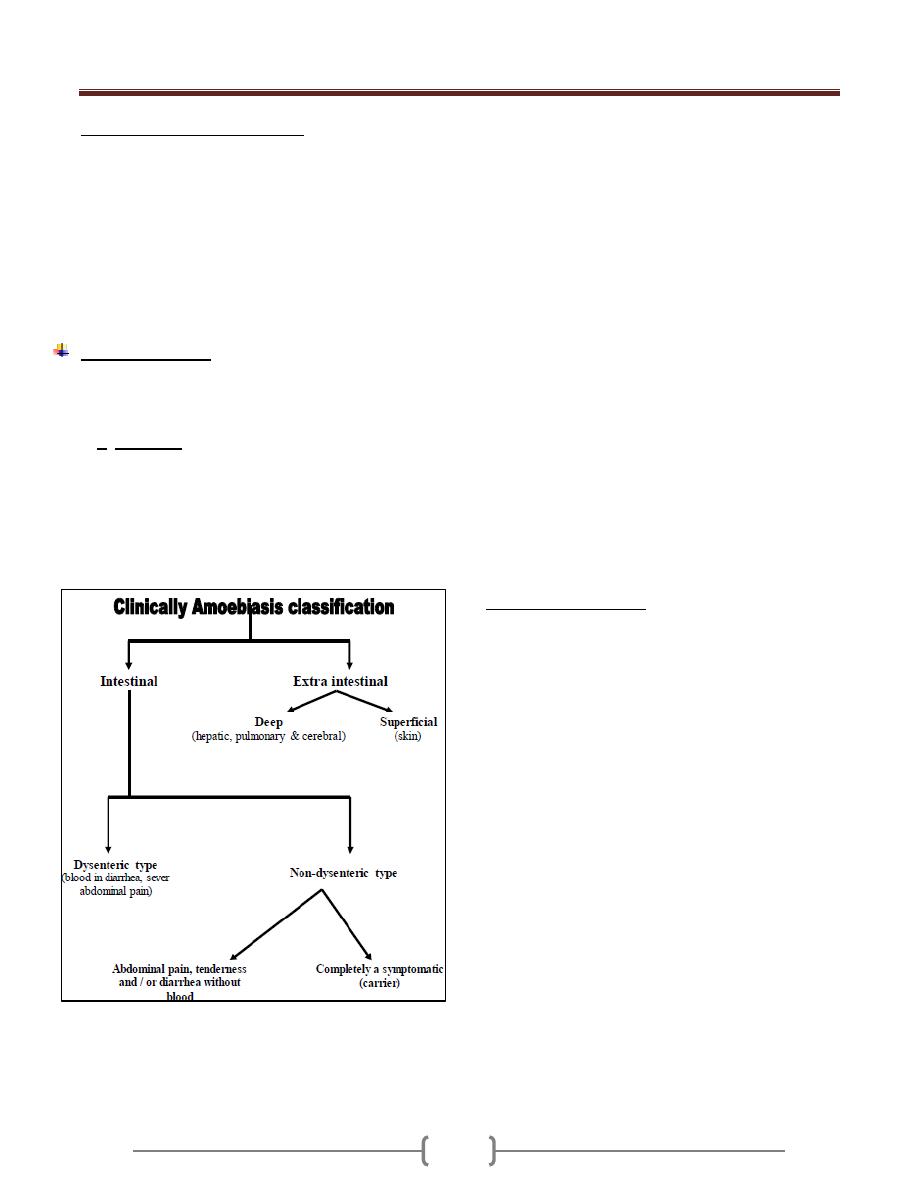
Entamoeba histolytica
Disease:
Amebiasis
Geographical distribution
Cosmopolitan mainly in tropical & subtropical area.
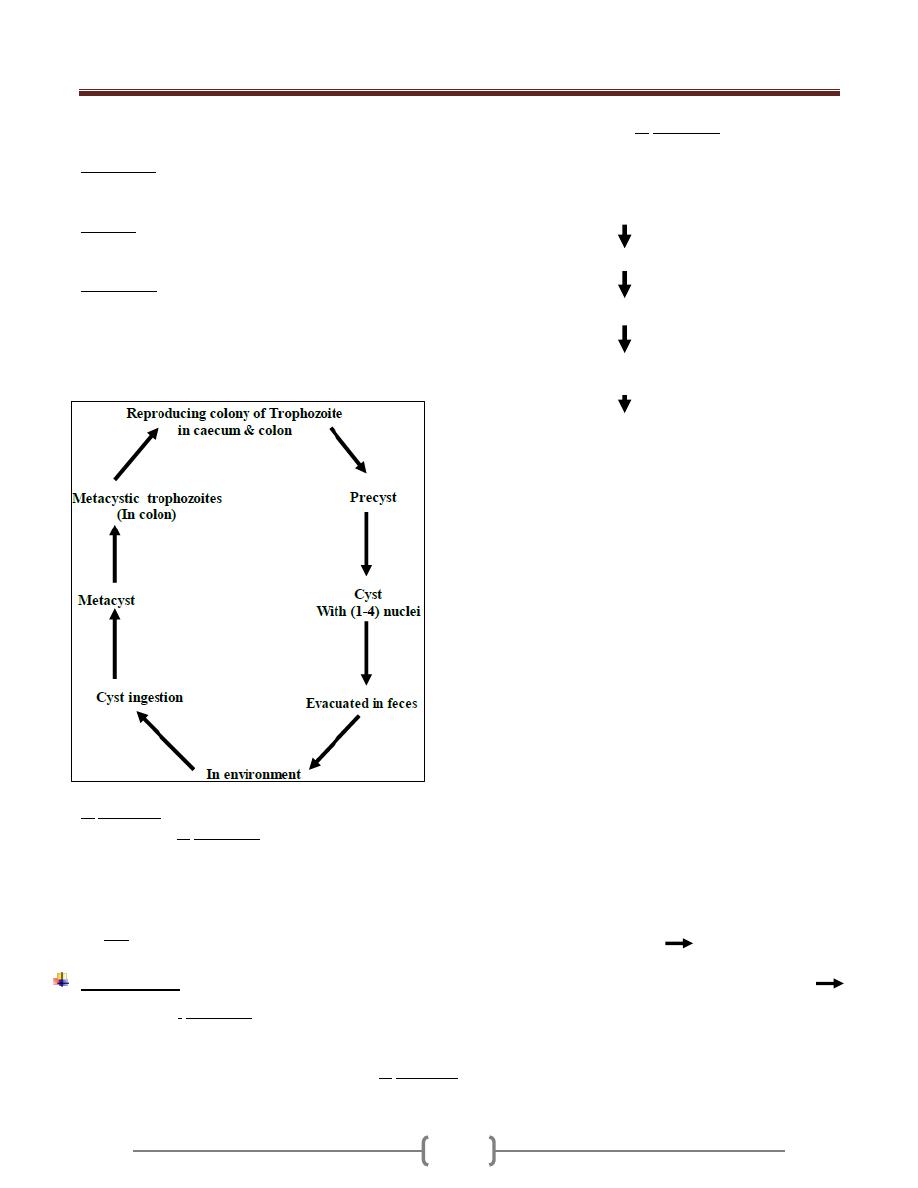
Morphology, Biology & Life cycle:
It has four stages: Trophozoite, precyst, cyst, metacyst
The stages recognized in the feces are trophozoites &
cysts. The other two found only inside the host body
(precyst, metacyst)
Trophozoite:
In its natural habitat, the large intestine & extra intestinal
foci the size about (12-
Trophozoite
up
dysenteric stools.
Trophozoite has finely granular, endoplasm & a clear,
grayish, green tinge ectoplasm.
The endoplasm contain many structures include nucleus &
food vacuoles within the food vacuoles we may see RBC.
The nucleus spherical, surrounded by delicate nuclear
membrane which on its inner surface there is fine,
regularly distributed chromatin granules.
In the center of the nucleus there is single dense
karyosome. Immediately around the karyosome there is
clear halo extending between this & the nuclear
membrane are radially exending a chromatin fibrils.
The pseudopodia have 2 types
1) Lobopodia: for the locomotion
2) Filopodia: for attachment to cells
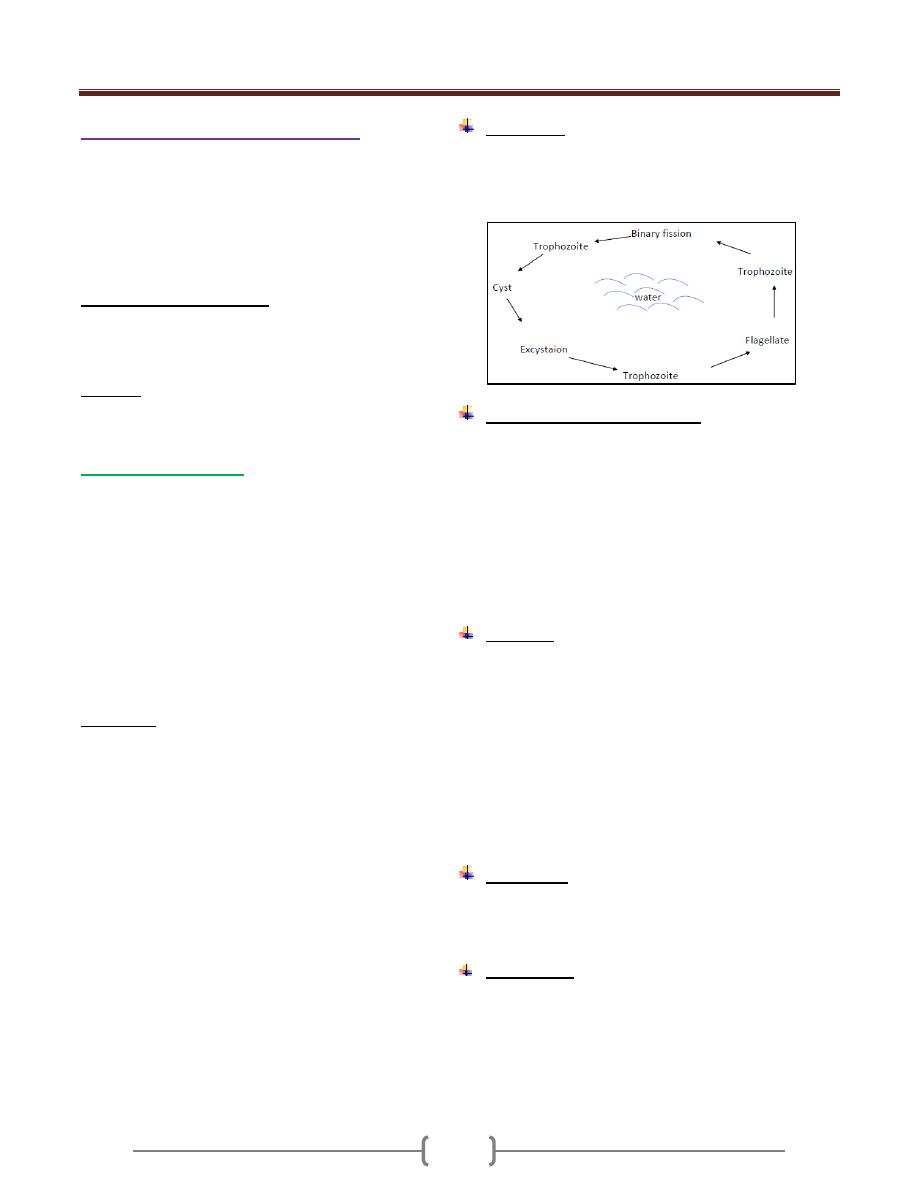
Life Cycle
Trophozoite stage convert to precyst stage which convert
to cyst stage with mononucleus, this divided into 2
nuclei, then these 2 nuclei will divide into 4 nuclei which
now called mature cyst.
The infection is usually started with the cyst stage by
contaminated food or water ingested by man. The cyst
usually not affected by the juice of stomach.
Unit 2: Protozoa
7
When it goes to or through intestine it under go 3
processes which are:
a) Excystation: Means the liberation of the metacyst from
cyst wall, then the cytoplasm divided forming metacystic
trophozoites. So after that 4 trophozoites are formed.
b) Invasion: The trophozoite invades the wall of large
intestine, particularly the caecum & colon & then
colonization results from multiplication by binary division.
c) Encystation: Occur in the large intestine, when the
Trophozoite dehydrated in bowel lumen, encystation
started.
Trophozoite, precyst considered as diagnostic stage.
Mature cyst as infective stage.
E. histolytica habitat is Caecum & flexure colon.
Viable cysts of E. histolytica in external environment are
soon killed by drying, direct sun light, heat, hypertonicity
& bacterial putrefaction.
Cyst passed in semiformed, form, solid, semisolid stool.
Trophozoite & precyst dies rapidly (so non-infective)
but cyst is resistance (infective stage).
Pathogenesis
Infection with E. histolytica leads to formation of
colonization.
The speed & depth of penetration depends on:
Pathogenic capacity of the particular strain of E. histolytica
General resistance of the host.
The damage caused by E. histolytica is:
1) Chemical (Enzyme action)
2) Mechanical (engulfing of the parasite)
Trophozoite may lodge in the crypts of large intestine
Lesion result from invasion leading to superficially
minute cavity (are result of lytic necrosis of the parasite
on colon mucosa).
More colonizing & more lytic action leading to narrow
channel, which lead to base of the mucosa.
The invasion extend laterally which leads to Flask
shaped ulcer, and the repair may take place to lytic
necrosis, lesion leads to extensive functional damage to
the mucosa.
In many cases, the Amoeba erode a passage into
muscularis mucosa then sub mucosa.
It can spread radially to surrounding tissues. (If there is
no secondary bacterial infection, there is no tissue
reaction).
From submucosa, the invasion extends to muscular coats
& penetrate to serosa (Perforation). It may perforate
mesenteric venules or lymphatics & carried into the liver
& other extraintestinal sites (brain, lung).
Any extraintestinal lesion is secondary to primary lesion
in large intestine except cutaneous lesion of the genitalia.
So the early uncomplicated amebic lesions are minute
opening with slightly raised yellowish ring in mucosa
leading into a deeper enlargement in the submucosa with
tunneled connection between two or more lesions leading
to cuts off the blood supply sloughing of overlying
layers.
As the lesion becomes chronic by bacterial infection
tissue reaction & cell infiltration, with neutrophilic
leukocytes & fibroblasts tend to form a wall around the
ulcer & over hanging edges become thickened.
Feeding
Colonized
Lytic activity
Invasion
Unit 2: Protozoa
8
Extraintestinal amebic lesion
At first consists of a small lesion where are a more ameba
enter the blood vessels & lodged into the liver or other
organ proceed to colonize producing necrosis of
surrounding host cells.
In the liver, tendency for lesion to be multiple-later one or
at most few become enlarged to develop amebic liver
abscess, this lesion bacteriologicaly sterile, but the
amount of tissue necrosis stimulates local & systemic
Leukocytosis.
Symptomatology
The incubation period (the time or duration from time of
exposure till first symptoms appears) is varying from few
days to 3 months or even a year.
In E. histolytica it is difficult to determine the interval
between exposure & first symptoms. The onset may be
insidious with vague (not sharp) abdominal discomfort or
soft stools for variable period or it may be sudden with
dysentery or acute abdominal pain.
In hepatic amebiasis, frequently there is no history of
amebic infection in colon.
Amebiasis may be only one of two or more concurrent
disease processes, example, Shigellosis, Salmonellosis,
carcinoma, appendicitis, peptic ulcers, cholecystitis.
At time or more amoebic granulomas (amebomas)
develop in the wall of colon or rectum.
The patient with amebic dysentery has tenesmus,
abdominal cramps. No systemic intoxication as seen in
bacillary dysentery.
The abdominal pain & tenderness are mostly in the lower
quadrants of the abdomen, on the right side. Clinically
sometime mistaken for appendicitis.
Extraintestinal symptoms depend on the organ affected.
Hepatic abscess presents with fever, enlarged tender are
mostly in the lower quadrants of the abdomen, on the
right side, clinically sometimes mistaken for appendicitis.
Extraintestinal symptoms depend on the organ affected.
Hepatic abscess present with fever, enlarged tender liver,
bulging & fixation of the right leaf of the diaphragm &
serious effusion of the right pleura.
Skin amoebiasis occurs due to damaged skin come in
contact with trophozoite stage.
Most common skin infection seen in:
1) Perineum, secondary to amoebic dysentery.
2) Penile lesion, acquired by anal intercourse.
3) The abdomen, at the mouth of fistulous tract from
colon or from hepatic abscess.
Pulmonary amoebiasis
Is a consequence of rupture of hepatic abscess into the
chest cavity, the lung & the bronchus. Therefore, the
patient present with signs of pneumonia & expectoration
of characteristic bitter, bile flavored liver-colored pus
passing through the hepatobronchial fistula
Rarely, amoebic lung abscess occurs by hematogenous
spread from the colon.
The amoeba secrete proteolytic enzymes that produce
necrosis of tissue & steps involved in amoebic killing the
target cell are:
1) Receptor mediated adherence of Entamoeba to target cell
2) Amoeba cytolysis of target cells
3) Amoebic phagocytosis of killed as viable target cells.
Attachment of Entamoeba histolytica to mucosa mediated
by amoebal galactose-inhibatable adherence Lectin.
Colonization of E. histolytica depends on:
1) Normal motility of intestine.
2) Proper establishment of metabolic requirements
3) Infected dose
4) Adequate bacterial flora.
5) Low oxygen tension
6) PH
In the cecum sometime, adjacent ulcers caused by
E.hitolytica may coalesce causing sloughing of

Unit 2: Protozoa
9
intervening mucosa to form the typical Dyak hair ulcer as
buffalo skin ulcer.
Factors determining development of amoebiasis:
1) Strain variation
2) Role of bacteria
3) Infective dose
4) Nutritional status
5) Associated disease (such as diabetes, tuberculosis &
malignancy), pregnancy
6) Immunity
7) Intestinal mucus
Complications of intestinal amoebiasis:
Local complication: Perforations, peritonitis, prolapse of
rectum, hemorrhage & obstruction stricture
Systemic complications: hepatic amoebiasis, pulmonary
amoebiasis, cerebral amoebiasis, splenic amoebiasis &
cutaneous amboebiasis
Amoebic liver abscess
Early stages appear homogenous area of yellow color
surrounded by zone of hemorrhagic liver tissue & nicrotic
material thick.
In advance cases, liquefaction of the central necrotic area
with cavitation may be visible. The abscess gives honey-
comb appearance as the connective tissue is more
resistant to liquefaction.
The pus on the cavity is thick fluid whose color may vary
greyish-yellow to chocolate & it is called anchovy sause-
like pus.
Microscopically, the pus reveals degenerated liver cells,
RBCs, WBCs & bacteriologically sterile.
Microscopic pathology to of amoebic liver abscess:
In a section passing through the margins of the liver
abscess. There are 3 zones can be differentiated:
1) Central zone of cytolysed material with no amoeba.
2) Intermediate area consisting of degenerated liver cells,
RBCs, WBCs, connective tissue & occasionally
trophozoites of E.histolytica
3) A peripheral zone consisting of congested capillaries with
necrotic liver cells with many trophozoite of E.histolytica
Diagnosis
Intestinal amoebiasis cannot be diagnosed on clinical
ground only, primary depend on direct microscopic
examination of the stool to recover motile trophozoite &
charcot-leyden crystals.
In extra intestinal amoebiasis, routine work of
Histopathology using (Best’s carmine) stain must be perform
Treatment
Depend on clinical type. In severe amebic dysentery the
purpose of treatment is not only to provide relief of
discomfort but also improve eradication of amebic infection.
Intestinal amoebiasis, the drug of choice is
Metronidazole 750 mg, Tid for (5-10) days.
Diiodohydroxyquin.
Antibiotic (Tetracycline).
Emetine hydrochloride, also effective.
Non dysenteric symptoms:
Diloxanide furoat & Diiodohydroxyquin.
A symptomatic cases:
Must be treated by Metronidazole.
Extraintestinal:
Metronidazole.
Emetin hydrochloride.
Chloroquine.
Or all of them
Epidemiology
High prevalence in warm climates & people of all races,
sexes & ages are subjected to infection.
Mode of transmission by:
1) Contamination of water with viable cyst.
2) Person-to-person contact.
3) Food handler’s.
4) Filth flies.
Control
1) Treatment of patient.
2) Screening of food handlers & treat the infected cases.
3) Improvement of hygiene & sanitation.
4) Human excreta must be disposed properly.
Unit 2: Protozoa
10
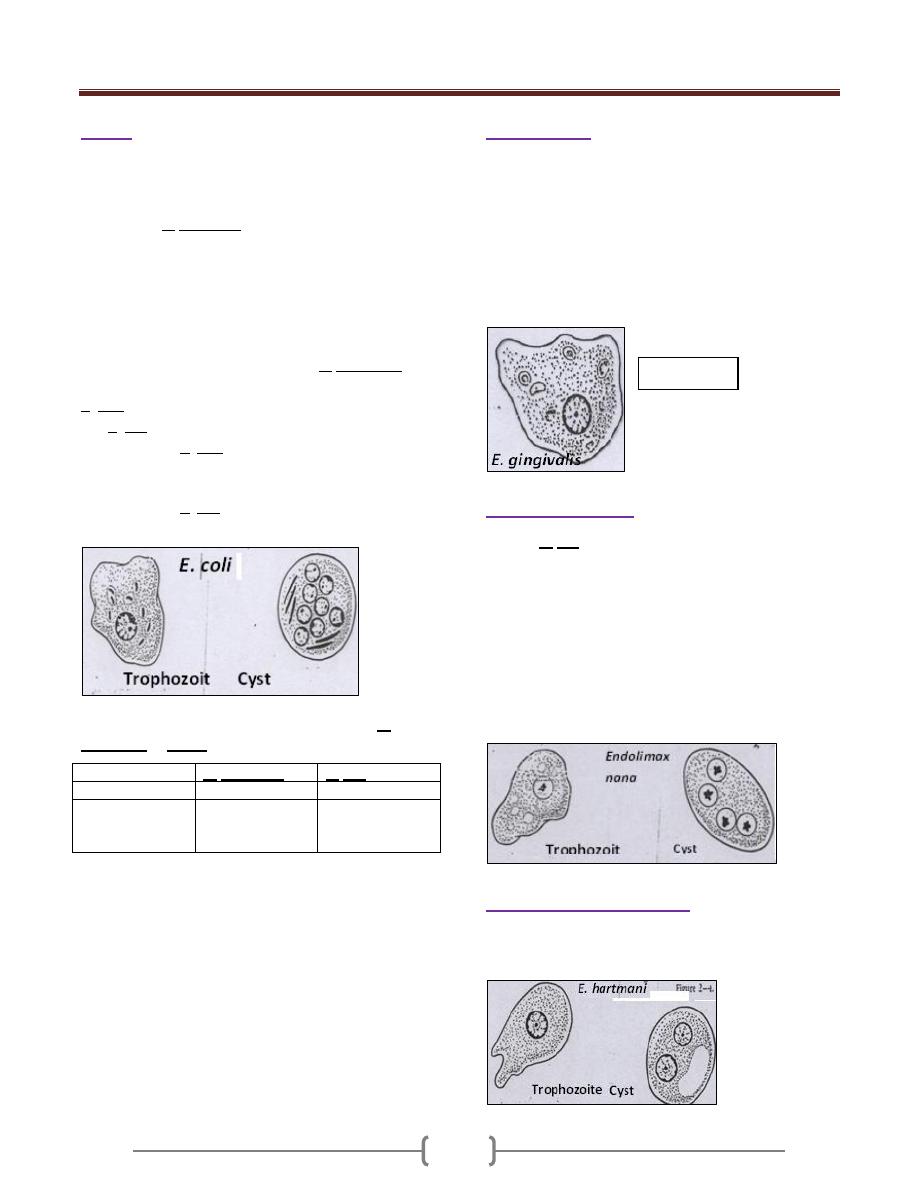
E.coli
The most common amoebic parasite of man (commensal).
It habits large intestine.
It has trophozoite & cyst stages, both of them are larger
than those of E. histolytica. The trophozoite size is (15-
50) µ, no RBCs seen in food vacuoles. There is no sharp
point between ectoplasm & endoplasm in trophozoite
stage.
In cyst stage (its size 10-33 µm), the mature cyst contains
8 nuclei, each of them has same feature of trophozoite
nuclei.
The shape of chromatoidal bodies in of E. histolytica is
cigarette, rounded in shape, but it is needle shaped in the
E. coli if presented.
The E. coli is not parasitism but commensalisms.
The presence of E. coli in stool of some bodies means the
food of this patient contaminated with feacal material,
how? By the Musca domestica, filth fly, or others.
The presence of E. coli in the host means his food been
contaminated.
There are 2 things for differentiation between E.
histolytica & E. coli
The difference
E. histolytica
E. coli
Karyosome
Central
Eccentric
Chromatin line
nuclear
membrane
Fine & regular
distributed
Course &
irregular
distributed
E.gingivalis
Only trophozoite been reported in E. gingivalis .
The size of the trophozoite is (15-30) µm.
It is nonpathogenic but opportunistic (in diseased gum or
tonsils).
The karyosome is central or somewhat eccentric.
It is found in diseased gum & tonsillitis as a phagocytic
(opportunistic).
It is transmitted through saliva droplets or intimate contact.
Endolimax nana
Like the E. coli, its presence means the food of the person
been contaminated with stool (feacal matter) of other person
It has trophozoite & cyst stages. The trophozoite has one
nucleus, and the cyst has 4 nuclei. The karyosome
consisting from one or more granules, commonly
eccentric in position.
The size of the trophozoite is (8-10) mm, the endoplasm
finally granular with numerous vacuoles.
In the cyst chromotoidal bodies, if present are short
curved rods or comma shaped.
Entamoeba hartmanni
Small race of E. histolytica, sometimes it is mistaken with
E. nana, fortunately both of them are nonpathogenic.
Trophozoite

Unit 2: Protozoa
11
Iodamoeba buetschlii
Cosmopolitan, commensal, living in lumen of large intestine
It has 2 stages:
Trophozoit: (8-
evidence of pseudopodial extensions.
Cyst: (5-
m.
We can differentiate between I. buetschlii & others by:
The trophozoite & cyst have one nucleus & both of them
have glycogen vacuoles, so in stain with iodine to give
brown mass.
A large karyosome in nucleus found centrally or
somewhat eccentrically.
Only the trophozoite of this amoeba has one or two
distinct glycogen vacuoles.
The cyst has only one nucleus, it has large glycogen
vacuoles which stained with iodine in deep brown color.
So these differences are very important.
Blastocystic hominis
It is parasitic amoeba.
Discovered in 1912.
Its life cycle is not clear.
Since discovered, B. hominis has been the subject of
controversy, initially described as algae, them as harmless
intestinal yeast, and since around 1982 as a protozoan
parasite.
Although a number of different forms of it are known, so
only vacuolated form is more common & easy to
recognize, therefore only this form is described.
The different form of B. hominis:-
1) Vacuolated type, which is the
most common type.
The size range from (5-
, but
the average is (7-
vacuole in the center forming about
10% at the periphery with 4 nuclei
situated at same level.
2) Granulated type.
3) Amoeboid type.
Life cycle
The life cycle has not been universally described, but it
may participate in a sexual reproduction.
Main method of reproduction is by binary division &
sporulation & it transmitted through contaminated food &
water, and it is prevalent in tropical & subtropical areas.
Pathogenesis
Is not well known & need to be determined.
Symptoms
Mainly diarrhea, nausea, vomiting, fever, as well as
abdominal pain & cramps.
Treatment
The best drug of choice is a combination of Flagyl &
Septrin
Essential differences between bacillary &
amoebic dysentery
Bacillary
dysentery
Amoebic dysentery
Number
> 10 per day
(6-8) per day
Amount
Small
Relatively copious
Appearance
Consist of
blood & mucus,
hardly any
faecal matter
Feces with stratum of
blood & mucus seen over
the surface
Color of
blood
Bright red
(fresh blood)
Dark red
(latered blood)
Consistency
Viscid, mucous
adherent to
container
Liquid or formed, mucus
not adherent to container
Odor
Odorless
Offensive
Chemical
reaction
Alkaline
Acidic
Microscopic examination
Pus cells
Numerous
Scanty
Red blood
cells
Discrete
In clumps, discolored
Eosinophils
Absent or rare
Present
Macrophages
Present
showing
ingested
erythrocytes
Absent
CL crystals
Absent
Present
E. histolytica
Absent
Trophozoites Present
Bacteria
Scanty, non-
motile
Numerous & motile
Cultural examination
Growth on media
for E. histolytica
Negative
Trophozoites grown
Growth on MacConkey agar
Positive
for
Shigella
spp.
Negative
Unit 2: Protozoa
12
Pathogenic free living amoebae
Free –living organisms that are capable of adapting to a
parasitic existence are called amphizoic. Meaning that
they can multiply both in the body of the host (endozoic)
and in free living (exozoic) condition.
Species of the two general Naegleria and Acanthamoeba
are among such organism
Geographical distribution:
Infection with free living amoebae was first discovered in
1965, in Australia, USA them in Europe, Africa, Asia,
newzealand.
Habitat:
in fresh water (lakes, ponds), Sea water,
sewage water, brackish water.
Naeglaria fowleri:
Causes, primary amebic meningoencephalitis (PAM)
which produces death within 5-7 days after the onset of
symptoms
Most cases have occurred during the hot summer months
in young people who within the preceding week swam or
dived in fresh or brackish water, lakes streams, hot
springs also, swimming pools have been apparent source
of the infection, few cases by inhalation of infected dusts
are also recorded
Portal of entry to the brain and meninges found to be the
nasal mucusa and cribiform plate.
Morphology, Naegleria fowleri, live well in fresh water
and moist soil, also grows well in tissue culture and
artificial media.
Occurs in trophozoite and cyst forms Motile trophozoites
from cerebrospinal fluid are elongate, broad anteriorly,
with single broad pseudopod at the forward end, the size
around 7 by 20 Micro meters, one nucleus with a large
central karyosome (endosome).
When the species is transferred to water, contractile
vacuoles become evident and flagellate forms with two
flagella begin to appear among ameboid forms.
The ameba flagellate forms are a transient forms , pear in
shape with two flagella at its broad , anterior end , they
moves rapidly forward or spin slowly in a circle ,
Under adverse circumstances it converted into cystic form
, which are spherical with a smooth thick wall ,
uninucleate and 7 to 10 Micro meter in diameter .cyst are
not formed in the tissue ,
Life Cycle
The amoebae invade the nasal mucosa; pass through the
olfactory plate into the meninges.
Multiplication is by binary fission, there is no sexual stages,
mitosis occurs and the nuclear membrane is retained.
Pathogenesis and symptoms
The amoeba colonize the nasal tissue and connected
sinuses and through the olfactory nerves into the brain.
At autopsy, the affected areas of the brain are soft, and the
meninges are congested and purulent. In tissues sections
the parasite found in the perivascular tissues where the
inflammatory cells are few or absent.
The symptoms of the disease are those of bacterial
meningitis such as frontal headache, high fever nausea,
vomiting, vision problems and mental problems.
Diagnosis
A history of exposure to stagnant or thermal water (springs)
3-6 days before the onset of symptoms of meningitis or
meningoencephalitis suggests the infection with naegleria.
In unstained wet preparation the trophozoite amoeba can
be recognized by their motility.
While they are recognized with difficulty in gram
negative or wright stained smears.
Culture isolation from cerebrospinal fluid or tissues can
be attempted on a plate of 1.5% non-nutrient agar seeded
with living Escherichia coli.
Treatment
To the few survived people amphotericin B & miconazole
was given intravenously and intra thecally, rifampin orally
& sulfisoxazole intravenously was found to be useful.
Epidemiology
PAM is a relatively rare disease with worldwide in
distribution ,infections where acquired while swimming
,diving with fresh water lakes, ponds, streams, warmed by
industrial effluent ,or natural thermal springs.
N. fowleri grows well at high temperature and tolerate
temperature up to 45° C.

Unit 2: Protozoa
13
Although cysts in air-borne dust are a theoretical source
of infection, but the trophozoite stage taken into the nose
in water is usual infective source.
Prevention
The best protective measure is to avoid exposure in warm
natural water, especially thermal springs, streams and
adequate chlorination of public water supply including
swimming pools.
Acanthamoeba
Disease:
Granulomatous amoebic encephalitis, uveitis, corneal
ulceration. Several species are considered pathogenic to
human such as A. culbertsoni and A. castellanii.
It causes a chronic disease which may last for more than a
week or even monthes before causing death.
Morphology
Acanthamoeba active trophozoite (12-45 µm) in diameter
and has irregular shape with spine-like pseudopodia called
acanthopodia arising from lobopodia and other areas of
the body
There is no flagellate form, contain one nucleus,consisting
of a large karyosome. No peripheral chromatin granules,
the cytoplasm appears granular and vacuolated.
Cyst stage: spherical (8-25 µm) with double wall forming
a smooth wrinkled outer wall (ectocyst) and a stellate or
roughly polygonal inner wall (endocyst).the single
nucleus is similar to that of trophozoite.
Life cycle
Infection can be acquired by inhalation, ingestion or
through traumatized skin or eyes. CNS infection is not
associated with swimming. invasion of CNS is secondary
to infection elsewhere in the body, Amoeba reach the
brain by the way of the blood stream from the lower
respiratory tract or through skin or mucosa.
A rapid transformation from cyst to trophozoite in the
nasal mucosa with subsequent CNS involvement.
Direct invasion of the eye are contracted in persons who
swim in ponds water with their contact lenses in place.
Improper cleaning of the lenses after their removal allows
the trophozoite to grow and multiply.
The trophozoite attach to the contact lenses after
reinsertion of the lenses. The trophozoite invades the eye
and digests corneal epithelial cells.
Pathogenesis and symptomatology
Invasion of the brain occure in chronically ill and
immunosuppressed patients. When the primary site of
invasion is the skin the onset of CNS symptoms comes
several month to a year later, the symptoms include
headache, sore throat, fever, neck stiffness, seizures,
nausea and vomiting. Cyst and trophozoite found in the
granulomatous lesions of the brain.
The lesions also found in skin, kidney, liver, lymph nodes,
ear, heart, prostate and eye.
Infection of the cornea of the eye (amoebic keratitis)
caused severe ocular pain and vision problems.
Penetration of the cornea may result in loss of vision.
Diagnosis:
The specimen of choice for diagnosing of trophozoite and
cyst is CSF also brain tissue, corneal scrapings and
suspected scraping may be cultured on agar plates seeded
with gram negative bacteria then transferred to a liquid
broth containing antibiotics.
Special (immunoenzymatic) staining of formalin fixed
tissue may be useful.
Treatment
No satisfactory GAE treatment available. Most patients
died before any diagnosis or treatment. Cases of
Acanthamoeba keratitis has been treated with a
combination of Neomycin drops, dibromopropamide
ointment and Brolene.
Epidemiology and control
Saurce of infection was presumed to be dust or water.
Infection found in people who are immunocompromised
or contact lenses wearer. Poor hygiene practice such as
using home made saline or tap water as rinsing solution
are major risk factor that may lead to infection.

Unit 2: Protozoa
14
Lecture 6 – Subphylum Mastigophora
Characterized by having flagellae in its trophozoite stage,
connected to an axonemes and kinetoplast (like brain in
human). The microorganism flagellum, an axonemes and
kinetoplast performing the neuromotor apparatus. The last
one kinetoplast is energizing & the first one is motor part.
The kinetoplast formed from the blepharoplast &
parabasal body, The blepharoplast either connected
together or scattered.
Some of flagellate is free living, and other are parasitizing
arthropods, plants, animal & man.
The flagellates which parasites human are:
1) Flagellate of digestive tract & urogenital system.
2) Flagellate of blood (haemoflagellate) & tissues.
The flagellate of digestive tract & urogenital systems:-
Live in the lumen.
Not tissues invader, but the (Giardia lamblia) &
(Trichomonas vaginalis) may evoke symptoms.
The flagellate of digestive tract &
urogenital systems:-
1)
Giardia lamblia
It also called Giardia intestinalis, a parasite of small
intestine, cosmopolitan, common in warm & temperate
climates.
Morphology
Both trophozoite & cyst stages are considered as a
diagnostic stages, while the infection stage is only the
cyst stage, because the trophozoite stage when ingested it
will killed by the gastric acid.
The trophozoite stage
The shape of trophozoite is pear shape, broad rounded
anteriorly, tapered to point posteriorly. Anteriorly
there is a sucking disk & side bilaterally, so the
trophozoite is described by bilaterally symmetrical. In
middle of sucking disk situated 2 nuclei in stage of
trophozoite. In middle of trophozoite from anterior to
posterior is complex system of an axoneme.
There is transverse curve broad, called the parabasal
body. The trophozoite length (9-
-15
-
The profile also called side view, show outside
curvature, anterior concavity and posterior curvature,
flagellae are distributed on many sites.
Giardia lamblia trophozoite stage: (1), ventral view; (2),
lateral view
The cyst stage
Thick hyaline membrane around avoidal shape cyst stage.
When parasite facing un suitable or unconventional
condition, it convert to cyst stage by retracting the
flagellae back on axoneme to form the parallel curved
fibrils, and each of 2 nuclei divided into 2 to form 4 nuclei
in cyst stage.
The cyst length (8-
-
The Life cycle
Usually infection arise from ingestion of contaminated
food or water, and after passing the stomach the
Exystation occur in small intestine and immediately 2
trophozoites arises from the cyst. Then the trophozoite
starting the multiplication and forming the colonies in
small intestine. When trophozoite goes down the
Encystation occur. So the summary of life cycle is:
1) Exystation
2) Multiplication
3) Encystation
Unit 2: Protozoa
15
Pathogenesis & Symptoms
Infection with Giardia called Giardiasis. The majority of
Giardiasis is asymptomatic, but some of them presented
with symptoms like fatty diarrhea, weight loss (due to
malabsorption), epigastric pain, abdominal cramps.
Giardia lamblia is not tissue invader, but by (E.M) had
shown that the trophozoite attached tightly to mucosa, so
covering and damaging to mucosa, so causing functional
derangement & reducing brush border enzymes. Diarrhea
& malabsorption may be caused by this mechanism.
Course of giardia infection in humans
Clinical manifestations in Giardiasis
Acute
Chronic
Diarrhea ( foul smelling
)
Greasy stools
Weight loss
Anorexia , vomiting
Headache
Low grade fever
Chills
Mucous in stools
Abdominal cramps
Recurrent diarrhea
Periodic constipation
Abdominal distension
Nausea
Substernal burning
Urticaria
Erythema nodosum
Malabsorption
syndrome
Fatigue
Diagnosis:
Recovering of cyst stage & trophozoite stage by:
General stool examination, or by
Concentration method
Treatment:
By Metronidazole, 250 mg/ 5-10 days.
Epidemiology
Infection occurs by viable cyst from human sources-
human faeces.
Giardiasis most common in warm moist climates. Type of
living is effecting its transmission, large families, and
children asylum.
Heavily infected groups, the infection start with infants &
goes to juveniles stage, and then go down to adult stage.
This occurs mainly in travelers or resort population.
Control
1) Treatment of patient.
2) Ordinary or chlorination water be found not enough to kill
cyst stage in endemic or hyperendemic, there for boiling
of water is important to kill the cyst stage.
3) Improving the habits of the person & community.
2) Chilomastix mesnili
Cosmopolitan, more prevalent in warm climates.
Morphology
It has 2 stages in its life cycle, trophozoite & cyst stage.
The trophozoite stage
Pear in shape, anteriorly broad and rounded, has one
nucleus, beside the nucleus is a groove called cytostome,
which represent the mouth of it. Regarding the flagella,
most of them directed forward & one directed backward
toward the cytostome , It has spiral groove which pass in
spiral path & its function is the movement. The
multiplication is by longitudinal binary division. The
movement is a jerky movement with spiraled path.
The cyst stage
Smaller than trophozoite, lemon shaped, (7-
length & (4.5-

Unit 2: Protozoa
16
hyaline wall, there is anterior projection like a lemon. There
is a groove in cyst stage which represents the cytostome.
Regarding the flagella, it retracted backward into the
organism and appears as a fibril inside the organism as well
as inside the cytoplasm. Also it has one nucleus.
3) Trichomonas
Pear shaped, have a single nucleus, infront the nucleus
situated the blepharoplast.
Most of flagella directed forward & one of them directed
backward, and this backward directed flagella forming
undulating membrane, which is a fold of membrane of
organism.
It characterized by presence of axostyle, which is semi
rigid translucent supported structure.
There are 3 species adapted to the human host, and only
these species contain axostyle.
There is a cytostome on the lateral side.
Line drawing of the three Trichomonads that parasitized
human beings. (1) Trichomonas vaginalis; (2)
Trichomonas tenax; (3) Trichomonas hominis.
Trichomonas hominis
shaped, marginal flagellum which forms undulating
membrane, the undulating membrane extend short
distance behind the posterior end. The axostyle is also
protruding behind the posterior extremity. It is non-
pathogen, although it feeds on bacteria, mucous & RBCs
if present. It live in large intestine.
Trichomonas buccalis
It also called Trichomonas tenax, smaller than T.
hominis, has small undulating membrane. It is non-
pathogenic, but it is found in diseased gum, tartar around
the teeth and carious teeth, so it is opportunistic parasite.
It is existence indicates poor oral hygiene.
Trichomonas vaginalis
It present in male & female, the diseased caused is called
Trichomoniasis or Trichomonas vaginitis.
Cosmopolitan parasite of man, size frequently larger than
other Tricomonas, it reach up to 27 mm in size. Maginal
flagellum does not extend byyond the undulating
membrane.
It inhibits vagina in female, and urethra + prostate in
male. It transmitted by sexual intercourse, although may
transmitted by other way (fomits). This parsite can
survive for few hours on dry fomites & longer if moist.
In male, is often asymptomatic, although it may cause
urethritis, also called non-specific urethritis.
In female, again may be asymptomatic or may produce
vaginitis complicated by bacteria, fungus & spirochete.
The chief complaints are dysuria, leukorrhea (white
discharge), urticaria, and acute vulvitis. The symptoms
vary from mild to sever, but the disease is annoying rather
than disabling.
Phagocytosis & killing of Gono cocci by Trichomonas
vaginalis have been reported.
Diagnosis: Made in male by recovery of the organism in
urine, prostatic or urethral discharges, by add normal
saline to dry smear, we show T. vaginalis. In female, by
recovery urine, vaginal discharge or vaginal swabs by also
adding normal saline to wet smear.
Treatment: Metronidazol 250 mg TID (three times per
day) for 7 days. In resistant cases, vaginal suppositories of
Metronidazol are useful.
Diantamoeba fragilis
Causes disease called Diantamoebiasis. It was considered
as an amoeba until 1974, when Honigberg put it in Order
– Trichomonadida & Species-Trichmonas
Only trophozoite stage is known, (5-
In stained preparation, 2 nuclei are evident, but with no
chromatin granules at nuclear membrane. The karyosome
being large central & appear to consist from 4 granules.
The Diantamoeba frgilis colonizes ceacum & upper colon
& does not invade the mucosa. It may cause anorexia,
abdominal discomfort & diarrhea and can be treated with
Tetracycline or Metronidazole.
Diantamoeba frgilis
trophozoite

Unit 2: Protozoa
17
Lecture 7 - Phylum ciliophora
Balantidium coli
Subphylum : Ciliophora
Class : Ciliata
Disease : called Balantidiasis or Balantidial dysentery
Have a cosmopolitan distribution in ahogs and a common
parasite of several species of Monkeys. In man is found in
warm climates.
2 stages occur in the life cycle, trophozoite or vegetative
& cyst stage. The trophozoite is ovoidal in shape (50-100
-
Anterior somewhat conical anteriorly and rounded
posteriorly, cilia present all around the body of the
microorganism. Anteriorly has funnel-shape called
peristome leading to Cytostome (mouth of the cell) and
posteriorly Cytopyge (anus of the cell). It has 2 nuclei,
large one Macronucleus (kidney shaped) & small one
Micronucleus. Also it has 2 contractile vacuoles which
called pulsating vacuoles, those disappeared in old cyst.
Two types of multiplication:
1) A sexual, micronucleus divides mitotically and
Macronucleus divides amititically followed by the
division of cytoplasm result in 2 organisms.
2) Conjugation (sexual), has been observed in B. coli but
not essential for its propagation.
has no cilia (double outline wall). Although the markings
on the cell remain 2 nuclei, 2 Pulsating vacuoles, although
they disappeared in the old cyst. Invasion by motility &
cytolyticenzyme.
Loalized to large intestines, rarely seen in Extra intestinal.
Ulcer:wide mouth. May cause diarrhea or dysentery.
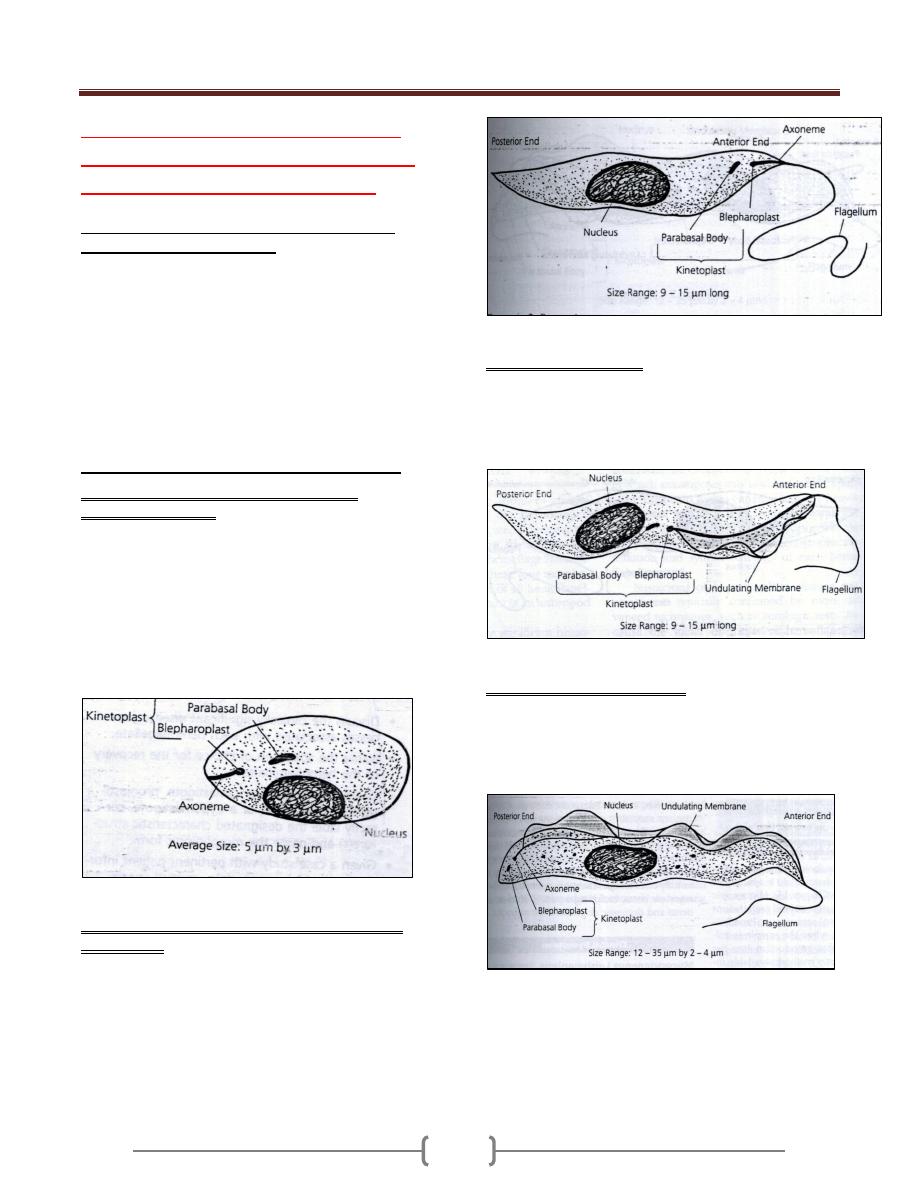
Trophozoite & cyst stage of Balantidium coli
Morphology
The organism has 2 stages, trophozoite and cyst.
The trophozoite is the largest of the protozoa that
parasitized man .It is ovoid covered with short cilia, the
organism exhibited rotary boring motility (size 50-100
µm in length by 40-70 µm in width).The anterior end is
conical and the posterior end is rounded .Near the anterior
end of the body, there is funnel –shaped peristome, which
leads into the cytostome.A minute cytopyge is situated at
the opposite end (anal opening).Two large contractile
vacuoles present in the cytoplasm. Also there is two
nuclei, micronucleus (small, spherical) lies adjacent to
kidney –shaped macronucleus.
Cyst: ovoid or spherical (43-56 µm in diameter) and it’s
the transfer stage, a double protective cyst wall surrounds
the organism; the cilia disappear.
The natural habitat of B.coli is the caecal and sigmoid-
rectal region of hogs, man is an incidental host.
In the trophozoite, asexual reproduction consists of
transverse binary fission in which the micronucleus first
divides mitotically, then the macronucleus amitotically
followed by the cytoplasm resulting in two daughter
organisms. Also conjugation has been observed in B.coli
but it is not essential for its propagation.
Pathogenesis and symptomatology:
B.coli penetrates the mucosal layer with extensive
submucosal destruction and causing ulceration. The
parasite may move through the muscularis mucosa into
the submucosa, where it spread radially causing rapid
destruction of the tissue. Unlike E.histolytica, it rarely
invades the muscular coat and extraintestinal infection is
very rare. The symptoms in balantidiasis vary from
fulminating dysentery or acute diarrhea to an
asymptomatic carrier state.
Diagnosis
Examination of stool for the presence of trophozoite and
cyst stage.Multible samples may be required to determine
the presence or absence of the parasite.
Treatment
1- Diiodohydroxyquin.
2- Tetracycline.
3- Metronidazole.
Unit 2: Protozoa
18
Lecture 8+9+10+11 – Subphylum
Mastigophora (Flagellates of blood
and tissues "Hemoflagellates")
The hemoflagellates of human include the genera
Trypanosoma and Leishmania.
1) All these organisms require 2 hosts in their life cycle.
Man or other mammals on the one hand and blood
sucking insect (intermediate host) on the other.
2) They live in the blood and tissue of man and other
vertebrate hosts, and in the gut of the insect vector.
3) Multiplication in both vertebrate and invertebrate hosts is
by binary fission.
4) They exist in two or more of the four developmental
forms or stages.
Hemoflagellates have 4 developmental forms:
1) Round intracellular stage called amastigote
(Leishmania)(Fig. 1). The amastigote is spherical or
subspherical, it lives and reproduces by longitudinal
binary fission in macrophage of skin, mucosa, lymph
node and RES.In preparation stained with Giemsa´s or
Wright's stain the cytoplasm is pale blue and the large
nucleus is red stain. In their cytoplasm in the median line
of the cell, there is a deep red rod like structure called
kinetoplast; a delicate filament called axoneme extends
from near the kinetoplast to the cell membrane.
Figure 1: Amastigote
2) Flagellated extracellular stage called promastigote
(leptomonas) .It is is the basic of the hemoflagelate.It is
pyriform without an undulating membrane with a
kinetoplast at the anterior end. A free flagellum near the
anterior end of the cell. There is no undulating membrane
(Figure 2).
Figure 2 Promastigote
3) Epimastigote (crithidia). Elongated extracellular stage
with kinetoplast placed more posteriorly close to and in
front of the nucleus. The flagellum runs alongside the
body as short undulating membrane before emerging from
the anterior end (Figure 3).
Figure 3: Epimastigote
4) Trypomastigote (trypanosoma) .The cell is elongated;
spindle shaped with a central nucleus and the kinetoplast
posterior to the nucleus situated at the posterior end of the
body .There is a long undulating membrane and a free
flagellum (Figure 4).
Figure 4: Trypomastigote
Unit 2: Protozoa
19
Leishmania
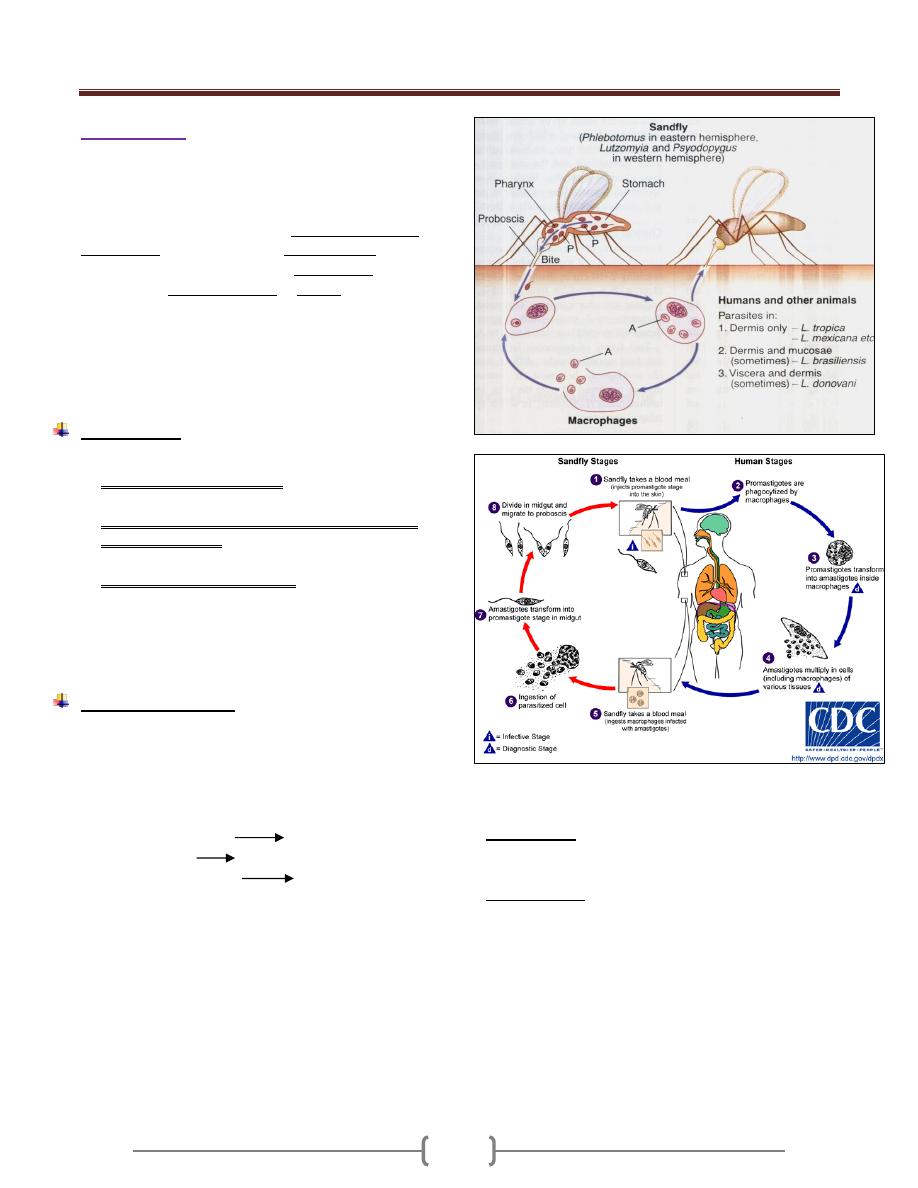
The genus Leishmania has been named after Sir William
Leishman, who discovered the species (Leishmania
donovani) that cause kala-azar.
Genus Leishmania are parasites of man, dog, gerbil, and
other rodents which represent the definitive hosts whereas
blood sucking sandfly of the genus Phlebotomus ( female
only) serve as intermediate hosts or vectors which
transmit the parasite from one mammalian host to another.
In man and reservoir host (dogs, rodent) , the organism in
the amastigote form is a parasites of macrophage cells
which multiply by binary fission and cause death of the
host cells.
Morphology:
The leishmania of man have been grouped into 3 species.
1) Leishmania tropica complex cause old world
cutaneous leishmaniasis.
2) Leishmania braziliensis complex and Leishmania
mexicana complex cause new world cutaneous and
mucocutaneous leishmaniasis.
3) Leishmania donovani complex cause visceral
leishmaniasis.
These species are morphologically similar but they
differ clinically, epidemiologically, immunologically
and biochemistry.
Life cycle (Figure 5)
2 stages in the life cycle are known:
1) Promastigote stage in sandfly and culture.
2) Amastigote in mammals and reservoir animal.
I. When a sandfly bite an infected person or reservoir host, it
sucks up parasitized macrophage or temporarily free
amastigote in the blood,
Midgut of the fly flagellated promastigote and after
rapid multiplication infective promastigote and
migrate forword. From the foregut they are regurgitated or
otherwise introduced into the skin of the next individual
when the sandfly takes another blood meal.
II. When a sand fly bites another healthy persons, the fly
inject the promastigote into the skin, the promastigote
rapidly changed to amastigote after phagocytosis by
macrophage, amastigote multiply filling the cytoplasm of
macrophage. The infected cells burst, the released
parasites are again phagocytosed and the process is
repeated. Cutaneous or visceral leishmaniasis depending
upon the species of the parasites and the host response.
Figure 5: life cycle of leishmania (Cutaneous and
Visceral)
Blepharoplast: A basal body in certain flagellated
protozoans that consists of a minute mass of chromatin
embedded in the cytoplasm at the base of the flagellum.
parabasal body, a cytoplasmic body of varying
appearance, structure, and function closely associated
with the nucleus, kinetoplast, and basal body in certain
parasitic flagellate protozoa- it is usually connected to the
basal body by a fibril or thread, which together are known
as the parabasal apparatus. More than one such structure
may be present in each organism. Some authorities
consider the parabasal body to be the Golgi complex of
these cells.

Unit 2: Protozoa
20
Leishmaniasis
A group of diseases caused by protozoa of the genus
Leishmania.
Leiahmaniasis classified into:
1) Cutaneous leishmaniasis.
2) Mucocutaneous leishmaniasis.
3) Visceral leishmaniasis.
1) Cutaneous leishmaniasis
Is classified into:
a) Old world cutaneous leishmaniasis (oriental sore, Delhi
boil)
b) New world cutaneous leishmaniasis.
The insect (vector) is a sandfly called phlebotomus
sandfly in old world cutaneous leishmaniasis and
lutzomyia for new world cutaneous leishmaniasis.
a) Old world cutaneous leishmaniasis.
1) Leishmania tropica minor (dry or urban cutaneous
leishmaniasis, oriental sore, Aleppo button, Jericho boil,
Delhi boil, Baghdad boil).It is anthroponotic disease
transmitted from human to human. L.tropica may
become viscerotropic.
2) Leishmania tropica major (rural or wet cutaneous
leishmaniasis).It is zoonotic disease.
3) Leishmania aethiopica (cutaneous and diffuse or
disseminated cutaneous leishmaniasis of Ethiopia, anergic
cutaneous leishmaniasis).
Incubation periods 2 weeks – 3 years
In Leishmania tropica and Leishmania aethiopica as log
as 3 years.
In Leishmania major 2 weeks.
Life cycle: As in figure 5.
Clinical features and pathogenesis(Leishmania tropica
and Leishmania major):
The lesion occur at the dermis at the site of the
inoculation of the promastigote.Mucous membrane are
rarely involved.
The lesion appears first as macule, then papule with
slightly raised center covered by a thin blister like layer of
epidermis.
The lesion then breaks down with discharge of a small
amount of clear or purulent exudate. At the ulcer crater
like base in the dermis, a granulation layer is formed and
the margin becomes indurated by infiltration of fibroblast.
In the dry type , the disease is chronic ,occurs in the
urban area , single lesion ,face is affected ,the ulceration is
slow and may not occur with little surrounding tissue
reaction ,the healing may take > 1 year, human is the only
reservoir.
In the wet type, the disease is acute, occurs in the rural
area, lower limb is affected, the ulceration is multiple and
prone to early ulceration with high degree of surrounding
tissue reaction and liable for secondary bacterial infection,
the healing may take 3-6 months, gerbils and other rodent
are the main reservoir.
Leishmania aethiopica
Cause cutaneous and diffuse or disseminated cutanous
leishmaniasis of aethiopia or called anergic cutaneous
leishmaniasis.
It occurs in the highlands of Ethiopia, in Kenya, and
possibly Yemen.
Morphologically it is indistinguishable from Leishmania
tropica.
The animal reservoirs are species of hyrax.
It cause disseminated leishmaniasis because of deficient
cell mediated immunity due to some characteristic of the
parasites itself.
The patient is not anergic to other infective agent, react
normally to, tuberculin skin test and have normal IgG
level but negative leishmanin skin test.
Clinically, three types have been described (lepromatoid,
intermediate and tuberculoid).
b) New world cutaneous leishmaniasis
(American cutaneous leishmaniasis).
They are caused by Leishmania mexicana complex and
Leishmania braziliensis complex. The American strains of
leishmania causing cutaneous leishmaniasis differ in their
tendency to involve the mucous membrane of the mouth
and nasopharynx by extension or metastasis.
Leishmania mexicana complex
1) Leishmania mexicana mexicana, found in Mexico,
Guatemala and Belize. It cause chiclero ulcer or bay sore
(name is derived from high occurance of the infection in
chicle collectrors) which affect the face, ears and does not
spread to the nasopharynx. The disease is mild and self-
limiting consisting of single papule, nodule or ulcer.

Unit 2: Protozoa
21
2) Leishmania mexicana amazonensis cause cutaneous
lesion with no nasopharyngeal involvement. It occurs in
Amazon basin.
3) Leishmania mexicana pifanoi cause disseminated
cutaneous disease in Venezuela.
Leishmania braziliensis complex:
1) Leishmania braziliensis guyanensis in Guiana,
Venezuela and Brazil.
2) Leishmania braziliensis panamensis in panama and
Colombia.
3) Leishmania braziliensis peruviana which cause uta
(resemble oriental sore) without nasopharyngeal
involvement.
2) Mucocutaneous leishmaniasis
It is caused primarily by Leishmania braziliensis which
cause Espundia start as papule at the site of bite and then
metastatic lesion forms, usually at the mucocutaneous
junction of the nose and mouth leading to disfiguring
granulomatous ulcerating lesion destroying the nasal
cartilage but not adjacent bone. Death occurs from
secondary bacterial infection.
Immunity to cutaneous leishmaniasis:
Host recovery in cutanous leishmaniasis depends on the
development of cell mediated immunity.
The usual cutaneous lesion heals spontaneously.
In certain instances, healing does not occur; these cases
may represent the 2 poles of the spectrum of response.
The first spectrum is the anergy as in leishmania
aethiopica.
The second spectrum represent the hypersensitivity
reaction in which the patient is capable for excellent Ab
and cellular responses but cannot completely eliminate the
parasites , so as the central lesion heals, active peripheral
ones continue to form . This stage is called leishmaniasis
recidiva or lupoid leishmaniasis (in Leishmamnia
tropica).
Diagnosis:
1) Specimens: Lymph node aspirate, scrapings and biopsies
from the margin of the lesion. The center of purulent
discharge is of no value.
2) Microscopic examination: The specimen smeared onto a
clean glass slide, the slide stained with Giemsa´s stain for
demonstration of amastigote within the macrophage or
spread out from ruptured cells.
3) Culture in NNN (Novy-MacNeal-Nicolle) medium or
inoculation in hamster.
4) Leishmanin skin test (The test done by i.d injection of a
suspension of killed promastigote) is positive in high % of
L.tropica and >95% of L.braziliensis.It is positive in
patient with active, healed or cured lesions and negative
in anergic persons (in diffuse leishmaniasis).False positive
seen in patient with tuberculosis, leprosy and mycosis.
Treatment:
1) Small lesion:
1. Freezing with liquid CO2 (cryotherapy).
2. Curettage
3. Infiltration with 1-2 ml of Na stibogluconate (pentostam) ®.
2) Drug therapy:
1. Na stibogluconate (pentostam) ®.
For multiple lesions or in disfiguring site.
The dose given i.m or i.v 20 mg /kg body weight for 20
days, repeated 10 days interval in resistant cases for
maximum 3 courses.
It acts by inhibition of the glycolytic enzyme and fatty
acid oxidation in leishmanial amastigote.
Na stibogluconate (pentostam) ® used for the treatment of
all form of cutaneous leishmaniasis except leishmania
aethiopica which respond to pentamidine.
2. Oral ketocanazole used daily for 4-8 weeks in treatment
of long standing cutaneous leishmaniasis.
3. Amphotericin B in cases not responding to pentostam.
4. Iraconazole in India to treat cutaneous leishmaniasis.
5. Clotrimazole 1% cream in Saudi Arabia.
6. Oral dapsone.
7. Intradermal injection of gamma interferon around the
lesion caused by L.tropica and L.guyanensis.This will
promote healing of ulcer.
8. Combined vaccine (heat killed L.amazonensis+viable
BCG) in treatment of American cutaneous leishmaniasis.
3)
Steroid
for treatment of leishmaniasis recidiva.
Epidemiology:
Cutaneous leishmaniasis is found around the
Mediterranean littoral, throughout Middle East and central
Asia as far as Pakistan and in Sub-Saharan West Africa
and Sudan.
Vaccination is practiced in certain areas by inoculating
serum from naturally acquired lesion into an
inconspicuous location on the body of a non-immune
person.
Unit 2: Protozoa
22
3) Visceral leishmaniasis
It is caused by at least three species belonging to the
Leishmania donovani complex with different geographical
distribution. The three species are (Leishmania donovani,
Leishmania infantum, Leishmania chagasi).
The causative agent is a parasites of the RES not confined
to the mucous membrane and subcutaneous tissue but
throughout the body.
a) Leishmania donovani.
occur in India, east Pakistan, Sumatra, Thailand, and the
central Africa, Chad, Ethiopia, Somali republic, Djibouti,
Kenya, Sudan , Gabon, Gambia and Niger .
It affects all age group.
Human is the only reservoir in India.
Various rodents in Sudan.
Dogs in china.
The vector is phlebotomus.
b) Leishmania infantum.
It is found along the whole Mediterranean littoral, near
east and Africa.
It occurs in children.
Human is an accidental host.
Dogs are the Reservoir.
The vector is phlebotomus.
c) Leishmania chagasi.
In central and South America.
Infect children.
Foxes, domestic dogs and cats are naturally infected.
The vector is lutzomyia.
Life cycle and pathogenesis:
Leishmania donovani has a predilection for the
reticulendothelial cells of the spleen, liver, bone marrow,
and visceral lymph node and as the promastigote stage of
the parasite introduced into the outer dermis by an
infected sandfly, the promastigote rapidly changed to
amastigote after phagocytosis by macrophage, amastigote
multiply filling the cytoplasm of macrophage. The
infected cells burst, after colonization in the dermis which
is in apparent, some of the organisms gain access to the
blood stream or lymphatics and are transported to the
viscera where lodge in fixed tissue macrophage and
rapidly multiply.
As the number of amastigote increase, those lead to
intense phagocytic activity and a remarkable increase in
the number of macrophage, increasing neutropenia, and
anemia.
The decrease in the bone marrow activity + cellular
destruction in the spleen , this lead to anemia, leukopenia
and thrombocytopenia leading to secondary bacterial
infection and bleeding tendency.
Spleen is enlarged due to a combination of proliferating
macrophage and sequestered blood cells.
Liver is enlarged.
Tonsils and lymph nodes are involved.
In fatal cases, the dermis contains large amounts of
amastigote inside the macrophage.
Note: In dogs, conspicuous lesions are on the skin,
cutaneous leishmaniasis due to infection with L.tpropica
and kala-azar caused by L.donovani are difficult to
distinguish.
Clinical features:
Incubation period = 10 days – many months.
The onset may be insidious (which is the usual) or acute.
In the insidious onset , the symptom begin with
intermittent fever (36.7-42C°) with 2 peaks daily ,
weakness , weight loss, massive enlargement of the spleen
, hyperpigmentation of the skins is seen in light skinned
patient ( kala-azar means black sickness) ,abdomen is
protuberant.
The course of the disease runs for months - years, initially
the patients feel well despite the fever. As the anemia,
leucopenia and thrombocytopenia increase, this lead to
weakness, infection, bleeding from the gums, lips, nares,
and the GIT. Untreated cases are fatal due to secondary
infection.
Complications:
1) Diarrhea or dysentery.
2) Bronchopneumonia.
3) Cancrum oris (gangrene of the oral cavity) less
frequently.

Unit 2: Protozoa
23
Dermal leishmaniasis:
Post kala-azar dermal leishmaniasis, present first as
hypopigmented or erythematous macules on any part of
the body or as nodular eruption especially on the face.
The organism may be present in the lesion. It is a delayed
hypersensitivity to parasite antigen and is interpreted as
an indication of inadequate treatment with residuance of
parasites that continue to propagate.
Diagnosis:
1) Nonspecific tests. Pancytopenia, reversed
Albumin/Globulin ratio.
2) Specimens. Bone marrow, spleen and lymph node
biopsies for detection of intracellular amasatigote (L-D
bodies).Splenic aspirates are the most sensitive method
for the diagnosis.
3) Culture in NNN (Novy-MacNeal-Nicolle) medium.
Note: The diagnosis is established by visualization of
amastigotes in smear, biopsies, or by growth of
promastigote in culture.
4) Serological tests: for detection of antigen or antibody.e.g.
I) indirect fluorescent Antibody test (IFAT).
II) Enzyme Linked Immunosorbant Assay (ELISA).
III) Direct agglutination test (DAT).
IV) Immunochromatographic K39 strip test
{Recombinant leishmanial antigens or synthetic
peptides (rK39)} (dipstick test).
The recombinant antigen is a 39 amino acid (rK39) cloned
from the C-terminus of the kinesin protein of Leishmania
species that cause visceral infecction. This test is used to
detect antibodies against K39 antigen in patient with
visceral leishmaniasis.
The sensitivity of the test is 100% and the specificity is
97%.
The test is simple, rapid (10 minutes), inexpensive,
requires no other reagents or instruments that can be
performed in the field by the paramedics.
These tests (serological) remain positive for several
months after cure has been achieved, so don’t predict
response to treatment or relapse.
5) Napier's Aldehyde test. One ml of the patient´s serum
mixed well with a drop of 40% formalin, shaken and kept
at room temperature.
A positive reaction is gellification and opacification of the
serum appearing in 2-20 minutes. This test is based on the
principle that a patient with visceral leishmaniasis has a
high concentration of IgG (hypergammaglobulinemia)
.This test is not diagnostic as other diseases cause also
hypergammaglobulinemia e.g. Multiple myloma, cirrhosis
of the liver and Schistosomiasis.
6) Leishmanin skin test is negative in the acute disease but
positive 2 months after recovery.
7) Polymerase chain reaction (PCR).
Treatment:
A. Pentavalent antimonial or pentavalent antimony
compounds (abbreviation: pentavalent Sb or Sbˇ), they
include:
1) Na stibogluconate (pentostam)® given by slow I.V infusion
2) Meglumine antimonite (Gliucantim) ® by I.M injection.
- Na stibogluconate for 28 days is the drug of choice.
B. B)Diamidines:Sudanese infections are generally
resistant to antimonials, and treatment, should be initiated
with pentamidine® at the rate of 2 to 4 mg /kg body
weight i.m for 10 – 15 days.
C. Amphotericin B can be used.
D. Allopurinol can be used in the treatment of visceral
leishmaniasis in patient with AIDS.
E. Antimony and Gamma interferon can be used
F. Miltefosine .It is antineoplastic and used as
antiprotozoal.It is used in the treatment of cutaneous and
visceral leishmaniasis in India, Colombia.
Immunity:
Lifelong immunity.
Massive polyclonal hypergammaglobulinemia with little
evidence of cell mediated immunity is the role in visceral
leishmaniasis. The elevated immunoglobulin level
diminishes rapidly when treatment begins.
Epidemiology:
Kala-azar is endemic in northern china, eastern india,
Afghanistan and Turkestan, Sudan, many foci around the
Mediterranean sea, Ethiopia, the east and west coasts of
Africa, Paraguay, Bolivia, northern Argentina, eastern
Brazil, and minor foci elsewhere in South and Central
America.
In most of these areas, the infection is endemic or
hyperendimic but on occasion it may become epidemic.
Control and prevention.
1) Destroying the stray dogs.
2) Using an insecticide.
3) Mosquitoes net.
4) Insect repellent cream.
5) Treatment of infective patient.

Unit 2: Protozoa
24
Trypanosoma
The hemoflagellates of the genus trypanosoma occur in
the blood of mammals as mature elongated
trypomastigote.
The trypomastigote is an Elongated bodies supporting a
longitudinal lateral undulating membrane and a flagellum
that borders the free edge of the membrane and emerge at
the anterior end as a whip like extension. The kinetoplast
is a darkly staining body lying in the posterior end of the
body immediately adjacent to tiny node (blepharoplast)
from which the flagellum arise, this form called
trypomastigote (Figure 1).
Figure 1: Trypomastigote
The forms that are seen in the vectors are called
epimastigote (crithidia)
Elongated extracellular stage with short undulating
membrane and a kinetoplast placed posteriorly in the
anterior end near the nucleus, this form called
epimastigote (Figure 2)
(Figure 2: Epimastigote)
Some species of trypanosomes apparently lives in their
natural vertebrate hosts without causing evident disease,
other cause variable degree of tissue pathology.
Three species of trypanosomes that commonly parasitize
man are all pathogenic and not infrequently cause death.
The three species are:
1) Trypanosoma brucei rhodesiense.
2) Trypanosoma brucei gambiense.
3) Trypanosoma cruzi.
Trypanosoma brucei brucie, Trypanosoma brucei
rhodesiense, Trypanosoma brucei gambiense and
Trypanosoma rangeli are called salivarian trypanosomes
because the forms of parasite that are infective for the
mammalian host develop in the saliva.
Trypanosoma cruzi differ in two aspects:
1) It is leishmania like in having dividing amastigote tissue
forms.
2) The infective stage develops in the hindgut of the vector
and emerges from the intestine (posterior station) in the
feces and this is called stercorian trypanosome.
Trypanosoma cruzi (Schizotrypanum)
Disease: chagas´ disease or American trypanosomiasis.
Life cycle (Figure 3):
Reservoirs are domestic cats, dogs and wild species such
as armadillo, raccoon, and rats.
Two main stages of Trypanosoma cruzi are found in
mammalian host, amastigote and trypomastigote, while
epimastigote (crithidial) and trypomastigote are found in
triatomine bug.
The reduviid bugs ingest trypomastigotes in the blood of
the reservoir hosts. In the insect gut, they multiply and
differentiate first into epimastigotes and then into
trypomastigote.
When the bug bites again, the site is contaminated with
feces containing metacyclic trypomastigotes, which enter
the blood of the host (or reservoir) through the bite wound
or intact mucous membrane (conjunctiva, mouth). The
parasites are engulfed by macrophages and become
amastigotes. After 4 or 5 days of multiplication by binary
fission, amastigotes again become trypomastigotes,
disrupt the cell, and enter the blood stream and other
tissues where the cycle continues as long as the host lives.
Many cells are affected, but myocardial, glial, and
reticuloendothelial cells are the most frequent sites.
To complete the cycles, amastigotes differentiate into
trypomastigotes, which enter the blood and are taken up
again by the reduviid bug.
In tissues the accumulation of multiplying parasites
produces pseudocysts; the amastigote are
Unit 2: Protozoa
25
indistinguishable from those of L.donovani but in
L.donovani, it invades only macrophages whereas in
T.cruzi, amastigote invades the cells of any tissue.
Other less frequent modes of human infection are by
blood transfusion and by congenital or transmammary
transmission, organ transplantation, rarely by eating food
contaminated with infective bug feces or by ingestion of
infected meat. Accidental laboratory infections have been
reported.
Infective stage = Metacyclic trypomastigote.
Pathogenesis:
The amastigote can kill cells and cause inflammation,
consisting mainly of mononuclear cells. Cardiac muscle is
the most frequently and severely affected tissue. Neuronal
damage leads to cardiac arrhythmias and loss of tone in
the colon (megacolon) and esophagus (megaesophagous).
During the acute phase, there are both trypomastigote in
the blood and amastigote intracellularly in the tissue .In
the chronic phase, the organism persist in the amastigote
form.
Clinical features:
Incubation period = 5-12 days
The disease is seen most commonly, and in its severe
form, in children younger than 5 years, in whom CNS
symptoms predominate, while in older children and
adults, the disease may be mild, subacute or chronic.
The first signs of acute Chagas´ disease develop at least 1
week after invasion by the parasites. When the organisms
enter through a break in the skin, an indurated area of
erythema and swelling (the chagoma), accompanied by
local lymphadenopathy, may appear.
Romana’s sign—the classic finding in acute Chagas´
disease, which consists of unilateral painless edema of the
palpebrae and periocular tissues—can result when the
conjunctiva is the portal of entry.
These initial local signs may be followed by malaise,
fever, anorexia, and edema of the face and lower
extremities. A morbilliform rash may also appear.
Generalized lymphadenopathy and hepatosplenomegaly
may develop. Severe myocarditis develops rarely; most
deaths in acute Chagas disease are due to heart failure.
Neurologic signs are not common, but
meningoencephalitis occurs occasionally. The acute
symptoms resolve spontaneously in virtually all patients,
who then enter the asymptomatic or indeterminate phase
of chronic T.cruzi infection
Symptomatic chronic Chagas´ disease becomes
apparent years or even decades after the initial infection.
The most commonly involved is the heart, also dilatation
of hollow viscera (megaesophagus, megacolon and
megaureter).
Death from chronic chagas´ disease is usually due to
cardiac arrhythmias and failure.
Laboratory diagnosis:
1) Acute disease
a. Wet blood preparation for motile organisms
b. Thick and thin blood film for demonstration of C-
shaped trypomastigote.
c. Culture in NNN medium
d. Muscle biopsy for amastigote
e. Polymerase chain reaction (PCR) when repeated
attempts to visualize the organisms are unsuccessful
2) Chronic Chagas´ disease
It is difficult because few trypomastigote in the blood
It is diagnosed by the detection of specific antibodies that
bind to T. cruzi antigens (serology) and by xenodiagnosis.
a) Serological test.
a) ELISA
b) Indirect fluorescent – antibody test
c) Indirect haemagglutination and
d) Complement fixation test.
b) Xenodiagnosis for chronics disease which consists of
allowing an uninfected, laboratory – raised reduviid bug
to feed on the patient and, after several weeks, examining
the intestinal contents of the bug for the organism.
Treatment:
The drug of choice for the acute form is nifurtimox, kills
the trypomastigote.
Benznidazole is an alternative drug.
No drug for chronic disease.

Unit 2: Protozoa
26
Trypanosoma gambiense and
Trypanosoma rhodesiense.
Disease:
They cause sleeping sickness (African trypanosomiasis).
Trypanosoma gambiense (Gambian or West African
sleeping sickness).
Trypanosoma rhodesiense (Rhodesian or East African
sleeping sickness).
Trypanosoma rhodesiense
(Rhodesian or East African sleeping sickness)
Life cycle (Figure 1)
The vector for both is the tsetse fly, but different species
of fly are involved.
Humans are the reservoir for Trypanosoma gambiense,
whereas Trypanosoma rhodesiense has reservoirs in
both domestic animals (especially cattle) and wild animals
(e.g., antelopes).
Tsetse fly ingests trypomastigote in blood meal from a
reservoir host.
They multiply in the insect gut and then migrate to the
salivary glands, where they transform into epimastigotes,
multiply further, and then form metacyclic
trypomastigotes, which are transmitted by the tsetse fly.
The development within the fly requires 3 weeks.
In man:
Metacyclic trypanosomes injected in the skin by tsetse fly,
multiply at the site of injection. Inside human,
multiplication in blood, lymph nodes, and spleen is by
binary fission as trypanosomal form.
Pathogenesis:
In the acute form, a cyclical fever spike occurs that is
related to antigenic variation of the surface
glycoprotein. The antigenic variation of surface
glycoprotein is explained by the fact that trypanosomes
may have 1000 or more variant surface glycoprotein
(VSG) genes and trypanosomes change their surface
antigen glycoprotein by turning off one gene coding for a
(VSG) and turning on another. As a result, one antigenic
type will coat the surface of the parasites for
approximately 10 days, followed by other types. These
antigenic variations allow the organism to evade the host
immune response (Figure 2).
Figure 2: antigenic variations in African trypanosome
VSG=Variant surface glycoprotein
A self-limited inflammatory lesion (trypanosomal
chancre) may appear a week or so after the bite of an
infected tsetse fly. The chancre subsides in a week or two
as the trypanosomes gain entry to the circulating blood.
Then they enter the lymph nodes, where a second focus of
inflammation occurs, with hyperplasia of the endothelial
lining of the blood sinuses and perivascular infiltration of
leukocytes. Thos process is rapid, fulminating, and often
causes death in a few months. Rarely the patient lives
long enough for the trypanosomes to invade the central
nervous system and produce the signs of the third stage of
the infection (Sleeping Sickness).
Clinical features:
Incubation periods= 1-2 weeks.
After the incubation period, the patient suffers from
headache, febrile paroxysms that recur frequently,
weakness, loss of weight. Lymph node enlargement is not
pronounced. Skin rash, odema and myocarditis may be
present. CNS signs are not common.
Death occurs within 1 year due to intercurrent infections.

Unit 2: Protozoa
27
Trypanosoma gambiense
(Gambian or West African sleeping sickness
)
Life cycle: As for Trypanosoma rhodesiense (Figure 1)
Pathogenesis:
As for Trypanosoma rhodesiense, but the difference is
that T. gambiense enter the arachinoid spaces of the CNS
and the in brain substance. Thus following the initial
lesion in the skin, three progressive stages occur:
Parasitemia, Lymphadenitis, and CNS involvement.
Clinical features:
Incubation period = 6-14 days.
The initial lesion is an indurated painful skin ulcer
(trypanosomal chancre) at the site of bite. A Blood film
reveals trypanosomes. This period of symptom free last
for weeks or months, then the infection may be abortive
or the parasites invade lymphatic tissues leading to
intermittent weekly fever( febrile attack of about a week's
duration and then afebrile period) and lymphadenopathy
develop. Enlargement of the posterior cervical lymph
node (Winterbottom´s sign) is commonly seen. Axillary
and groin lymph nodes are also involved with
splenomegaly
After some months in the absence of treatment, the central
nervous system is invaded and is characterized initially by
headache, insomnia by night and sleepiness by day, mood
changes followed by muscle tremor, slurred speech and
apathy progress to somnolence, coma and death.
Diagnosis:
1) Thick or thin blood film, an aspirate of the chancre or
enlarged lymph node, bone marrow biopsy to reveals
the trypomastigote.
The likelihood of finding parasites in blood is higher in
stage I,II than in stage III disease and in patients infected
with T. b. rhodesiense rather than T. b. gambiense.
2) CSF in case of encephalitis reveals trypanosomes with
elevated protein level, pleocytosis, high IgM and CSF
pressure.
3) Culture in NNN medium.
4) Serological tests: card indirect agglutination test for
trypanosomiasis, and ELISA for IgM Ab.
Treatment:
Suramin is the most effective drugs but does not cross the
blood brain barrier.
Pentamidine is an alternative drug.
Melarsoprol in case of CNS involvement
Comparison of West African and East African
Trypanosomiases
Point of
Comparison
West African
East African
(Gambiense)
(Rhodesiense)
Organism
T. b.
gambiense
T. b. rhodesiense
Vectors
Tsetse flies
(palpalisgroup)
Tsetseflies
(morsitansgroup)
Primary reservoir
Humans
Antelope and
cattle
Human illness
Antelope and
cattle
Acute(early CNS
disease)
Duration of illness
Months to
years
<9 months
Lymphadenopathy Prominent
Minimal
Parasitemia
Low
High
Diagnosis by
rodent inoculation
No
Yes
Epidemiology
Rural
populations
Workers in wild
areas, rural
populations,
tourists in game
parks
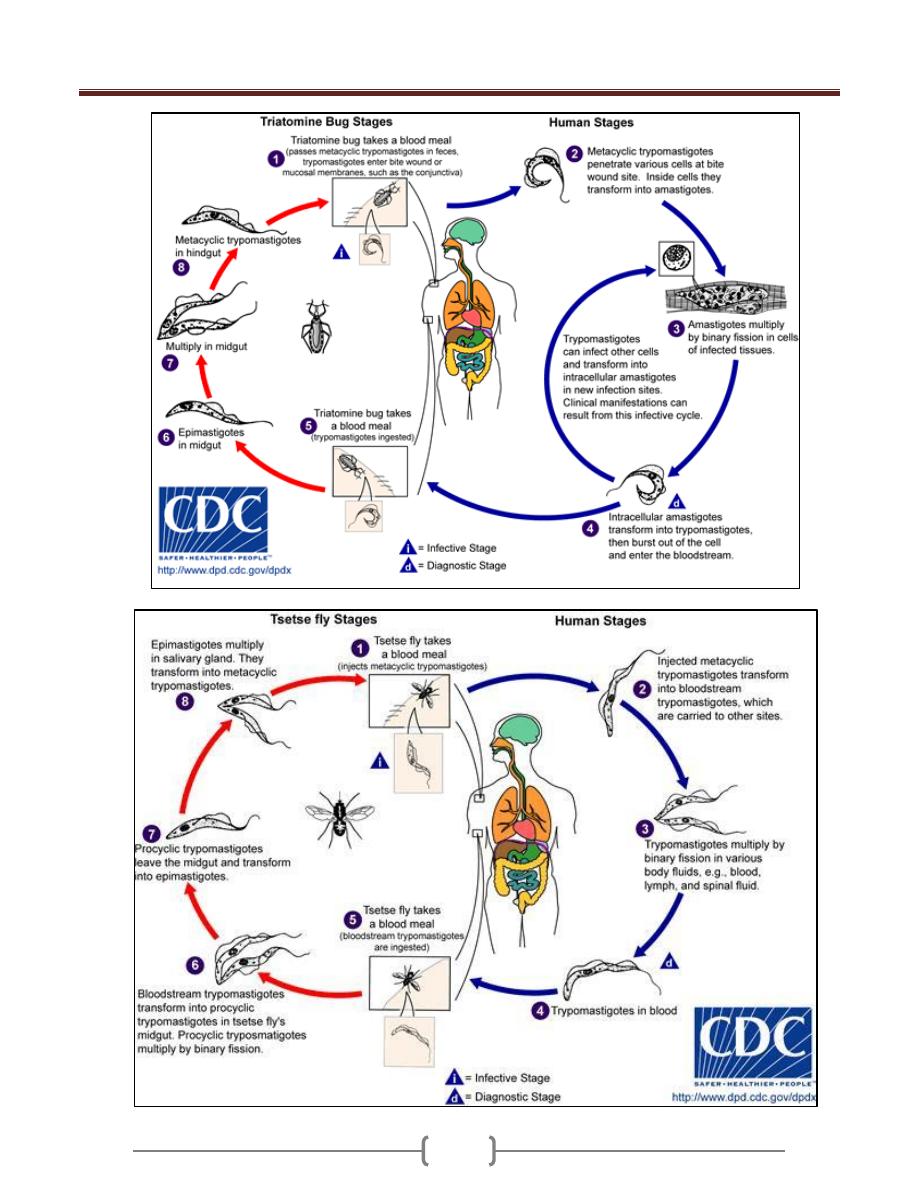
Unit 2: Protozoa
28
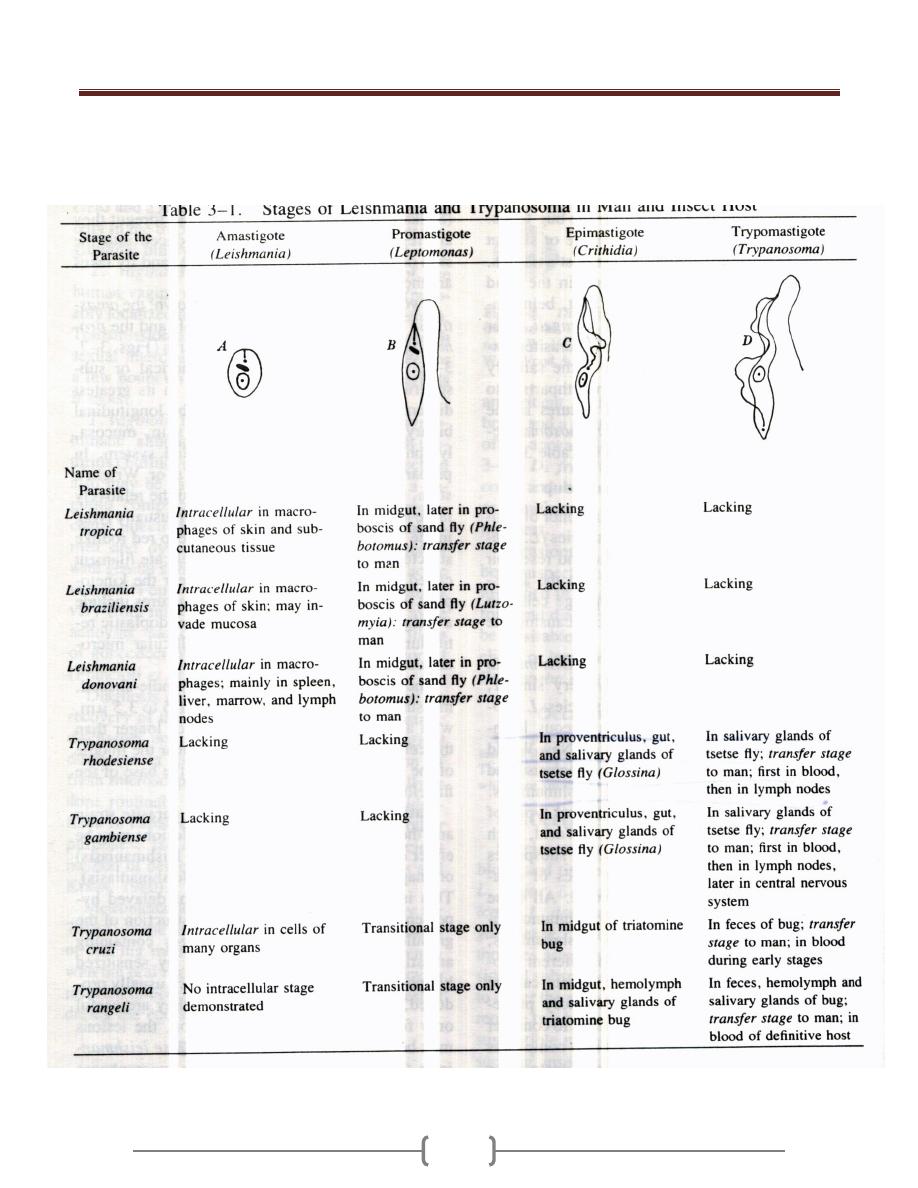
Unit 2: Protozoa
29
Unit 2: Protozoa
30
Lecture 12+13+14 - Suborder:
Haemosporina (Genus Plasmodium)
Subkingdom: Protozoa.
Phylum: Apicomplexa.
Class: Coccidia.
Order: Eucoccidia.
Suborder: Haemosporina.
Genus: Plasmodium.
There are 4 species of the genus plasmodium that infect
human, these are:
1) Plasmodium vivax (benign tertian malaria)
2) Plasmodium ovale (ovale tertian malaria)
3) Plasmodium falciparum (malignant tertian malaria)
4) Plasmodium malariae (quartan malaria).
The disease caused by the genus plasmodium is called
Malaria.
Genus plasmodium require 2 hosts for the life cycle, the
intermediate host which is the vertebrate host where
asexual phase develop and the definitive host which is the
female of Anopheline mosquitoes where sexual phase
develop.
Life cycle (Figure 1)
1) In mosquitoes:
Once the ripe gametocytes are ingested by a female
anopheles in a blood meal and reach the midgut, they
transform into mature gametes. One macrogametocyte
develops a single macrogamete (oocyte or unfertilized
ova), and one microgametocyte produces several
flagellated microgametes. A microgamete then enters a
macrogamete, resulting in a zygote that becomes a motile
ookinete, migrates through the stomach wall, & becomes
an oocyst just under the outer membrane of the stomach.
The oocyst grows rapidly and develops internal nuclear
centers. Each center then produces a large number of
delicate, spindle – shaped sporozoites. By the time the
sporozoite become mature, the wall of the greatly enlarged
oocyst bursts, releasing the sporozoites into the hemocele of
the mosquito. The sporozoites then migrate to the salivary
glands, which they enter, and from there they pass down
through the salivary ducts into the median tube of the
mosquito's proboscis. When the mosquito next takes a
blood meal, sporozoites are injected into the cutaneous
blood vessels of the victim and initiate a new infection.
Time & temperature for complete sexual phases in mosquito:
Plasmodium falciparum 10 days, 30 C
o
Plasmodium vivax 11 days, 25 C
o
Plasmodium ovale 14 days.
Plasmodium malariae 18-21 days, 22 C
o
2)
In humans
:
Three phases
a) Exoerythrocytic or preerythrocytic schizogony.
Bite of the female of anopheline mosquito's
sporozoite to human blood circulation and remain
for 30 minutes liver parenchymal cells in which
Exoerythrocytic cycle occur and result in the production
of tiny merozoites in each schizont, rupture of the liver
cells merozoites released to the circulation.
Time for asexual cycle in the liver (Prepatent period):
Plasmodium falciparum 6days
Plasmodium vivax 8 days
Plasmodium ovale 9 days
Plasmodium malariae 13 days
In case of Plasmodium vivax and Plasmodium ovale, there
is a varying proportion of sporozoites enter a resting stage
before undergoing asexual multiplication, this stage is
known as hypnozoite. The hypnozoites may undergo
reactivation after weeks or months which bring about the
relapse which is the characteristic of these 2 species but
not for Plasmodium falciparum and Plasmodium
malariae.
b) Erythrocytic schizogony
Merozoites that have developed in preerythrocytic cycle
enter the RBC; they transform into trophozoites, which
grow and develop into schizonts, each producing a no. of
merozoite characteristic of the spp. of plasmodium. When
fully matured, the merozoites break out of the parasitized
RBC and soon actively enter other RBC to repeat the
asexual cycle. The time required to complete an asexual
cycle in the RBC depends on the species of the
plasmodium.
Plasmodium falciparum it is every 36 - 48 hours.
Plasmodium vivax it is every 48 hours.
Plasmodium ovale it is every 48 hours
Plasmodium malariae it is every 72 hours
c) Early gametogony.
In individual bitten by infected mosquitoes, gametocytes
begin to appear in circulating erythrocytes after a few to
several asexual multiplications, those of P.falciparum
usually appearing relatively late. These cells do not
multiply or develop further unless taken up by a suitable
mosquito host.
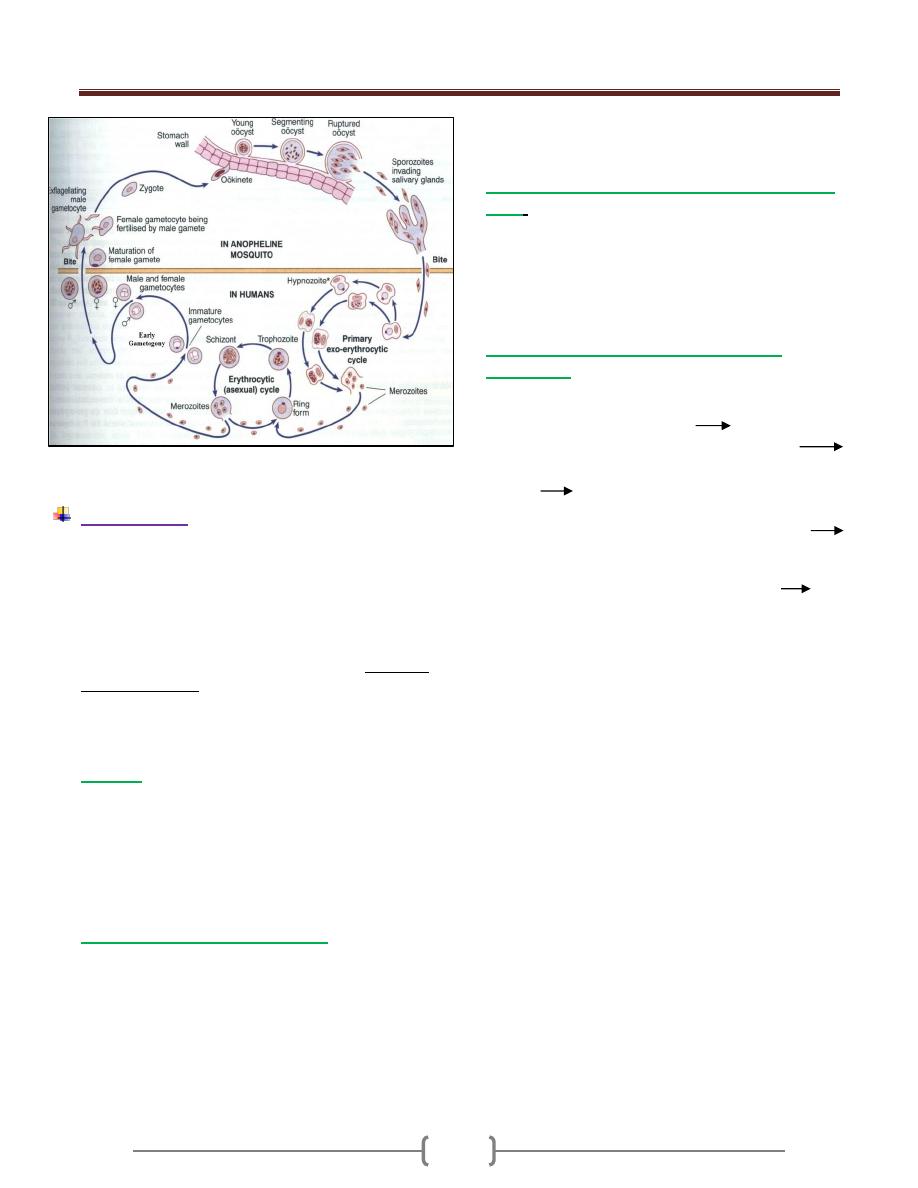
Unit 2: Protozoa
31
Figure 1 Life cycle of plasmodium
Pathogenesis
The pathogencity of malaria is related to RBC infection.
Tissue destruction in exoerythrocytic cycle appears not to
produce sign and symptoms. The plasmodia in RBC grow
and segment at the expense of the host cells and the
malarial parasite progressively consumes and degrades
intracellular proteins, the hemoglobin. The potentially
toxic heme is polymerized to biologically inert hemozines
or malarial pigment.
Plasmodium falciparum infects RBC at all ages.
Plasmodium vivax and ovale infects only reticulocytes.
Plasmodium malariae infects only mature RBC.
1) Anemia:
as the no. of parasites increase with each successive
schizogony, the no. of RBC decreases due to rupture of
parasitized and non-parasitized RBC.The mechanism by
which non parasitized RBC ruptured is through the
production of autoAb to the RBC during infection or
through the binding of soluble material Ag or the
circulating Ag-Ab complex to the cell surface.
2) Chills and fever of malarial attack:
When the debris of the ruptured cells, merozoites and
their metabolite by product is set free into the blood
stream, it stimulates chemoreceptor of the temperature
regulating mechanism of host to conserve heat. The
amount of pyrogen released initially is not enough to
produce a marked reaction, but they cause prodromal
symptoms. As the no. of the invaded RBC increases and
the asexual cycle of the parasites become more
synchronized, the quantities of the pyrogen become
sufficient to produce the characteristic chills and fever of
a malarial attack.
3)
Infection with P.falciparum differs from the other
types
:
a) It invades erythrocytes of all ages and thus causing
very extensive parasetemia.
b) The schizogonic cycle in the bloodstream requires not
more than 48 hrs but is frequently less synochronized.
c) There is a tendency for more than one parasite to
develop in a single RBC.
4)
cytoadherance phenomena in Plasmodium
falciparum :
it is the result of expression of knobs
(ligands) on the surface of the parasitized RBC which
adhere to specific receptors on the endothelial cell, the
RBC tend to adhere to each other rosetting and
agglutination, and to the lining of the blood vessel
capillary blockage in the vital organ e.g. brain, lungs,
kidneys. Tissue anoxia.
5)
Agglutination of RBC in Plasmodium falciparum + loss
of plasma from the blood vessel in all spp. of malaria
phenomena called
sludging
of the RBC in the vessel.
6)
The decrease in the no. of RBC + decrease in the quality
of the circulating RBC with decrease in oxygen
multiple thromboses
in the smaller blood vessel and
decrease in the circulating blood volume.
7)
Spleen is enlarged
, congested, soft and hemorrhagic in
acute stage and hard in chronic stage.
Splenomegaly is due to congested of sinusoids with
RBC+ hyperplasia of the lymphocyte and macrophage.
8)
Liver
is hypertrophic and congested.
9)
Kidneys
are congested, glomerular capillaries become
thrombotic with accumulation of parasitized RBC, free
hematin, macrophage.
Plasmodium malariae have been associated with
nephrotic syndrome (quartan nephrosis) in children with
peak incidence at the age of 5 years.
10)
Pulmonary capillaries
are congested.
11)
Brain
is edematous and grayish in color.
12)
All mucous membranes
show patechial hemorrhage
13)
Heart
shows fatty degeneration.

Unit 2: Protozoa
32
Clinical features
Prepatent period
It is defined as the time between sporozoite inoculation
and the appearance of parasites in the blood and
represents the duration of the liver stage and the number
of merozoites produced.
Incubation periods
It tends to be a little longer than prepatent period and
is defined as the time between sporozoite inoculation and
the onset of symptoms.
Incubation periods
= exoerythrocytic cycle (usually 2)
+1 or 2 erythrocytic cycle.
Plasmodium falciparum = 12 days.
Plasmodium vivax and P.ovale = 13-17 days.
Plasmodium malariae = 28-30 days
I. Nonspecific symptoms (prodromal stage)
Malaria begins with nonspecific initial symptoms that last
several days, including for instance headache, pain in limbs,
general fatigue, chills, and occasionally nausea as well as
intermittent fever, either continuous or at irregular intervals,
this is because the cycles are asynchronous. Several days to
a week after onset of parasitemia, the schizogonic cycle
synchronizes: in infections with P. vivax, P. ovale, and P.
falciparum, a cycle is completed within 48 hours, in
infections with P. malariae within 72 hours.
II. Malarial paroxysms or febrile attack.
The Classic malarial paroxysm started suddenly with:
a) Shaking chills (cold stage)
Lasting 15min-1 hr, begins as the dividing generations of
parasites rupture their RBC and escape into the blood. The
patient complains of extreme cold, although the temperature
is elevated at the onset and rises during the period of chill.
The skin is pale and cyanotic and the patient huddled under
a pile of blankets.
Nausea and vomiting also occur at this stage.
b) Febrile stage (hot stage)
Lasting several hrs, characterized by a spiking fever that
reaches 40 C or more. The skin becomes flushed, the patient
is agitated, restless, disoriented or even delirious. Severe
frontal headache and pains in the limbs and back. It lasts 2-6
hours in vivax and ovale malaria, 6 hoursor more in
quarten, and longer in falciparum.
During this stage, the parasite invades their RBC.
c) Sweating phase.
The fever subsides and the patient falls asleep and later
awakes feeling well.
Note:
1) In P.falciparum infection the initial chill is usually less
pronounced and the fever more prolonged.
2) In mixed infections with 2 or more species, or in the
early stages of infection with one species there may be
daily (quotidian) paroxysms or even double paroxysms in
one day. Occasionally, two 48- hour parasite broods may
be asynchronous by 24 hour , producing regular , daily
fever in tertian species.
Following an essentially symptomless remission which
varies with the species, there is a 2
nd
paroxysms followed by
several additional one over a period of up to 3 weeks or
more before the symptoms terminate. This series of
paroxysms constitutes the primary attacks.
The malarial paroxysms will become less severe and
irregular in periodicity as the host develops immunity.
Relapse:
Following the termination of the primary attack, either
naturally or following treatment, parasites are depressed
or may be completely disappear from the blood. In
P.vivax and p.ovale, one to several more attacks occur due
to hypnozoite in the liver.Relapses of P.vivax malaria
usually continue over a period of 2-3 years before the
infection is terminated, those of p.ovale malaria occur
infrequently and rarely persist longer than 1 year.
Recrudescence:
In P.falciparum and quartan malaria a renewal of clinical
manifestations after weeks, months, or years without re-
exposure is attributed to the persistence of parasites in the
blood at levels too low to be detected or to produce
symptoms. Such parasitemias may persist for up to one
year in P.falciparum infections and for many years in
quartan infection.
Note:
Only the sporozoites (introduced by the mosquitoes
themselves) can penetrate the liver cells. Thus, if malaria
is acquired by blood transfusion or transplacentally, no
infection of the liver occurs and relapses do not occur.
Primary vivax attacks if untreated, last 3 weeks – 2
months or longer. Relapse extending over a period of 5-8
years.
In ovale malaria, early spontaneous recovery after no
more than 5-10 paroxysms. Relapse no longer than a year
after the initial attack.
In quartan malaria, 3weeks- 24 weeks.
Unit 2: Protozoa
33
Un treated primary attack of plasmodium falciparum
tends to run its course quickly and seldom exceeds 2-3
weeks duration but coma or death occurs within this
period.
Complications of malaria
P.vivax, ovale is benign.
P.falciparum cause severe infection (severe malaria).
Complications of severe malaria include hypoglycemia,
severe anemia, renal failure and metabolic acidosis
Severe malaria should be considered in any non-immune
patient with a parasite count greater than 2%.
1) Cerebral malaria.
Severe complication of Plasmodium falciparum.it starts
suddenly as severe headache, drowsiness, confusion and
coma.
Pathogenicity: cytoadherance phenomena + decrease in
deformability of P.falciparum infected RBC, once the
parasite has matured beyond the ring stage.
Sequele: cortical blindness, hemiparesis, cerebellar ataxia
and severe headache.
Cerebral malaria is assumed when asexual parasites are
present in the blood film and the patient has impaired
consciousness and other encephalopathies have been
excluded, particularly bacterial meningitis and locally
occurring viral encephalitis.
2)
Anemia
which is more severe in Plasmodium
falciparum. What are the causes of anemia?
3)
Renal disease
occurs in severe Plasmodium falciparum
infection and in chronic Plasmodium malariae infection.
In Plasmodium falciparum there is acute tubular necrosis
and tissue anoxia because of RBC sludging.
Plasmodium malariae associated with nephrotic
syndrome due to acute glomerulonephritis due to
deposition of immune complex in the glomeruli. Seen in
children < 5 years associated with odema, proteinuria.
4) Black water fever (Hemoglobinuric fever)
It is seen in severe Plasmodium falciparum infection with
irregular treatment with quinine.
Pathogenesis: Massive intravascular hemolysis
haemoglobinuria.
It occurs in persons who have lived in areas where
P.falciparum infections abounds. The hemolysis is caused
by erythrocytic AutoAb derived from previous infections
and reacting with autoantigens initiated by fresh RBC
infection with the same strain.
Clinically: chills, rigor, high fever, jaundice, vomiting,
anemia and passage of dark red or black urine.
5)
Dysenteric malaria
: uncommon complication of
Plasmodium falciparum characterized by abdominal pain,
nausea and vomiting, upper GIT bleeding.
6) Algid malaria (shock).
Also seen in severe Plasmodium falciparum infection
characterized by hypotension, decrease temperature and
impairment of vascular perfusion. This is due to gram
negative septicemia, GIT bleeding, splenic rupture or
incorrect dehydration.
7) Tropical splenomegaly syndrome.
Seen in some hyperendemic area in which exaggerated
immune response to malaria is seen. There is high level of
IgM. IgM aggregated with other Ig or complement and
precipitate in cold. IgM aggregate phagocytosed by RE
cell in the spleen and liver leading to enlargement of the
spleen, reticulocytosis, thrombocytopenia.
Patients with Tropical splenomegaly syndrome present
with an abdominal mass or a dragging sensation in the
abdomen and occasional sharp abdominal pains suggesting
perisplenitis. Anemia and some degree of pancytopenia are
usually evident, but in many cases malarial parasites cannot
be found in peripheral- blood smears.
8)
Hypoglycemia
a) Hyperinsulinaemia due to treatment with quinine or quinidine
b) Impairment of hepatic gluconeogenesis
9)
Pulmonary edema:
Due to fluid overload in oliguric or anuric patient or due
to disseminated intravascular coagulation.
Malaria in hyperendemic area:
In population living in highly malarious area, they are
subjected to periodic re-exposure throughout the life and
the typical overt manifestations are observed in young
only. Deaths occur commonly in children. Older child and
adults who have survived the earlier attacks have
developed tolerance to the disease.
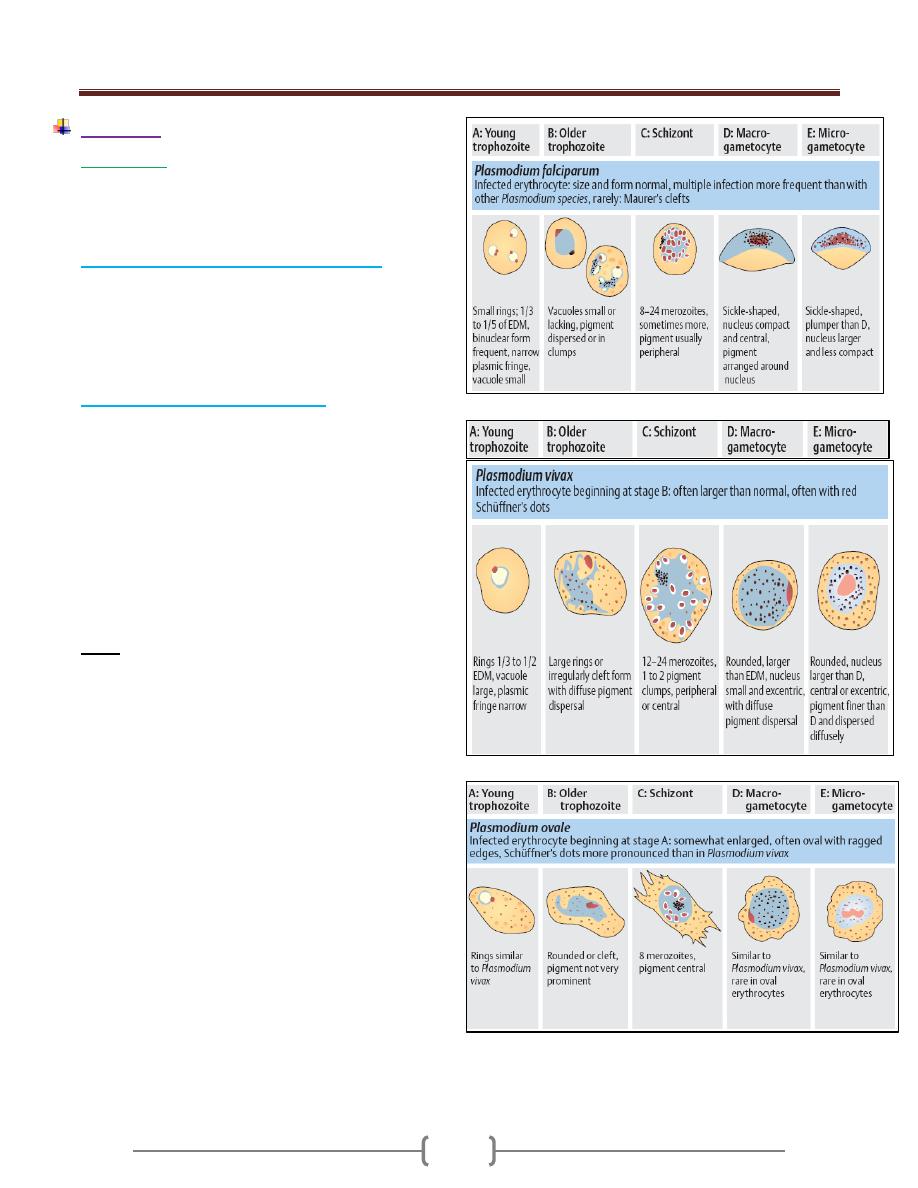
Unit 2: Protozoa
34
Diagnosis
1) Microscopic
Thick and thin blood films.
The careful examination of a well-prepared and well-
stained blood film currently remains the "gold standard"
for malaria diagnosis.
A. Thick blood film for screening of the organism.
Thick films allow the examiner to screen a larger volume
of blood and are about eleven times more sensitive than
the thin film, so picking up low levels of infection is
easier on the thick film, but the appearance of the parasite
is much more distorted and therefore distinguishing
between the different species can be much more difficult.
B. Thin blood film for spp. identification.
The timing of blood examination is important; blood film
taken just before or at the height of the malarial paroxysm
will contain a detectable number of parasites.
If the parasites cannot be detected in the 1
st
sample, so it
is advisable to do thick and thin blood films every 6-12
hrs. as long as 48 hrs.
The aim of the diagnosis is the presumptive
differentiation of Plasmodium falciparum from other
spp. and the diagnosis of plasmodium falciparum is based
on the detection of ring forms with or without
gametocytes and > 5% of the RBC are parasitized.
Note:
1) Parasites are not likely to be circulating in the blood
during suppressive therapy, immediately following
curative treatment, or soon after self-medication with
antimalarial drugs.
2) When two species of malaria are present, there appeaers
to be antagonism between them, Plasmodium falciparum
is predominantes over P.vivax which in turn
predominates over p.ovale and p.malariae . Thus in a
mixed infection with Plasmodium falciparum and
P.vivax, the latter would be initially suppressed and not
diagnosed. This could produce relapse later.
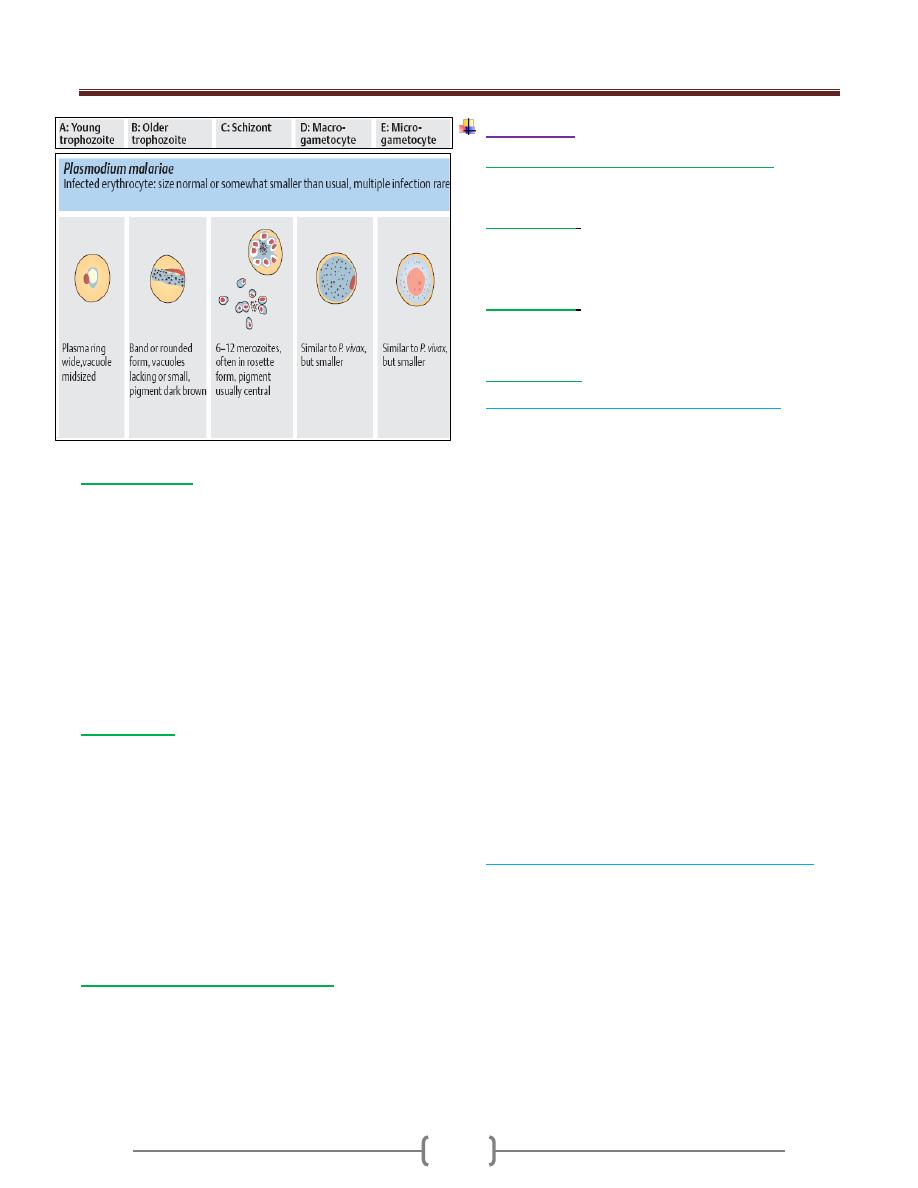
Unit 2: Protozoa
35
2) Serological test:
Serological tests provide confirmation of past malaria in
patients and are valuable for epidemiological studies.
These tests are also useful for screening donated blood
and diagnosing hyperactive malarial splenomegaly.
Among the tests used are the:
a) Indirect fluorescent antibody test (IFAT),
b) Indirect hemagglutination antibody (IHA) test,
c) Enzyme-linked immunosorbent assay (ELISA).
All these tests produce positive results several days after
malaria parasites appear in the blood and so do not help in
the diagnosis of the acute infection for treatment purposes.
3) Dipstick tests
Dipstick tests based on the detection of P. falciparum
histidine-rich protein-2 (PfHRP-2) antigen are specific for
P. falciparum infections and do not detect the other 3
species. Dipstick tests based on the detection of parasite
lactate dehydrogenase are now available; these tests can
detect both P falciparum and P vivax.
These tests have high sensitivity and specificity, require
no special equipment or training, and produce results
rapidly. However, they remain positive for a week or
more after the treatment and cure, and, in this situation,
can yield false-positive results.
4) Molecular biological detection tests:
DNA and RNA probes and polymerase chain reaction
(PCR) have good sensitivity and specificity but require
sophisticated expensive equipment.
Treatment
Suppressive therapy (chemoprophylaxis)
Kills the parasites as it enter the blood stream with small
doses of drugs effective against erythrocytic stage.
Clinical cure
:
Larger doses of the same type of the drug used in
suppressive therapy or different ones to eliminate the
large no. of erythrocytic parasites.
Radical cure
:
Elimination of not only the blood stream infection but the
tissue stage in the liver.
Drug therapy
1) Chemotherapy of mild P. falciparum malaria .
P. falciparum is now resistant to chloroquine almost
world-wide, so quinine is the drug of choice.
Quinine dihydrochloride or sulphate 600 mg salt 8-hourly
by mouth is given until the patient is clinically better and
the blood is free of parasites (usually 3-5 days).
This regimen should be followed by a single dose of
sulfadoxine 1.5 g combined with pyrimethamine 75 mg,
i.e. 3 tablets of Fansidar®.
If sulphonamide sensitivity is suspected, quinine may be
followed by doxycycline 100 mg daily for 7 days.
Alternatives to quinine plus Fansidar are atovaquone 250
mg plus proguanil 100 mg (Malarone), or artemether
orally for 5 days then mefloquine.
Mefloquine may occasionally cause alarming
neuropsychiatric side-effects which can persist for several
days due to its plasma half-life of 14 days.
artemisinin combination therapy. This combines an
astemisinin drug (artemether or artesunate) with another
drug. Currently co-artemether (artemether-lumefantrine)
and artesunate+amodiaquine are the most widely used
In pregnancy a 7-day course of quinine alone should be
given.
2) Management of complicated P. falciparum malaria.
Severe malaria is a medical emergency and cerebral
malaria is the most common presentation and cause of
death in adults with malaria.
The management of severe malaria should include:
A) Early and appropriate antimalarial chemotherapy,
B) Active treatment of complications,
C) Correction of fluid,
D) Electrolyte and acid-base balance.
E) Quinine is indicated if a chloroquine-resistant infection
is at all likely.
Unit 2: Protozoa
36
3) P. vivax, P. ovale and P. malariae infections should be
treated with chloroquine: 600 mg chloroquine base
followed by 300 mg base in 6 hours, then 150 mg base
12-hourly for 2 more days.
4) In case of p.vivax and p.ovale, primaquine (15 mg daily
for 14 days), which destroys the hypnozoite phase in the
liver should follow the chloroquione.
Side effect: hemolytic anemia so one should do the G6PD
enzyme assay.
Prevention and control
1) Chemoprophylaxis
Choice of regimen is determined by
a) Area to be visited.
b) Length of stay.
c) Level of malaria transmission.
d) Level of drug resistance.
e) Presence of underlying disease in the traveler and
concomitant medication taken.
I. Chloroquine remains the drug of choice for the
prevention of infection with drug-sensitive P. falciparum
and with the other human malarial species (although
chloroquine-resistant P. vivax has been reported from
parts of eastern Asia, Oceania, and Central and South
America). Unfortunately, there are now few areas of the
world with Chloroquine-sensitive P. falciparum.
Chloroquine is considered safe in pregnancy. Chronic
administration for >5 years, a characteristic dose-related
retinopathy may develop, but this condition is rare at the
doses used for antimalarial prophylaxis.
Primaquine (0.5 mg of base/kg or 30 mg, daily adult
dose) has proved safe and effective in the prevention of
drug-resistant falciparum and vivax malaria in adults.
II. In areas where Chloroquine resistant P.falciparum
(CRPF) is endemic, drugs effective against resistant P.
falciparum should be used [mefloquine, atovaquone-
proguanil (Malarone), doxycycline or primaquine].
Atovaquone-proguanil (Malarone; 3.75/1.5 mg per kg or
250/100 mg, daily adult dose) is a fixed-combination
once-daily prophylactic agent that is very well tolerated
by adults and children, with fewer adverse gastrointestinal
effects than chloroquine-proguanil and fewer adverse
central nervous system effects than mefloquine.
Daily administration of doxycycline (100 mg daily, adult
dose) is an effective alternative to mefloquine.
The regime of chemoprophylaxis
1-2 weeks before traveling to the endemic area +during
the stay in the area + 4 weeks after leaving the area
Primquine can be used for 2 weeks after the above regime
to kill the hypnozoite.
2) Mosquitoes nets, windows screen, protective clothes and
insects repellents.
3) Drainage of stagnant water in swamps and ditches
decreasing the breeding areas.
Epidemiology
Plasmodium falciparum and Plasmodium ovale are
diseases of the tropics
Plasmodium malariae seen in subtropics and temperate zone
Plasmodium vivax in all endemic area.
The transmission of all species depends on the presence of
suitable spp. of anopheline mosquitoes and infected
gametocytes bearing humans.
Transmission can occur also by
1) Blood transfusion.
2) organ transplantation and in
3) Drug addicts.
Carriers are Peoples in whom gametocytes are
commonly circulating in peripheral blood over a
considerable period of time.
Autochthonous malaria: malaria which is acquired
locally by mosquito bite.
Imported malaria: malaria which is contracted outside
the area and brought in.
Introduced malaria: malaria which is acquired from an
imported case.
Induced malaria: malaria which is contracted by
parenteral inoculation (e.g., by blood transfusion). It is
also called transfusion malaria .The incubation period is
short because there is no preerythrocytic phase.
Individuals with sickle cell trait (heterozygote) are
protected against malaria because their RBC has little
ATPase activity and cannot produce sufficient energy to
support the growth of the parasites. Homozygous sickle
cell anemia is also protected.
The receptor for Plasmodium vivax is the Duffy blood
group antigen. People who are homozygous recessive for
the genes that encode this protein are resistant to infection
by Plasmodium vivax. More than 90% of black West
Africans and many of their American descendants do not
produce the Duffy antigen, so they resist Plasmodium
vivax infection.
People with G6PD deficiency are also protected against
the severe effects of Plasmodium falciparum.
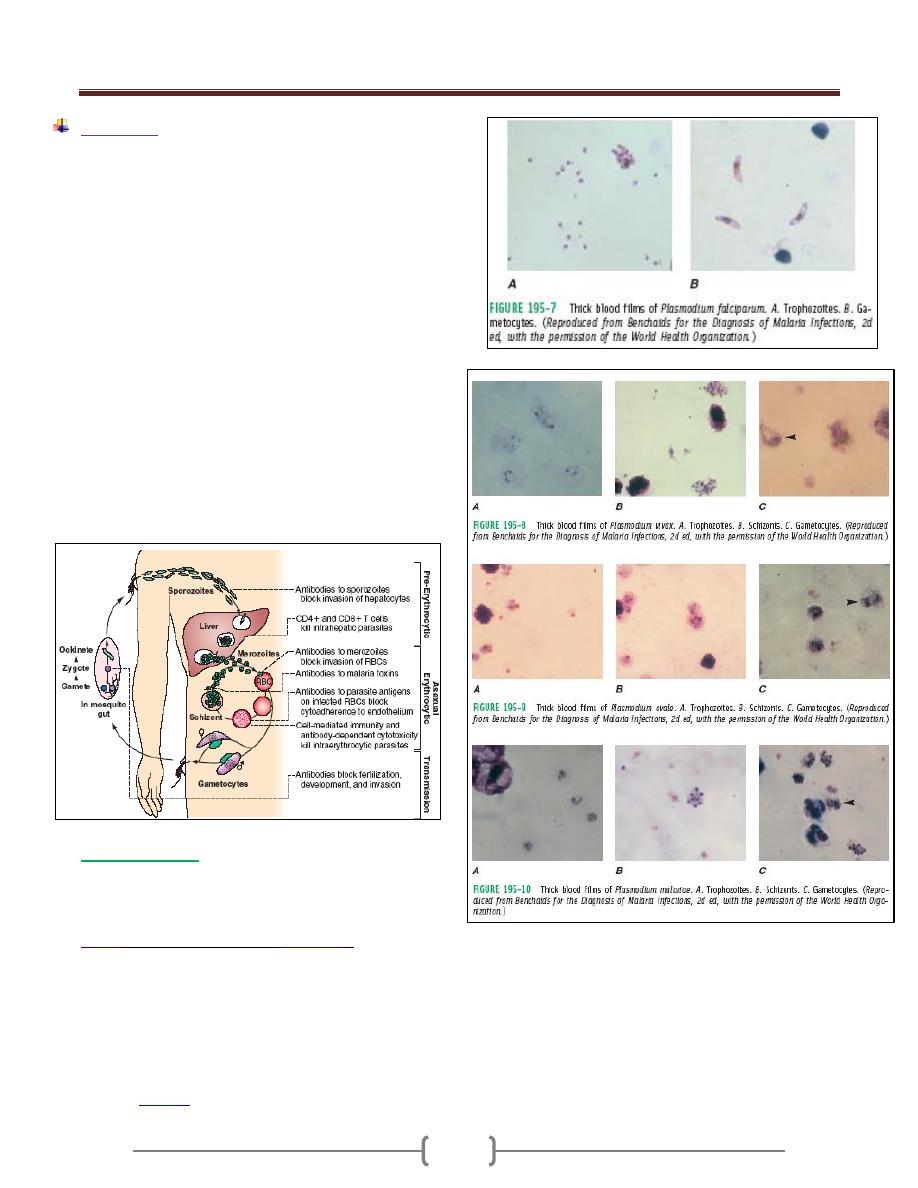
Unit 2: Protozoa
37
Immunity
The immunity against malaria is slow to develop and
requires multiple exposures. In highly endemic areas only
young children are at a high risk of developing severe
falciparum malaria whereas older children and adults are
essentially protected from severe disease and death.
However, this immunity is not a sterilizing immunity in
that persons can still become infected. In addition, the
immunity is short lived and in the absence of repeated
exposure the level of immunity decreases. For example,
previously semi-immune adults will often develop severe
malaria upon returning to an endemic area after being in a
non-endemic area for 1-2 years. This state of partial
immunity in which parasitemia is lowered, but not
eliminated, and parasitemia is better tolerated is
sometimes referred to as premunition. Premunition
refers to an immunity that is contingent upon the pathogen
being present. In the endemic area, a low level of
parasitemia and low grade symptoms results; this is called
the premmunition or concomitant immunity.
Malarial vaccine
• Malaria vaccines are an area of intensive research.
However, there is no effective vaccine that has been
introduced into clinical practice.
•
/AS01 (commercial name:
), which
started Pivotal Phase III evaluation in May 2009 and is
designed not for travelers but for children resident in
malaria-endemic areas who suffer the burden of disease
and death related to malaria.
• The RTS,S vaccine was engineered using genes from the
outer protein of Plasmodium falciparum malaria parasite
and a portion of a hepatitis B virus plus a
chemical
to boost the immune system response.
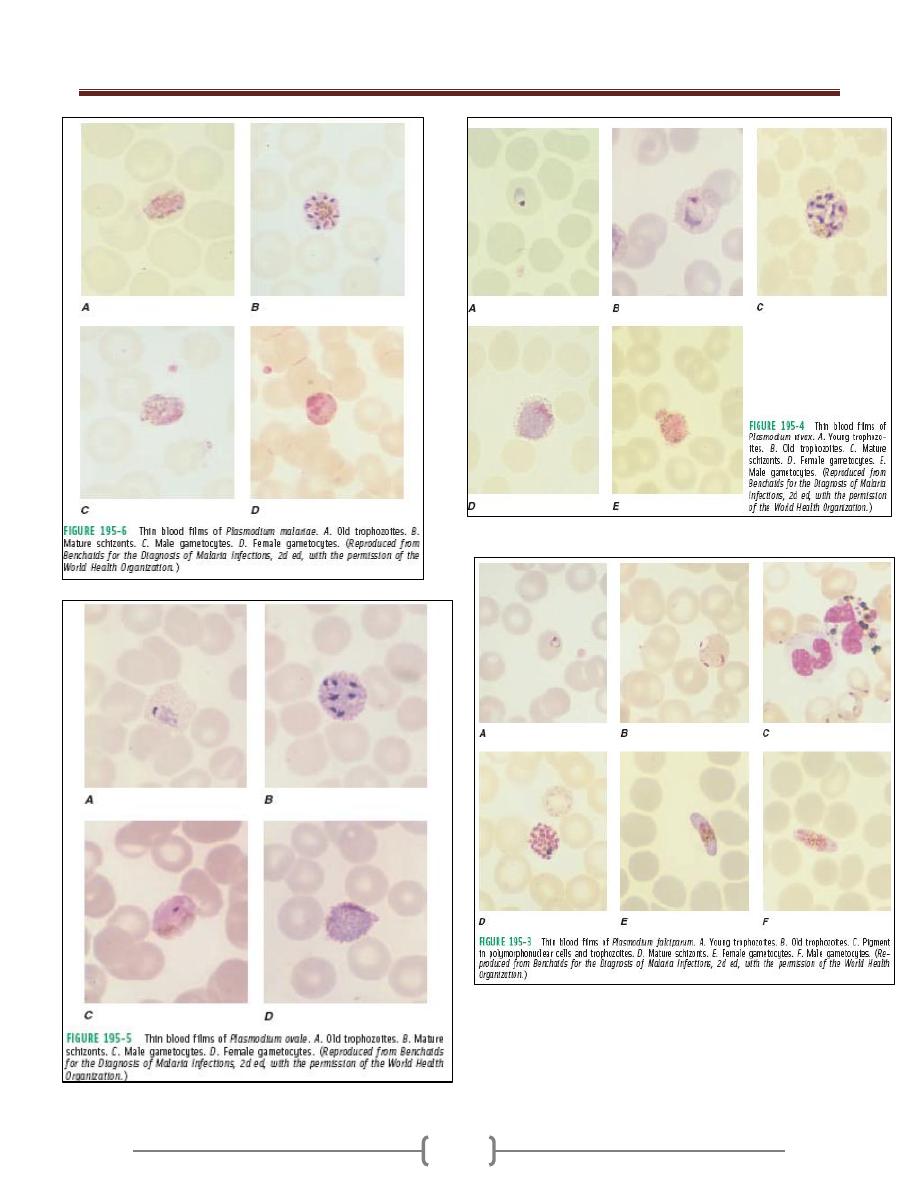
Unit 2: Protozoa
38
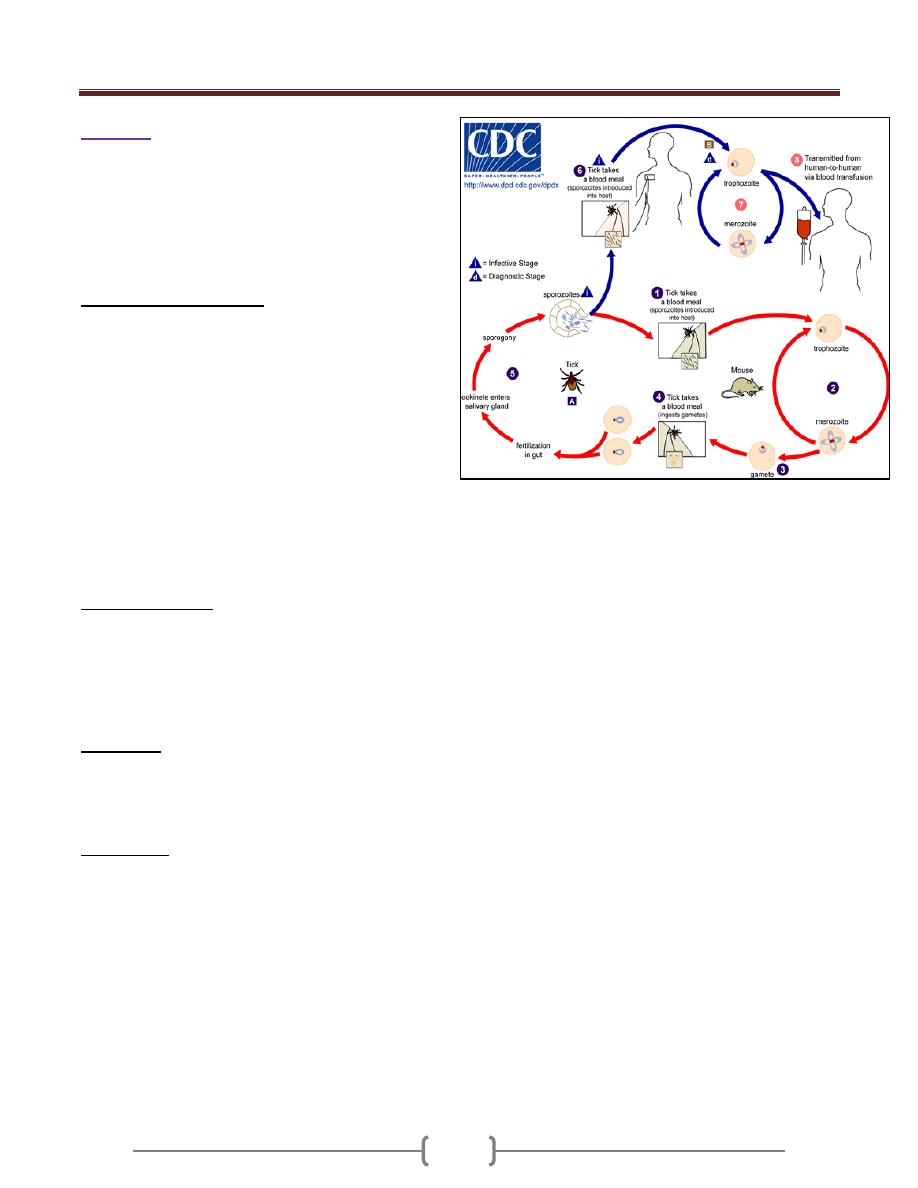
Unit 2: Protozoa
39
Babesia
Babesia species produce fulminating malaria-like disease
known as babesiosis, piroplasmosis, or red water fever.
The disease is important in cattle, dogs and rodents, cats
and horses.
The parasite requires a tick as a vector.
Life cycle :( Figure 1-B)
Ticks become infected by ingesting infected erythrocytes
but don’t themselves transmit the infection latter during
feeding. The organisms penetrate the developing ova of
the tick, and infecting the embryo which hatch from the
eggs, then the parasite penetrate the salivary glands of the
embryo where they continue reproduction and are
available for transmission to the mammalian host when
the tick feeds.
In man, Babesia occurs in the trophozoite stage- which may
be pear -shaped, spherical, ovoid, spindle-shaped, or
amoeboid – and only in erythrocytes. It undergoes asexual
reproduction, forming pairs, or tetrad groups within the cell,
organisms rupture the cell and infect other erythrocyte.
Clinical features:
The disease presented with acute onset of shaking chills,
headache, fever (40C
o
) and pain in the abdomen, muscles,
and back. Rapid onset of anemia and jaundice may occur.
The disease is more severe in splenctomized patient, but it
also occurs in normal patient with normal spleen.
Diagnosis:
Thin blood film to demonstrate a pale area in the RBC
that represents a vacuole. Babesia infection is more likely
to be confused with P. falciparum.
Treatment:
Quinine and clindamycin.
Figure 1-B - Life cycle of Babesia

Unit 2: Protozoa
40
Lecture 15+16 - Suborder Eimeriina
Genus Toxoplasm
Subkingdom Protozoa
Phylum Apicomplexa
Class Sporozoea
Subclass Coccidia
Order Eucoccidia
Suborder Eimeriina
Genus Toxoplasma
Cryptosporidium
Isospora
Sarcosystis
Toxoplasma gondii
is a coccidian parasite of
cosmopolitan distribution, able to develop in a wide
variety of vertebrate hosts, but the definitive host is the
house cat and certain felidae. The prevalence is highest in
hot, humid climates and lowest in dry, cold climates.
It was originally distributed in a North African rodent
called the gundii from which it derives its special name.
The definitive host is the house or domestic cat and other
felidae.
Intermediate host: man (considered as a dead end host)
and other mammals (cattle, sheep, mouse and pig).
Morphology
There are five forms in T. gondii life cycle:
1- Trophozoite (tachyzoite),
2- Tissue cyst (bradyzoite),
3- Schizont
4- Gametocyte
5- Oocyst .
Tachyzoites, tissue cysts and oocysts
are important stages seen during the life cycle of the
parasite, and all these stages are infectious to man.
Trophozoite (tachyzoite): Fig. 1-8
It is oval to crescent-shaped with a pointed anterior end
and a rounded posterior end. It is 4 -7 µ m long and 2 -4 µ
m wide. An ovoid nucleus is present in the posterior end
of the parasite. Tachyzoite is the multiplying form seen
during the acute stage of the infection. It can invade any
type of cell in a host and once inside a cell, it multiplies
within a vacuole by a process known as endodyogeny, or
by binary fission or schizogony. Tachyzoites divide until
they fill the host cell, which then liberates them, and they
reinvade (or ingested by) other macrophages, repeating
the process. The cell which contains them, when becomes
merely a bag full of tachyzoites, called(Pseudocyst).
Fig 1-8 tachyzoite
Tissue cyst:
It is spherical and may vary in size from 5 to 100 µ m in
diameter. This is the chronic stage of the infection. The
tissue cysts can be found in any organ of the body but are
commonly found in the brain ,skeletal and heart muscels.
An eosinophilic cyst wall surrounds each cyst. The cyst
contain hundreds of bradyzoite ( cystozoites). Bradyziotes
multiply slowly.
Oocyst:
This stage is only present in cats and other felines but not
in humans. It is oval and measures 10-12 µm diameters.
Each cyst is surrounded by a thick resistant wall which
encloses a spheroplast.The oocyst librated from the
intestinal epithelial cell while still immature; it completes
its development while passing down the gut and after
expulsion in the feces. Its contents are divided first into
two cells; these then secrete cyst walls to form 2
sporocyst. The content of each sporocyst then divide once
more to produce two infective sporozoites.
Life cycle (Figure 2-8 ):
In cat (definitive host):
Ingestion of the organism (either sporozoite from oocyst
or bradyzoite from tissue cyst) invades the mucosal cells
of the cat’s small intestine, and then they form schizont
(asexual) and gametocytes (sexual). After sexual fusion of
gametes, zygotes are formed and a thin wall protect the
zygote and an oocyst develops, then exit into the gut
lumen, then passes out via feces as an oocyst with single
sporoblast. Within each oocyst, 2 sporocysts form (zygote
divide into 2 sporoblast) in about 3-4 days, four
sporozoite forms within each sporocyst (i.e. 8 sporozoite)
(infective oocyst).
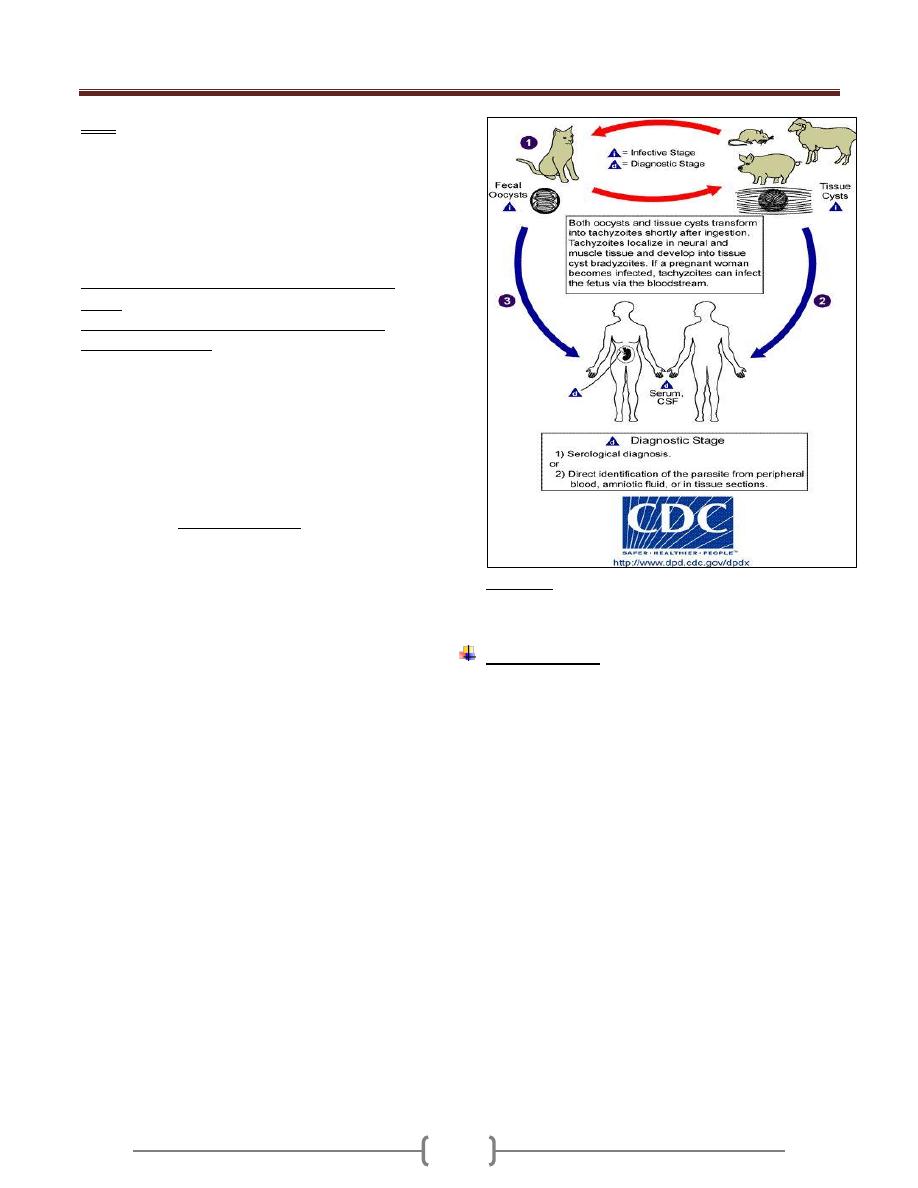
Unit 2: Protozoa
41
Note:
If the cat ingest infective oocyst and initiate intestinal
infection, the animal will pass oocyst within 21-24 days.
If the cat fed an acutely ill mouse with proliferative forms
in its tissue, oocyst will appear in the cat's feces within 9-
11 days, and if the cat fed on chronically ill mouse the
oocyst will appear in cat feces within 3-5 days.
In human and other mammals (intermediate
hosts)
Human infection may be acquired in several ways:
Mode of transmission
a) Ingestion of undercooked infected meat
b) Ingestion of the oocyst from fecally contaminated
hands (oocyst during the cleaning of cat litter box) or
food, milk and water;
c) Organ transplantation or blood transfusion;
d) Transplacental transmission;
e) Accidental inoculation of tachyzoites.
Swallowing the oocyst or tissue cyst initiate the
development of ASEXUAL CYCLE. This process occurs
mainly in macrophages.
The sporozoites from the ingested oocyst and bradyzoites
from the tissue cyst invade the mucosal epithelial cell of
the small intestine in which they multiply as tachyzoites
by endodyogeny. The tachyzoites divide until they fill the
host cell which then liberates them, and the tachyzoite
reinvade (or ingested by) other macrophages, repeating
the process and form pseudocyst.
The multiplying tachyzoites also spread to distant extra -
intestinal organ (e.g. brain, eye, liver, spleen, heart,
skeletal muscle and placenta of pregnant mother) by
invading lymphatics and blood.
The multiplication of tachyzoites constitutes the acute
phase of infection.
If the host lives, and the infection is untreated, the host’s
immune system becomes effective and tachyzoites are
destroyed, but the the parasite usually responds to this
by entering other cells (muscle cells, neurons, and
perhaps others) and secreting a thin but tough cyst
wall around itself form a tissue cyst and initiate the
chronic phase of the disease. A tissue cyst contains
hundreds of bradyzoites.
If another intermediate host eats uncooked meat
containing this tissue cyst , bradyzoites emerge in the
duodenum and repeat the cycle.
Figure 2-8 Life cycle of Toxoplasma gondii
Pathogenesis:
Upon the host’s ingestion of either tissue cysts containing
bradyzoites or oocysts containing sporozoites, the
parasites are released from the cysts by a digestive
process. Bradyzoites are resistant to the effect of pepsin
and invade the host’s gastrointestinal tract.
Within enterocytes (or other gut-associated cells), the
parasites undergo morphologic transformation, giving rise
to invasive tachyzoites. These tachyzoites induce a
parasite-specific secretory IgA response.
From the gastrointestinal tract, parasites are disseminated
to a variety of organs, particularly lymphatic tissue,
skeletal muscle, myocardium, retina, placenta, and the
CNS. At these sites, the parasite infects host cells,
replicates, and invades the adjoining cells. In this fashion,
the hallmarks of the infection develop: cell death and
focal necrosis surrounded by an acute inflammatory
response. In the normal immune host, both the humoral
and the cellular immune responses control infection. As
tachyzoites are cleared from the acutely infected host,
tissue cysts containing bradyzoites begin to appear,
usually within the CNS and the muscles initiating chronic
phase of the infection.
Unit 2: Protozoa
42
In the immunocompromised or fetal host, the immune
factors necessary to control the spread of tachyzoite
infection are lacking. This allows the persistence of
tachyzoites and gives rise to the progressive focal
destruction that result in organ failure (i.e., necrotizing
encephalitis, pneumonia, and myocarditis).
Persistence of infection with cysts containing bradyzoites
is common in the immunocompetent host. This lifelong
infection usually remains subclinical. Although
bradyzoites are in a slow metabolic phase, cysts do
degenerate and rupture within the CNS. This degenerative
process, with the development of new bradyzoite-
containing cysts, is the most probable source of
recrudescent infection in immunocompromised
individuals and the most likely stimulus for the
persistence of antibody titers in the immunocompetent
host.
In the brain, minute scattered necrotic areas may later
calcify.
Note:
Tachyzoites directly destroy cells, particularly
parenchymal and reticuloendothelial cells, whereas
bradyzoites released from ruptured tissue cysts cause local
inflammation with blockage of blood vessels and
necrosis.
Clinical features:
Toxoplasmosis is either acquired or congenital infections.
1) Acquired infection:
It is seen in adults
In immune competent, it is either:
a) Asymptomatic which is the majority of the cases or
b) It may persist as infectious mononucleosis like
symptom with negative heterophil antibody test.
In immunocompromised patient (as in AIDS patient):
a) Myocarditis, meningoencephalitis and atypical
pneumonia.
b) CNS involvement which is fatal.
c) Retinochoroiditis.
In immunocompromised patient, it is the tachyzoite form
rather than the bradyzoite form and cysts that commonly
are seen.
2) Congenital toxoplasmosis:
Congenital infection of the fetus occurs only when the
mother is infected during pregnancy.
If she is infected before pregnancy, the organism will be
in the cyst form and there will be no trophozoite to pass
through the placenta.
The mother who is reinfected during pregnancy but who
has immunity from previous infection will not transmit
the infection to her child.
One third of mother infected primarily during pregnancy,
give birth of infected infants, and only 10 % of these
infants are symptomatic.
Congenital infection leads to stillbirth, retinochoroiditis,
intracranial calcification, psychomotor disturbances and
hydrocephaly or microcephaly.
If the infection occurred in the first trimester, the
incidence of transplacental infection is lowest (_15%), but
the disease in the neonate is most severe. Stillbirth or
major C.N.S anomalies, encephalitis, retinochoroiditis,
hepatosplenomegaly, fever, jaundice, and intracranial
calcification.
Second- third trimester infection, the incidence of
transplacental infection is greatest (65%), but the infant is
usually asymptomatic at birth and the clinical
manifestation of these infections may be delayed long
after birth , even beyond childhood and manifested by
neurological problem or learning difficulties. They may
develop retinochoroiditis later.
Laboratory diagnosis
A. Pathogenic
1) Smears from Lymph node, bone marrow, spleen. For
organism detection.
2) Body fluids (in acute infection) or from tissue (chronic
infection) inoculated intraperitonealy into young
laboratory mice for demonstrations of the organisms.
B. Serological
Serology remains the primary approach for the diagnosis
of toxoplasmosis.
1) Indirect fluorescent antibody (IFA) test and ELISA
test including IgM and IgG kits.
In Immunocompetent adults.
Toxoplasmosis is normally diagnosed serologically by
detection of parasite-specific IgG and IgM antibodies.
IgM antibodies can be detected as early as one week after
the primary infection, peak within two to four weeks, then
drop to below the detection limit within a few weeks; in
some cases persistence at low titers lasts longer. IgG
antibodies appear somewhat later, peak after two to four
months and persist for many years. A high or rising IgG

Unit 2: Protozoa
43
titer with contemporal detection of IgM indicates an acute
primary infection.
Ocular toxoplasmosis normally cannot be diagnosed by
serological methods.
Serological findings are often not reliable indicators in
immunodeficient patients due to reduced antibody
production. The cerebral form of the infection seen
frequently in reactivated toxoplasmosis is therefore
usually diagnosed by means of clinical imaging method.
Prenatal toxoplasmosis in neonates is difficult, but highly
important. Since IgG antibodies are transmitted from
mother to child diaplacentally, detection of them in the
child cannot serve as a definitive diagnostic indicator.
IgM is only present in about 50% of prenatally infected
children. In suspected cases, the blood or cerebrospinal
fluid should be examined using the PCR.
Toxoplasmosis and pregnancy
Acute Toxoplasma gondii infection in early pregnancy
carries
the risk of transmitting the infection to the fetus
with serious
sequelae. Serological testing for IgG/IgM
anti-Toxoplasma
antibodies may fail to differentiate
between a recent and past
infection. IgM may remain
positive for up to 1 year. IgG Avidity test had been
developed based on the fact that following immune
response, the IgG antibodies produced initially bind
weakly to the antigen (low avidity). As the immune
response develops there is maturation of IgG antibody
response and the avidity increases progressively over
weeks or months (high avidity). The presence of high
avidity IgG excludes the possibility that infection
occurred within the previous five months.
2) sabin-feldman dye test
depends upon the appearance in 2-3 weeks of antibody
that will render the membrane of laboratory cultured
living Toxoplasma gondii impermeable to alkaline
methylene blue , so that the organism are unstained in
presence of positive specimen.
C. Molecular diagnosis by PCR
Treatment:
1) in adult with acute infection:
a) No symptoms, no treatment.
b) In case of severs symptom, active retinochoroiditis, and
immunocompromised patient, treatment should be given.
The regime include: sulphodiazine + pyrimethamine for 4
weeks.
Alternative drugs: spiramycin, clindamycin, trimethprim-
sulphamethoxazole
2)
In pregnancy
: Spiramycin (Rovamycin) ® continued
till delivery.
Prevention:
1) Meat cooked to 50-60 Cº for 4 – 6 minutes or freezing at
– 20 Cº for 48 hours.
2) Pregnant mother should avoid all contacts with cats.
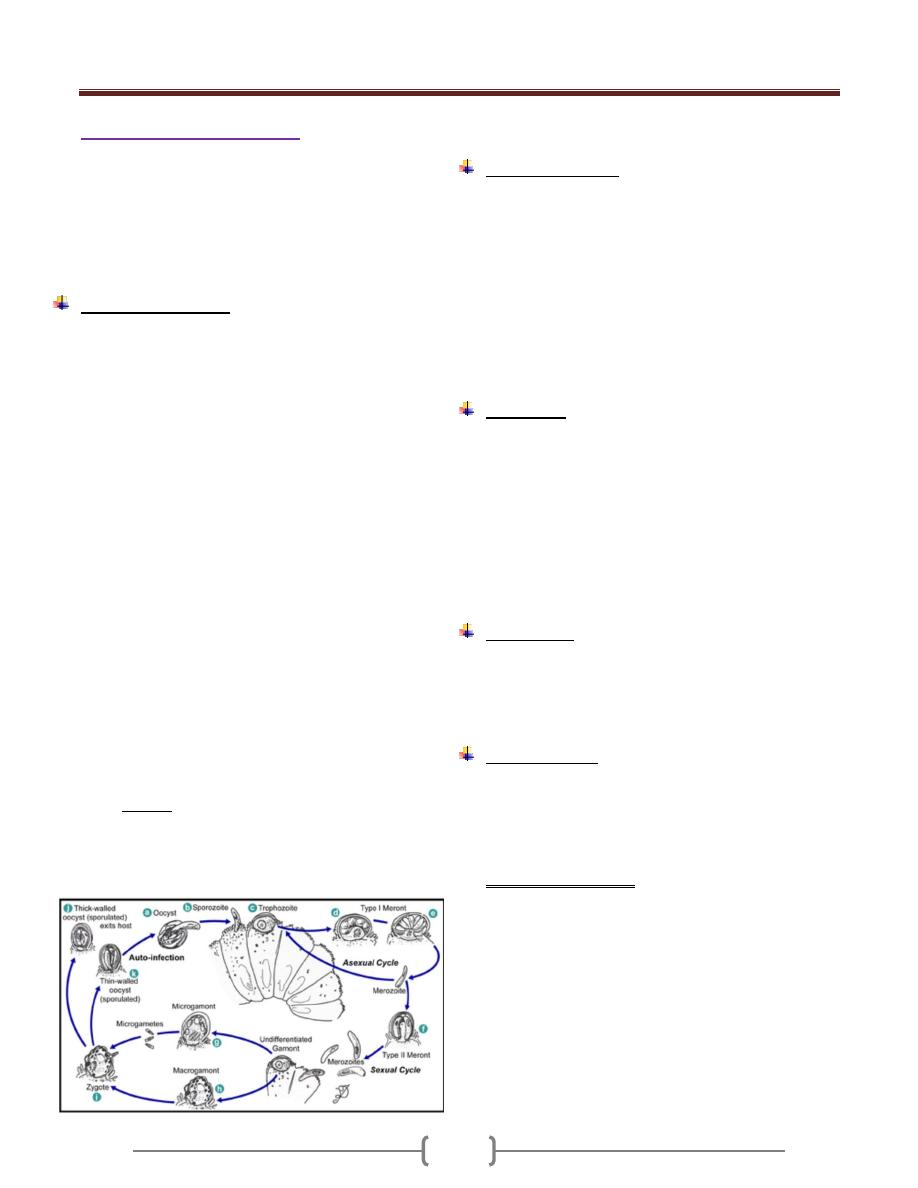
Unit 2: Protozoa
44
Genus Cryptosporidium
It is a minute coccidian parasite with worldwide
distribution. Twenty species of the parasite have been
described from a variety of vertebrates including birds,
fish, mammals and reptiles.
The species that infects humans and most mammals is
Cryptosporidium parvum.
Life cycle (figure 1)
Cryptosporidium species use a single host in their life cycle.
Development of cryptosporidia occurs within the brush
border of the epithelial cells of the intestine. The parasite can
cause damage to the microvilli where it attaches.
The life cycle of Cryptosporidium parvum consists of an
asexual stage and a sexual stage. After being ingested, the
oocysts excyst in the small intestine. They release
sporozoites that attach to the microvilli of the epithelial
cells of the small intestine. From there they become
trophozoites that reproduce asexually by multiple fission,
a process known as schizogony. The trophozoites develop
into Type 1 meronts that contain 8 daughter cells. These
daughter cells are Type 1 merozoites, which get released
by the meronts. Some of these merozoites can cause
autoinfection by attaching to epithelial cells. Others of
these merozoites become Type II meronts, which contain
4 Type II merozoites.These merozoites get released and
they attach to the epithelial cells. From there they become
either macrogamonts or microgamonts. These are the
female and male sexual forms, respectively. This stage,
when sexual forms arise, is called gametogony. Zygotes
are formed by microgametes from the microgamont
penetrating the macrogamonts. The zygotes develop into
oocysts of two types. 20% of oocysts have thin walls and
so can reinfect the host by rupturing and releasing
sporozoites that start the process over again. The thick-
walled oocysts are excreted into the environment. The
oocysts are mature and infective upon being excreted.
They can survive in the environment for months.
Figure 1. Life cycle of C.parvum
Clinical features:
Incubation periods = 7-10 days.
Cryptosporidium can be transmitted from human to
human and from animals to human.
In immunocompetent patient, it is a self-limited diarrhea
lasts about 2 weeks accompanied by abdominal
discomfort, anorexia, fever, nausea, and weight loss.
In immunodeficient patient, severe diarrhea which may be
life threatening. In such cases, cryptosporidia can affects the
biliary tree leading to acalculous cholecystitis or sclerosing
cholangitis, pancreatitis & respiratory tract infection.
Diagnosis:
1) Detection of oocysts in fresh stool samples.
2) Stool concentration techniques using a modified acid fast
stain.
3) Identifying the organism (meronts containing merozoit
and gamonts containing micro and macrogametes) in
intestinal biopsy.
4) Monoclonal antibody for detection of low level of
infection.
5) ELISA for detection of fecal antigens.
Treatment:
1) Immunocompetent patient, no treatment.
2) Immunocompromised patient e.g. AIDS patient,
spiramycin, paromomycin (Humentin), Nitazoxanide
(Cryptaz), or combination therapy with azithromycin.
Epidemiology:
Person – person transmission occurs in child day –care
centers and among house hold contacts and medical
providers.
Oocyst is highly resistant to chlorination but removal can
be achieved by filtration of water.
For patient with AIDS:
1) Boiling of water for 1 minute.
2) Water filtration.
3) Pasteurization is sufficient to destroy infectivity in milk.
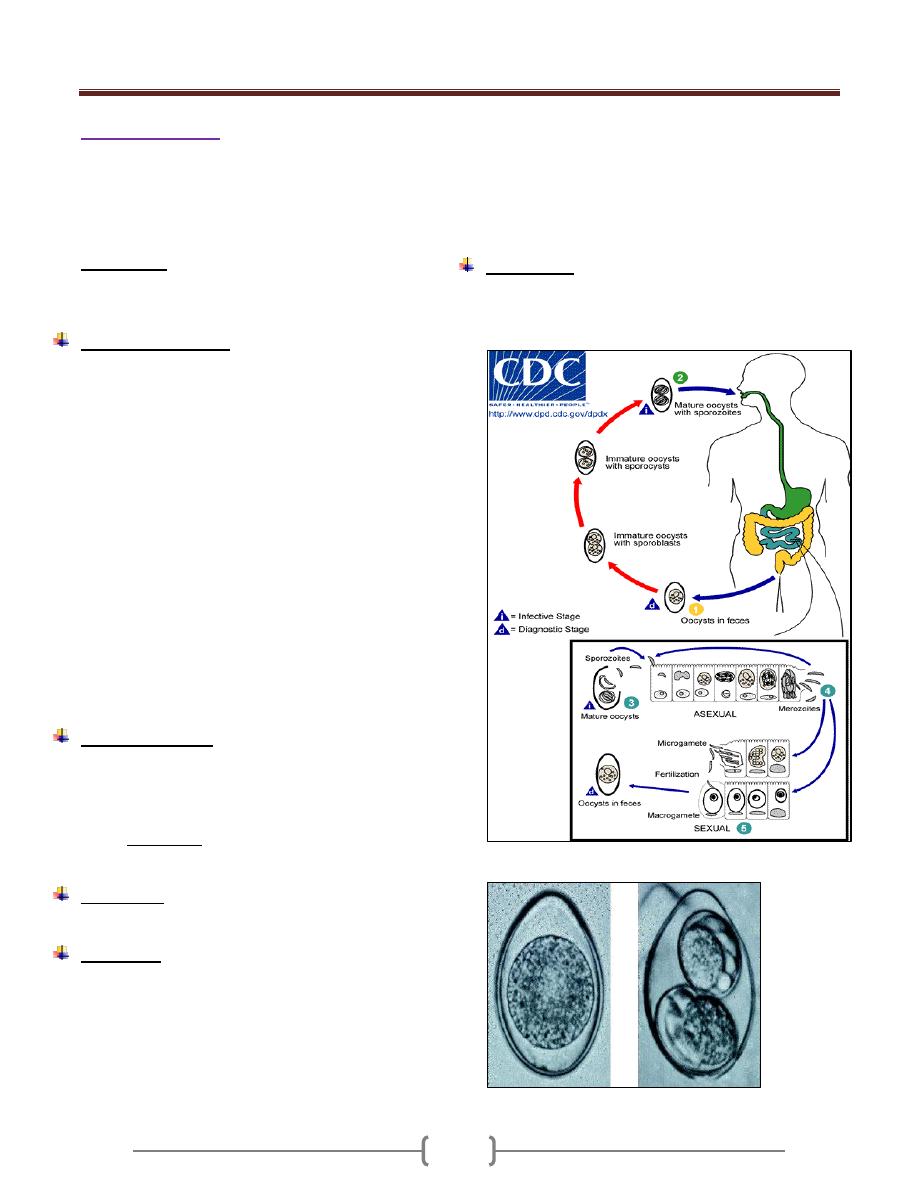
Unit 2: Protozoa
45
Genus Isospora
It is a coccidian parasite of the epithelial cells of the
intestine in which it may undergo repeated asexual
development (with consequent destruction of the surface
layer of considerable portion of the intestine) and sexual
stage which culminate in oocyst passed in the feces.
Isospora belli
It is a sporozoan of the human intestine leading to
coccidiosis (Isosporiosis) in human.
Life cycle (figure 2)
Infection occurs by ingestion of sporocyst-containing
oocysts: the sporocysts excyst in the small intestine and
release their sporozoites, which invade the epithelial cells
and initiate schizogony. Upon rupture of the schizonts, the
merozoites are released, invade new epithelial cells, and
continue the cycle of asexual multiplication. Trophozoites
develop into schizonts which contain multiple merozoites.
After a minimum of one week, the sexual stage begins
with the development of male and female gametocytes.
Fertilization results in the development of oocysts that are
excreted in the stool. At time of excretion, the immature
oocyst contains usually one sporoblast (more rarely two).
In further maturation after excretion, the sporoblast
divides in two, so the oocyst now contains two
sporoblasts. The sporoblasts secrete a cyst wall, thus
becoming sporocysts; and the sporocysts divide twice to
produce four sporozoites.
Isospora belli infects both humans and animals.
Clinical features:
Infection causes acute, non-bloody diarrhea with crampy
abdominal pain, which can last for weeks and result in
malabsorption and weight loss. In immunodepressed
patients, and in infants and children, the diarrhea can be
severe. Eosinophilia may be present (differently from
other protozoan infections).
Pathology:
Jejunal biopsy reveals villous atrophy.
Diagnosis:
1) Stool examination by modified acid fast staining for
oocyst (either immature or mature oocyst containing 2
sporocyst) (figure 3).
Immature oocyst is an ellipsoid or spindle shaped with
blunt ends. In the immature oocyst, is a spherical mass of
protoplasm which soon divide it form 2 sporoblasts which
in turn develop heavy cyst walls and are known as
sporocysts; within each sporocyst, 4 curved sausage
shaped sporozoites develop (mature oocyst).
The immature oocyst requires 4-5 days for maturation.
2) Duodenal biopsy if repeated stool examination is
negative.
3) Eosinophilia which is not found in other protozoal infection.
Treatment:
Trimethoprim – sulfamethoxasole for 3 weeks.
If the patient is hypersensitive to Trimethoprim –
sulfamethoxasole, pyrimethamine or ciprofloxacin can be used
Figure 2. Life cycle of Isospora belli
Figure 3. a immature oocyst, b mature oocyst .
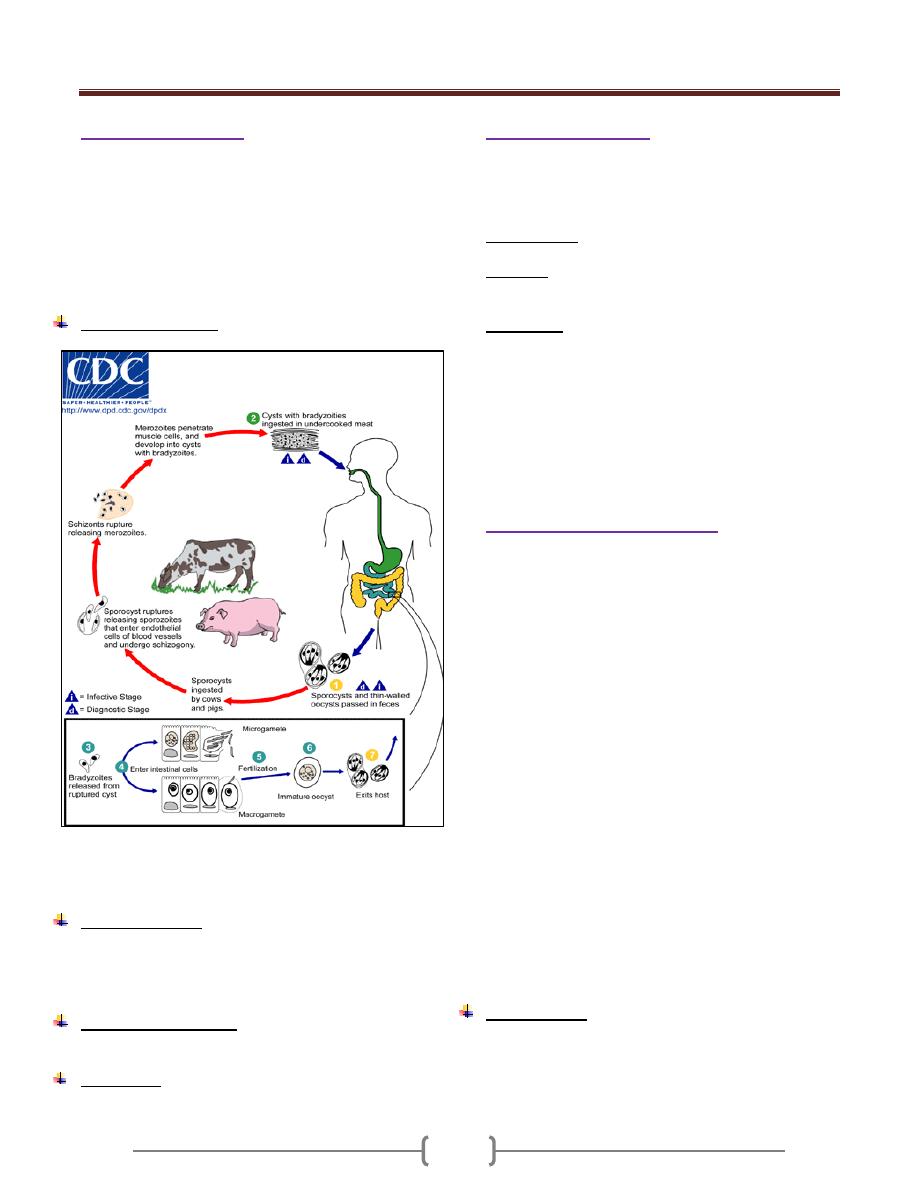
Unit 2: Protozoa
46
Genus Sarcocystis
Sarcocystis species are coccidian with a biphasic life
cycle: An intestinal (sexual) stage in gut mucosal cells of
carnivores, and an encysted tissue (asexual) stage in
muscle or other cells of herbivores animals. Humans serve
as both intermediate and final host depending on the
species. Human sarcocystosis develops from ingestion of
undercooked beef and pork meat.
Life cycle (figure 4)
Figure 4. Life cycle of sarcocystis (Human can serve as
intermediate host (with tissue sarcocystis) and as
definitive host (oocyst formed in the intestinal mucosa))
Clinical findings:
The infection causes no symptoms or signs in humans, but
sever symptoms may be developed in immunocompressed
person.heart failure may be attributed to sarcocystis.
Laboratory diagnosis:
Complement fixation test.
Treatment
: No effective treatment.
Genus Cyclospora
Cyclospra cayetanensis is an intestinal protozoan that
causes watery diarrhea in immunocompetent and
immunocompromised individuals
Transmission: through feco-oral route.
Diagnosis: By demonstration of spherical oocysts by
modified acid-fast stain of a stool sample.
Treatment: Trimetheprim- sulfamethoxazol
) (غير مطلوبMicrosporidia
Microsporidia are obligate intracellular spore forming
protozoa that infect many animals and cause disease in
humans, especially as opportunistic pathogens in AIDS. It
reproduces through formation of minute spores that have
polar tubules or filaments. Tubules are used to inject the
infective material (sporoplasm) into the host cells.
Microsporidia are members of a distinct phylum,
Microspora, which contains dozens of genera and
hundreds of species. The various microsporidia are
differentiated by their developmental life cycles, by
ultrastructural features, and by molecular taxonomy based
on ribosomal RNA. The complex life cycles of the
organisms result in the production of infectious spores.
Currently, eight genera of microsporidia—
Encephalitozoon,
Pleistophora, Nosema, Vittaforma, Trachipleistophora,
Brachiola, and Enterocytozoon and
Microsporidium. ) (لالطالع
Microsporidiosis is most common among patients with
AIDS, less common among patients with other types of
immunocompromise, and rare among immunocompetent
hosts.
Transmission:
1) Ingestion of spores in food or water.
2) Transplacental transmission
3) Ocular
4) Sexual.
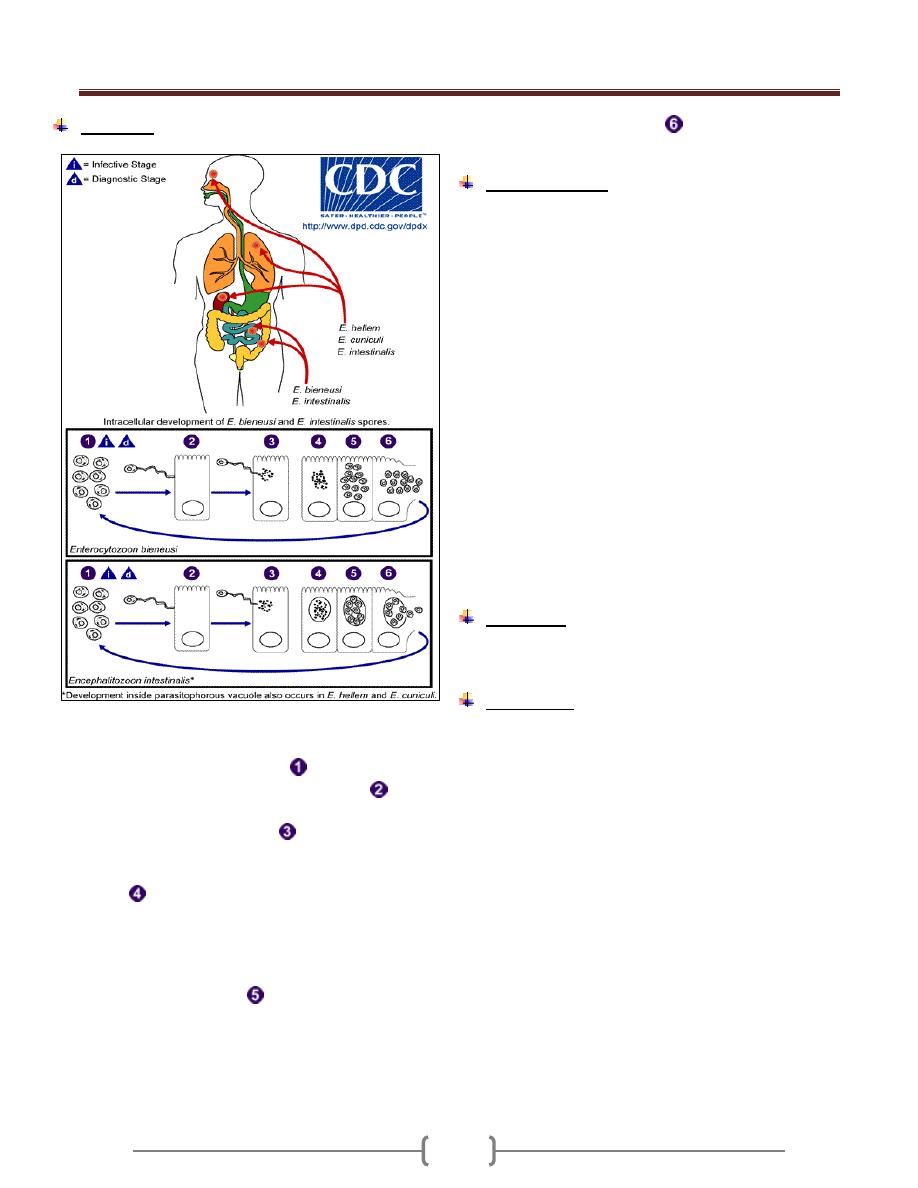
Unit 2: Protozoa
47
Life cycle:
Figure 4. Life cycle of Microsporidia: The infective form
of microsporidia is the resistant spore and it can survive
for a long time in the environment
. The spore
extrudes its polar tubule and infects the host cell
. The
spore injects the infective sporoplasm into the eukaryotic
host cell through the polar tubule
. Inside the cell, the
sporoplasm undergoes extensive multiplication either by
merogony (binary fission) or schizogony (multiple
. This development can occur either in direct
contact with the host cell cytoplasm (e.g., E. bieneusi) or
inside a vacuole termed parasitophorous vacuole (e.g., E.
intestinalis). Either free in the cytoplasm or inside a
parasitophorous vacuole, microsporidia develop by
sporogony to mature spores
. During sporogony, a
thick wall is formed around the spore, which provides
resistance to adverse environmental conditions. When the
spores increase in number and completely fill the host cell
cytoplasm, the cell membrane is disrupted and releases
the spores to the surroundings
. These free mature
spores can infect new cells thus continuing the cycle.
Clinical features:
Depends on the site of infection, which includes
intestinal, ocular, muscular and systemic.
In patients with AIDS, intestinal infections with
Enterocytozoon bieneusi and Encephalitozoon (formerly
Septata) intestinalis are increasingly recognized to
contribute to chronic diarrhea and wasting; these
infections are found in 10 to 40% of patients with chronic
diarrhea.
Both organisms have been found in the biliary tracts of
patients with cholecystitis. E. intestinalis may also
disseminate to cause fever, diarrhea, sinusitis, cholangitis,
and bronchiolitis.
In patients with AIDS, Encephalitozoon hellem has
caused superficial keratoconjunctivitis as well as sinusitis,
respiratory tract disease, and disseminated infection.
Myositis due to Pleistophora has been documented.
Nosema, Vittaforma, and Microsporidium have caused
stromal keratitis associated with trauma in
immunocompetent patients.
Diagnosis:
Visualization of spores in stool samples or intestinal
biopsy samples.
Treatment:
Albendazole in treating intestinal and disseminated
infection.
Topical fumagillin for treatment of ocular infection
caused by Encephalitozoon hellem.
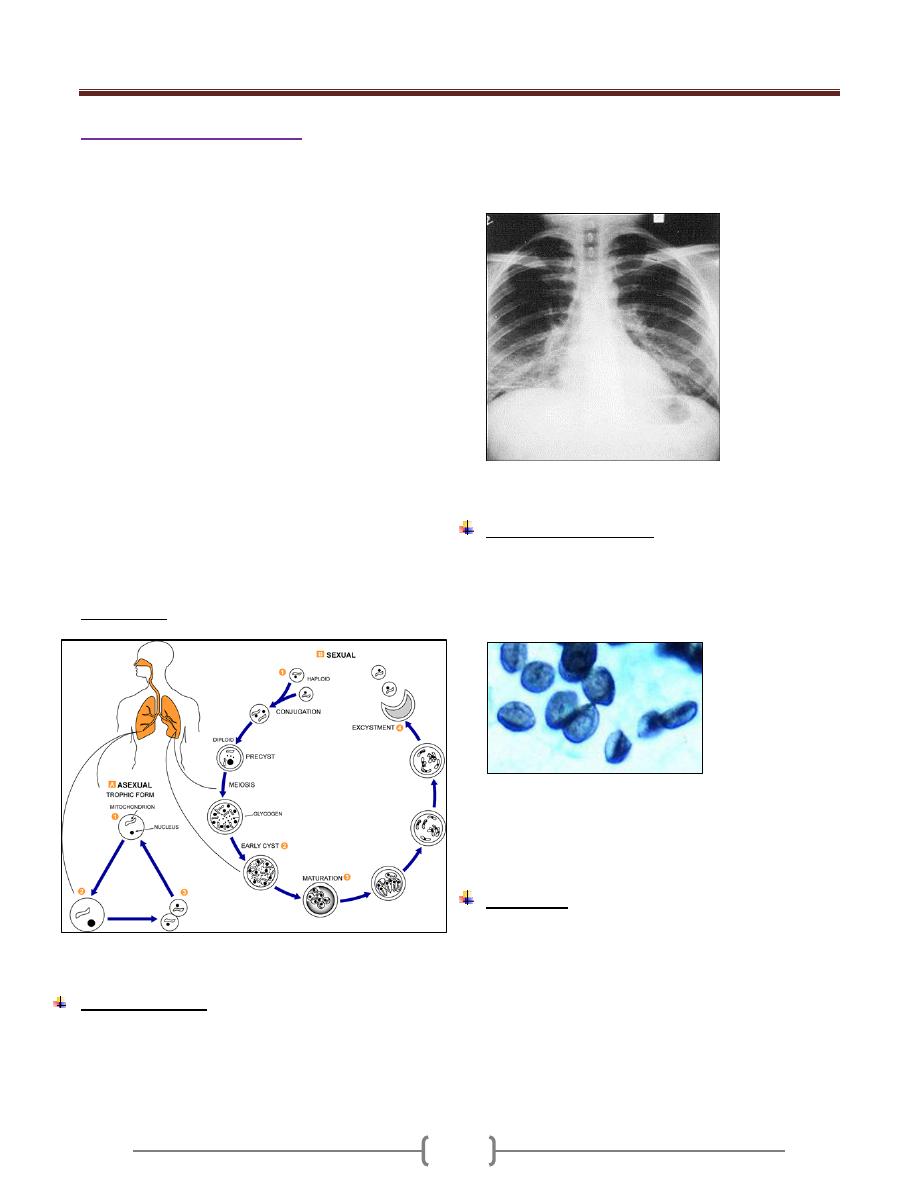
Unit 2: Protozoa
48
Pneumocystis ( )غير مطلوب
Pneumocystis jirovecii (jirovecii pronounced: yee row vet
zee) causes pneumonia in immunocompromised patients.
Before 2002, P.jirovecii was called P. carinii but after
2002, taxonomists renamed the human species of
pneumocystis as P.jirovecii and recommended that
P.carinii be used only to describe the rat species of
pneumocystis.
Until recently, P.jirovecii was thought to be a protozoan,
but molecular biologic studies have proved that it is a
fungus with a close relationship to ascomycetes.
Pneumocystis species are found in domestic animals such
as horses and sheep and in a variety of rodents, but it is
thought that these animals are not a reservoir for human
infection.
P.jirovecii has morphologically distinct forms (figure 5):
thin-walled trophozoites and cysts, which are thick,
walled, spherical to elliptical, and contain 4-8 nuclei.
P.jirovecii contains a surface glycoprotein that exhibit
antigenic variation.
It is an extracellular pathogen. Growth in the lung is
limited to the surfactant layer above the alveolar
epithelium.
Transmission: through inhalation.
Figure 5. Life cycle of P.jirovecii
Clinical features
In absence of immunosupression, P. jirovecii does not
cause disease. Pneumocystis is commonly found in the
lungs of healthy people. Most individual are infected in
early childhood.
In immunosupressed patient, it is presented with sudden
onset of
Fever, nonproductive cough, dyspnoea and tachypnoea
X-ray gives ground glass appearance (Figure 6).
Figure 6 .Chest x-ray showing ground glass appearance
Laboratory Diagnosis
1) Cysts by microscopic examination of lung tissue or fluids
obtained by bronchoscopy, bronchial lavage, or open lung
biopsy. The cysts resemble crushed ping-pong balls and
are present in aggregates of 2 to 8 (Figure 7)
2)
Figure 7. Cysts of P.jirovecii
3) Fluorescent – antibody staining.
4) Polymerase chain reaction (PCR).
Treatment
Trimetheprim- sulfamethoxazole.

49

50

Unit 3: Helminthes (Introduction)
51
Lecture 1 – Introduction to
Helminthes:
The term Helminths means Worms
It comprises of phyla
The two classes are parasitic in all or most of their life
cycle stages.
General characters of helminthes
Helminthes are macroscopic & multicellular.
Bilaterally symmetrical.
Have special adaptations for the parasitic mode of life &
for survival.
Like the complete absence of the Digestive tract or
greatly reduced consist of mouth & blind sac only this is
because of their location in the host intestine or tissue
where predigested nutrient are abundant.
In Trematodes the Digestive system is partially loss.
In Cestodes there is no
Digestive system.
While in Nematodes the Digestive system is complete.
There is special adaptation in Trematode & Cestode,
presence of a coat of microvilli on the outer surface of the
tegument for nutrient absorption.
Internal parasite possesses all sorts of adaptation like
hooks, suckers, boring apparatus, etc……….
Reproductive system is very well developed.
(Self-fertilization, cross fertilization may take place. They
are monicious or diecious).
Eggs are produced in large numbers or few of them
survive & manage to infect a suitable host.
Helminthes
do not multiply in human body so the
number of individuals in a worm population living within
a given host does not exceed the number of infective eggs
or larvae that enter from the external environment.
However reproduction by autoinfection to increase
parasite population occur e.g. pin worm by ingestion
eggs through contaminated fingers .
Helminthes (adult worm, their eggs, larvae) can be
distributed in various organs & tissues of the body.
Most of the infected people with helminthes are
symptomatic carriers, and the diseased individual
among the infected groups are those with heaviest worm
burden.
The terms Light, moderate, heavy applied to worm
burden are relative & differ for various species of
helminthes and in people of different age & physical
status.
The number of eggs or larvae eliminated in feces, urine,
sputum is roughly proportional to the number of worms
generating them.
In heavy infection (crowded worms) the collective egg
output is great but egg output\worm is low.
The coexistence of several species of helminthes in the
same individual is widely prevalent, we called it
Polyhelminthism.
Many worms may be quite restricted in distribution. Since
they require certain environmental conditions (temp,
humidity, nature of soil, etc…) also presence of
intermediate host.
(e.g. Schistosomes) require certain species of snails as
intermediate host which in turn require specific
environments for survival. Hook worms as e.g. during
their phase in soil require proper conditions of temp.,
moisture & soil texture in order to survive & continue
their life cycle.
The life cycle of the helminths is either Direct or simple
involving only one host species, or indirect or complex
involving more than one host.
Certain helminthes include in their life cycle special kind
of transmission called Paratenesis involving paratenic
host which provide the parasite with protection, support &
availability to its final host.
Helminths are transmitted through contaminated water,
soil, food. And the modes of infection are:-
a) Oral ingestion:
Most of the worm transmitted through ingestion of
infective stage (either egg, larva through intermediate
infective host)
b) Skin penetration
:
(e.g. Schistosome)
c) Inhalation
:
Very rare (pin worm)
d) Insect bites: (e.g. in Filariasis)
The portal of exit depend on the habitat or localization of
the adult worm, if it is in the intestine (eggs in feces), in
the urinary tract (eggs in urine), in the Respiratory
system (eggs in sputum).
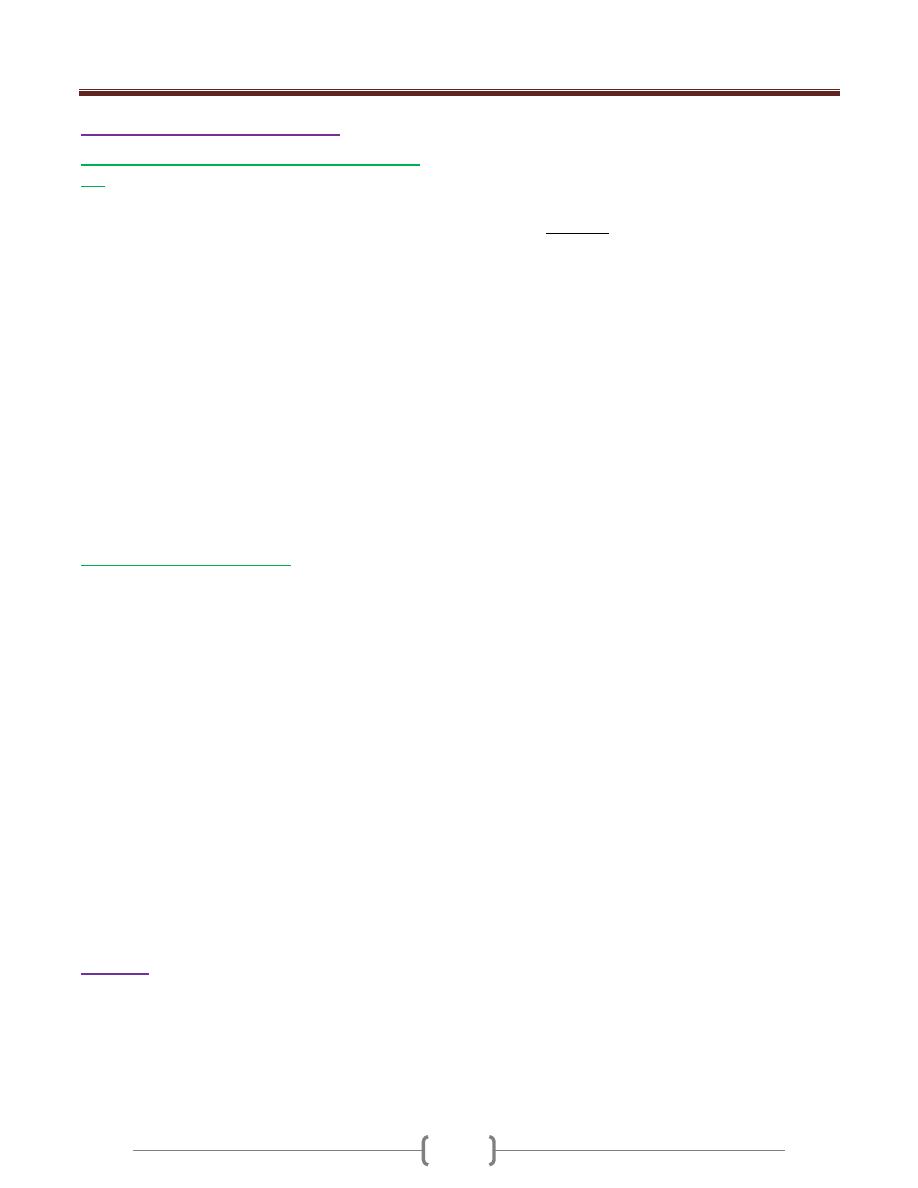
Unit 3: Helminthes (Introduction)
52
Pathogenesis of parasitic infection
The effects produced by parasitic worm depend
on:
1) The organs or tissues occupied or unusual site for
their occurrence
2) Number of adult worm harbored (severity or intensity
of infection).
3) Species of helminth parasite.
(some are potentially dangerous)
4) Re infection (repeated exposure)
5) Immunity of the host
Immunological responses are of great importance in
parasitic diseases. Since they present a large number of
antigens to their hosts. So in this case the host may
elaborate specific antibodies to counteract the antigen
produced as may wall off the pathogen or its products
cellular infiltration, proliferation & differentiation.
Some helminths parasite have special mechanism for
evasion the immune response (e.g. Schistosomes adsorb
host proteins on their surfaces).
Pathogenic effects of parasite
Physical trauma,or destruction of cells, tissues, organs by
mechanical or chemical means.
The parasite cause damage externally on the skin at the
site of invasion (e.g. hook worm, Schistosome cercaria)
or internally (attachment of hook worm, taeniasis).
Irritation of tissues by extruding their eggs into them
(e.g. Schistosome) or by movement & migration of adult
& larvae cause initiation of intestinal wall (e.g. Taenia
solium).
Mechanical pressure by the growing & developing of
cestodes larvae (e.g. hydatid cyst) causing atrophy of the
neighboring organ.
Intestinal obstruction:
Due the size & number (e.g. Ascaris “worm ball” )
Intestinal perforation (e.g. Ascaris, Strongyloides)
Production of anemia. Due to sucking blood (e.g. Hook
worm) or due to vit. B
12
depletion (e.g. Fish tape worm).
Reaction
All worms that live or migrate in blood or tissue sensitize
the body to their section or execution or to parasite surface
glycoprotein & polysaccharides causing allergic reaction
A characteristic feature of an allergic reaction is an
increase in the number of eosinophils so eosinophilia is a
general sign of helminthic infections.
Besides eosinophilia, common signal of occult helminthic
infection are hepatomegal y, pneumonitis, brouchial
asthma, urticaria, sub cutanous cysts, neurologic
disturbance & deviations in behaviour.
So must of the pathological features of helminthic
infection not due to direct action of the parasitic on
tissue but on host’s response to the product of parasite
eggs, larvae & soluble antigens.

53

Unit 3: Helminthes (Cestodes)
54
Lecture 1 - Introduction
Phylum Platyhelminthes
Class Cestoidea
General characters
The cestodes or tape worm are parasitic in all or nearly all
stages of the life cycle.
Adult worm live attach to mucosa of the small intestine of
vertebrates
The larval stage is parasitic in the tissue or body cavity of
vertebrate or invertebrate host.
All adult worm of man consist of the following parts:
Scolex (head) or hold fast organ.
A neck immediately behind the Scolex (a region of
growth and proliferation).
A strand of proglottids or segment
Immature ---- mature ----- gravid contain mature eggs.
Strobila is the entire series of proglottids and neck.
Number of proglottids varies from 3 or 4 segment in
Echinococcus species to 1,ooo or more in beef tape
worm and 3,ooo or 4,ooo in the fish tape worm .
The worm is flattened dorsoventrally, creamy to
chalky white in colour, covered with transparent
tegument,which is a syncytium with an outer covering
of microvilli (microtrichs)
Scolex: (head) knob like provided by 4 cupped suckers
2 ventrolaterally, 2 dorso laterally
Or the Scolex is spatulate provided with elongated median
vental and dorsal sucking groove
Excretory system: The major dorsal and vental
longitudinal excretory tubules run along the lateral margin
of the segment.These joined by anastomoses in the Scolex
and by transverse anastomoses near the posterior margin
of each segment which collects the waste products
through the terminal flame cells and numerous tubules.
Nervous system
:
consist of several ganglia with
commissure in the Scolex. Lateral longitudinal nerve
trunks connected by Transverse commissure.
Digestive system: Cestodes lack digestive organ
Tape worms that infect human, belong to 2 orders
Pseudophyllidea e.g Diphyllobothrium
Cyclophyllidea e.g Taenia ,Echinococcus,
Hymenolepis
Genital organ: Tape worm is a hermaphrodite. There is
one complete set of male and female organs for each
proglottids. The genital pores may be located mid lateral
(Taendia) or mid ventral in the anterior half of the
proglottids (Diphyllobothrium) in which the uterus is
provided with a pore through which unembryonated egg
are discharged.
In order Pseudophyllidea, the eggs are operculated and
unembryonated when laid.
In order Cyclophyllidea the eggs have two layers and
contain mature embryo, which contain 6 hooklets called
an Oncosphere.
Developmental stages of Tape worms
Eggs are passed in faeces or gravid proglottids become
detached and pass out of the bowel
Order Pseudophyllidea
: Solid form larvae
The oncosphere undergo development inside the egg in
fresh water and in one to two weeks a ciliated embryo
called Coracidium
hatches through the operculum
consists of typical hexacanth embryo surrounded by
ciliated membrane.
In the first intermediate host (Cyclops) the hexacanth
embryo develops into an elongated worm like organism
with large knob like structure (cercomer) on the posterior
end , which contains the emberyonic hooks and a slight
depression is formed at the anterior end in which lytic
glands develop.This larval form known as Procercoid
about o.5 mm in length.
In the Plankton
_
eating fish (2 intermediate hosts) the
procercoid develop into (Plerocercoid) which is
elongated flattened, wrinkly white mass of tissue with
inverted Scolex at anterior end.
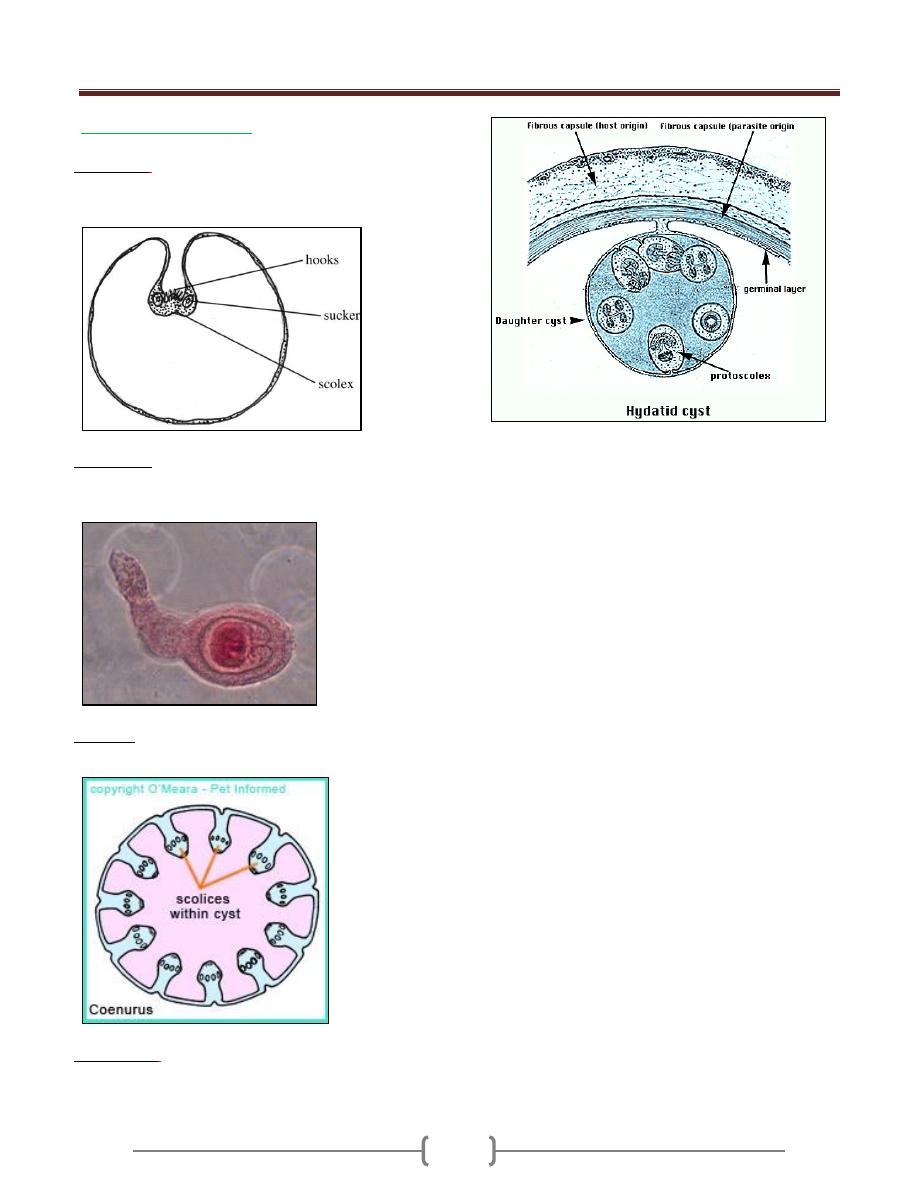
Unit 3: Helminthes (Cestodes)
55
In order Cyclophyllidea
The larval stage is bladder
or cyctic, vesicular form.
Cycticercus
:
refered to as a bladder worm. is a small
bladder filled with fluid and containing one invaginated
Scolex attach to the inner wall.
Cysticercoid
:
is a small solid larva with invaginated
Scolex lies within solid tissue instead of fluid and the body
tapers into a tail like projection.
Coenurus
:
large bladder worm with many invaginated
scolices in the fluid_ filled bladder.
Echinococcus
:
a large bladder or hydatid which internally
produces multiple scolices (protoscolices) and numerous
daughter bladders or brood capsules.
Unit 3: Helminthes (Cestodes)
56
Lecture 2 – Order Pseudophyllidea
Diphyllobothrium latum
Disease:
Fish tape worm infection, dipyllobothriasis .
Morphology, Biology
The adult worm in the intestine of man is very large,
measures ten meters in length and may have 3OOO_
4Ooo proglottids.
Scolex: (Head): Elliptical or
spatulate 2.5 mm in length by 1
mm in
Breadth, with median ventral and a
median dorsal grooved sucker
(botheria).
Mature segment broader than long
Male genital organ: spherical,
small Testes scattered throughout
the lateral dorsal region, Vas
deferens run forward to the
muscular cirrus which opens into
the genital pore in the middle of
the anterior region.
Female genital organ: Ovary bilobed in posterior region
of proglottids. Vitellaria small follicle scattered through
Out the lateral ventral region , Rosette uterus coiled with
uterine pore Egg : ovoid ,with operculum at one end and a
small thickening of the shell at the opposite end ,.Un
embryonated 58 to 76µm by 4o to 51 µm in width
.
Life cycle
Pathogensis and symptomatology:
D. latum produce no symptom, but infection may cause
digestive disturbance, diarrhea, hunger pain, nausea,
anorexia, vomiting. Sudden vomiting of a portion of
worm may occur. With symptoms suggesting peptic ulcer,
In certain instance carriers develop Pernicious anemia,
called bothriocephalus anemia.
In about 2 percent of the cases megaloblastic anemia
develop .When the worm high up in the jejunum it
absorbs vitamin B 12 from the intestine thus prevents its
combination with intrinsic factor, blocking the absorbtion
of vitamin B12 resulting in its deficiency.
Diagnosis:
Stool examination looking for eggs or segments also
Immunodiagnosis.
Treatment:
Niclosamide
Praziquantel, quinacrine hydrochloride
Epidemiology:
The parasite is prevalent in central Europe. America,
Japan, central Africa where raw fish are routinely
consumed.
Control:
Sanitary disposal of human feces
Thorough cooking of fish, freezing at temp _ 18 degree
centigrate for 48 hours will kill the infective larva.
Avoidance of eating raw or undercooked fish
.
Unit 3: Helminthes (Cestodes)
57
Lecture 3+4+5+6+7+8 – Order
Cyclophyllidea
Taenia saginata
Synonyms:
Beef tapeworm, Taeniarhynchus saginata.
Disease:
Taeniasis saginata.
Habitat:
Adult tapeworm is attached to the wall of small
intestine of man.
Morphology:
It is white tape like warm adult worm 5 meters long with
1000 -2OOO- proglottids.
Scolex: pyriform with 4 muscular suckers, no rostellum,
no hooks, there is a slight apical depression (unarmed tape
worms).
Strobila: neck, lmmature proglottids, mature
prohglottidds, about 12 mm in width with a full set of
male and female reproductive organs.
Male & Female Reproducti ve organs :
Ovary, bilobed, vagina, ootype, vitellaria, behind the
ovaries, blind uterus.
Testes, 300-400 follicle, vasa efferentia coiled vas deferense,
cirrus, genital pore on the lateral margin of the segment.
The genital pore on the lateral margin of the segment
alternate irregularly between the right & left margins.
As the segments move towards the posterior end of the
worm, they become more elongated & narrower (gravid.seg.)
In the gravid segment the uterus consist of central
longitudinal stem with 15-20 lateral branches on each side
which intern sub branch.
The terminal proglottid become separated singly or in
small groups and pass out with the stool.
Egg are liberated by rupture of the ripe proglottid 80,000
eggs in single proglottid, infected person can discharge
about 500,000 egg /day.
Egg: spherical 31 to 43 µm in diameter have thin
transparent outer, embryonal envelope .and thick brown
shell, composed of many slender rodes cemented together
, within the shell is a hexacanth embryo , which has 3
pairs of lancet shaped hooklets .
Life Cycle
Cysticercus bovis: Oval, elliptical in shape which
measures 5 by 10 mm and head likes the adult worm,
invaginated into fluid filled bladder. ,
Measly beef: meat that contain cysticercus bovis
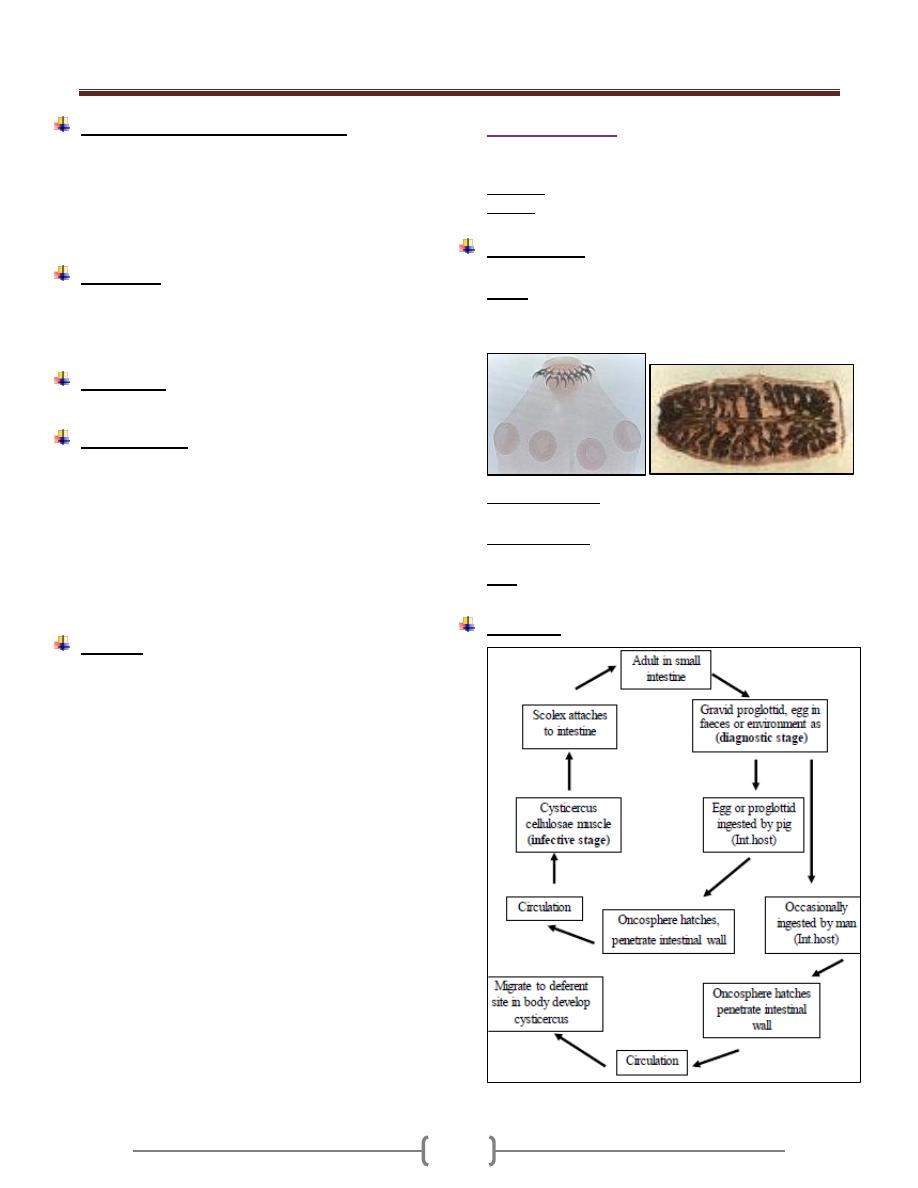
Unit 3: Helminthes (Cestodes)
58
Pathogenesis and symptomatology:
Infection with adult T.saginata is without symptoms
Abdominal discomfort
Diarrhea alternate with constipation.
Anorexia, hunger pain.
Intestinal obstruction (rarely)
Diagnosis:
Demonstration of proglottid or egg in faeces.
Serodiagnosis: IHA, IFA, ElISA.
Adhesive cellophane tape technique.
Treatment:
Niclosamide- praziquantel quinacrine hydrochloride
Epidemiology:
Cattle acquire the larval stage of T.saginata by grazing on
moist pasture contaminated with faeces or sewage
containing egg
EGG remains viable for 2 months in natural condition and
for 6 months under optimal condition of moisture & temp.
Man is the only natural definitive host of saginata. Man
acquires the infection by eating uncooked or under
cooked beef containing cysticerci.
Control:
1) Proper disposal of human faeces
2) Workers at cattle feed lots examined periodically for sign
of infection.
3) Thorough cooking of beef before consumption, heating
the meat to 65 degree centigrates is a safe guard.
4) Freezing the beef at -20 degree centigrates for 24 hours
or longer kill the cysticerci.
Taenia Solium
Pork tape worm, armed tape worm,
Disease
: Taeniasis solium , pork , tapewarm infection .
Habitat: small intestine of man.
Morphology:
2-3 meters in length, fewer than 1000 proglottid.
Scolex: Globular in shape, 4 suckers, of alternating large
and small hooks 22 to36 in number and measuring 140 to
200 µm and 100 to 150-µm long.
Mature proglottids: are wider than long and nearly
identical to those of T.saginata, Testes 150-200 follicles.
Gravid Segments: longer than wide, have a uterus, the
medial stem with 9 to 10 lateral branches.
Eggs: are morphologically similar to that of T.saginata
Life cycle
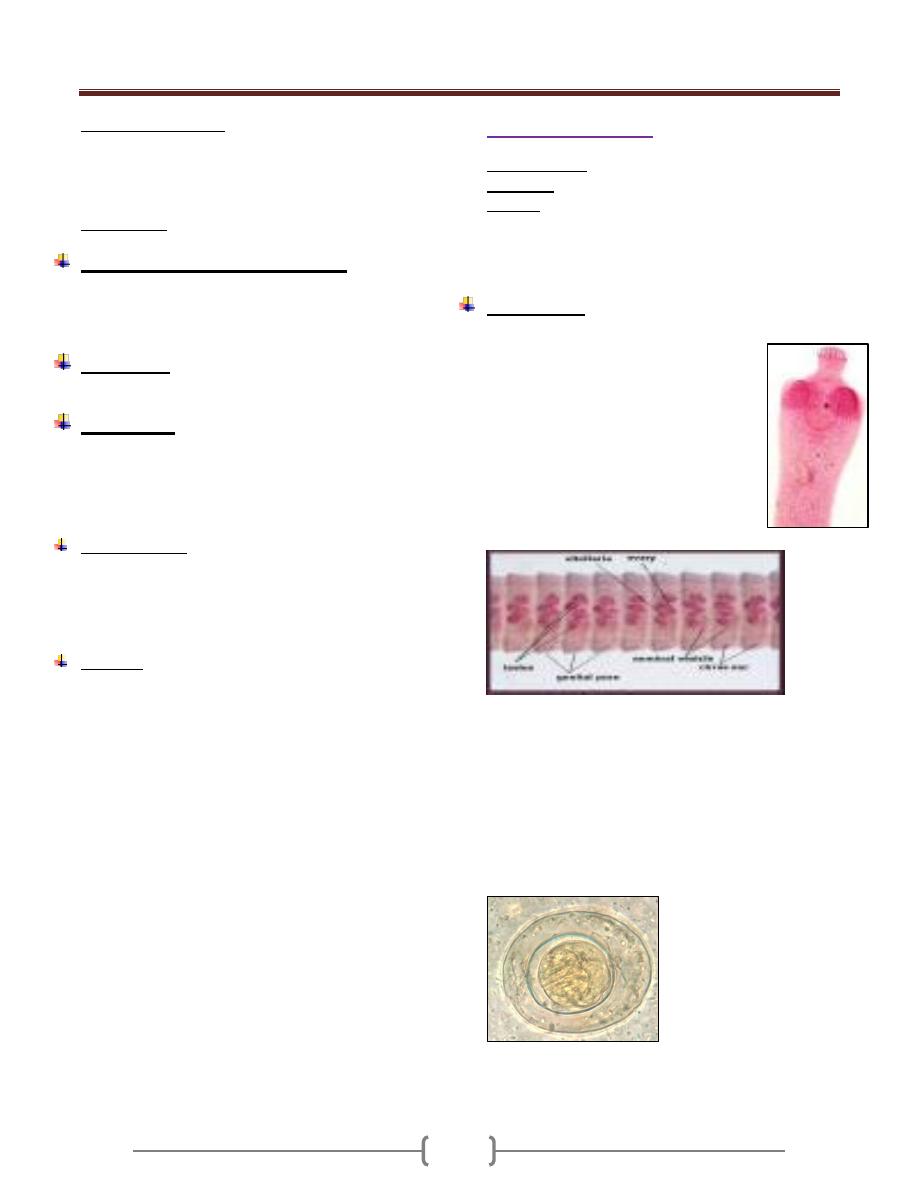
Unit 3: Helminthes (Cestodes)
59
Cysticercus cellulosae: Pearly white, measuring 5 mm
by 8 to 10 mm, the scolex deeply invaginated into fluid
filled bladder is provided with 4 suckers and a rostellr as
in adult is provided with 4 suckers and a rostellum as in
adult worm.
Measley pork: pork containing cysticercus cellulosae.
Pathogenesis and symptomatology:
Infectin with the adult T.solium produce the same clinical
manifestations as infection with T.saginata.
However , no intestinual obstruction.
Diagnosis:
Similar to that of T.saginata
Treatment:
Niclosamide , Praziquantel are the drug of choice
However Niclosamide is not recommended causes the
proglottids to disintegrate releasing the eggs to the bowel
lumen.
Epidemiology:
Human infection with adult T. solium results from
eating raw pork containing Cysticercus cellulosae.
Man is the only natural host of the adult worm. Man is
also a suitable host for the cysticercus.
Control:
Sanitary disposal of human faeces , Treatment of
infected person , Thorough cooking of pork or held in
a deep freeze for at least 24 hrs .
Hymenolepis nana
Common name: Dwarf tape worm
Synonyms: Vampirolepis nana
Disease: Hymenolepiasis nana, Dwarf tape worm
infection. Hymenolepiasis nana is an infection by adult
and larval stage of H. nana. It is found warld wide,
primarily limited to children in warm climate.
Morphology:
Small 25 to 4o mm in length, 1 mm in breadth
Scolex: Small, globular with short rectactile
rostellum, 4 sucker s and a single ring of 2o
to 3o minute hooklets
Strobila: 2oo segments, broader than long
Mature proglottids: single genital pore on
one side of segment
There are 3 round testes lie in the posterior
part of each segment, Bilobed ovary lie
posteriorly between the testes with compact
vitelline gland behind.
Gravid uterus forms a sac filled with eggs. Gravid
segment destroyed in the intestine releasing the eggs
which are found in faeces.
Egg: nearly spherical 3o _ 47 µ m in diameter. with two
thin membranous shell s . The inner one with 2 polar
thickenings, each provided with 4 to 8 long thread like
filaments extending into the space between the inner and
outer shells. The centrally located hexacanth embryo is
equipped with 3 pairs of hooklets
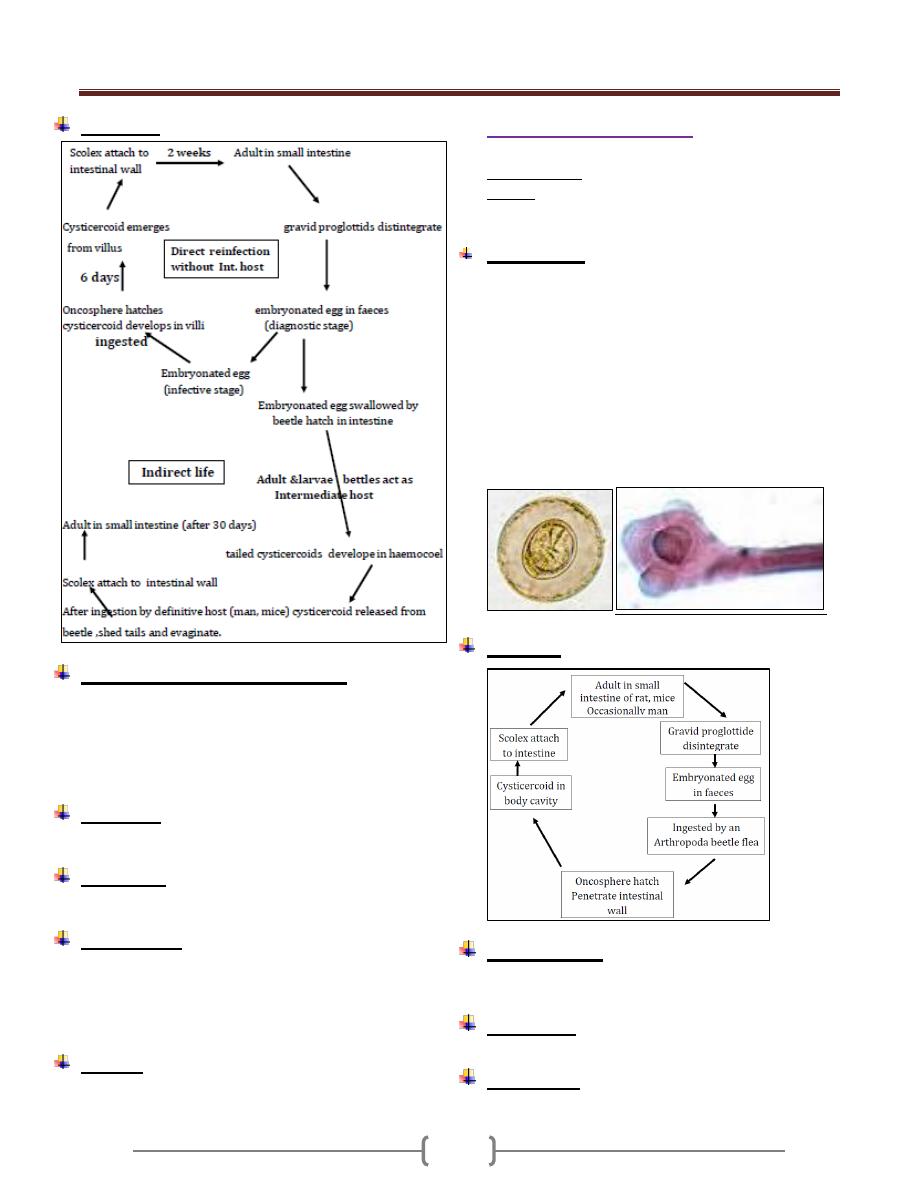
Unit 3: Helminthes (Cestodes)
60
Life Cycle
Pathogenesis and symptomatology:
Infection with H. nana produces No symptoms in light
infection or may be diarrhea, anorexia, vomiting, loss of
weight, pruritus of the nose and anus, urticaria.
Heavy infection causes diarrhea, abdominal pain,
anorexia and nervous disorders.
Diagnosis:
By demonstration of the egg in the stool.
Treatment:
Niclosamide is the drug of choice in a course of 5-7 days
Epidemiology:
Infection iscommonly acquired by anus to mouth
transmission of eggs. (Hand, food) is more common in
children.
Occasional infection may occur from rodent source.
Control:
a) Good personal hygiene and sanitation
b) Treatment of infected person.
Hymenolepis dimenuta
Common name: Rat tape worm infection
Habitat: in the small intestine of Rat and mice and
rarely in Human.
Morphology:
2o-6O Cm in length by 3.5 to 4.o mm in width, with
1,ooo proglottids
Scolex: o.4 mm wide with 4 suckers and retractable and
un armed rostellum.
Proglottids as in H.Nana.
Egg: Ovoid to sub spherical 72 to 86 µm by 6o to 79
µm with a space between the outer tanned egg
membrane and the hyaline inner membrane which
provided with a pair of pollar thickenings but lack the
polar filaments.
Life cycle
Pathogenesis:
Nonpathogenic but may produce mild diarrhea and
abdominal pain.
Diagnosis:
By demonstration of eggs in stool.
Treatment:
Similar to that for H. nana

Unit 3: Helminthes (Cestodes)
61
Epidemiology:
H. diminuta is worldwide in distribution. Human infection
is associated with the contamination of cereals, grains by
infected grain beetles. Infected fleas may transferred to
the mouth by dirty hands.
Control:
1) Eradication of rat around the home.
2) Protection of food such as grain and cereals from rat
dropping and from insect.
Dipylidium caninum
Common name: Dog / cat tape worm
Disease: Dipylidiasis, dog tape worm infection
Habitat: Adult in the small intestine of dogs and cats.
Occasionally in human mostly in children, infants.
Morphology:
Adult median size 1o _ 7o cm in length, 6o_17o
proglottids.
Scolex: rhomboidal in shape, o.3_ o.5 mm in diameter, 4
suckers, introversible apical club _shape proboscis with 6
rows of minute hooklets.
Mature proglottids: Contain paired reproductive organs
with a genital pore at each lateral margin.
Gravid proglottids:
Resemble cucumber seeds in shape, size. Uterus
disappear early in development and replaced by hyaline ,
non-cellular masses of egg capsules, each egg capsule
filled with 1 to 2 o fully embryonated eggs.
Egg: 3o_ 6o µm in diameter consist of typical 6 hooked
oncosphere .
Life cycle
Pathogenesis:
In a child may produce diarrhea, unrest, sometimes
urticaria, fever, eosinophilia and rarely convulsion.
Diagnosis:
Based on recovery of egg packets or gravid segment in
stool.
Treatment:
Niclosamide, praziquantel, quanacrine hydrochloride.
Epidemiology:
Human infection especially children occur upon ingestion
of the fleas intermediate host, by licking of an infected
dog or cat or by hand to mouth contamination
Control:
1) Infected dogs and cats should be treated.
2) Children should be taught not to let dogs or cats lick
them in their mouth.
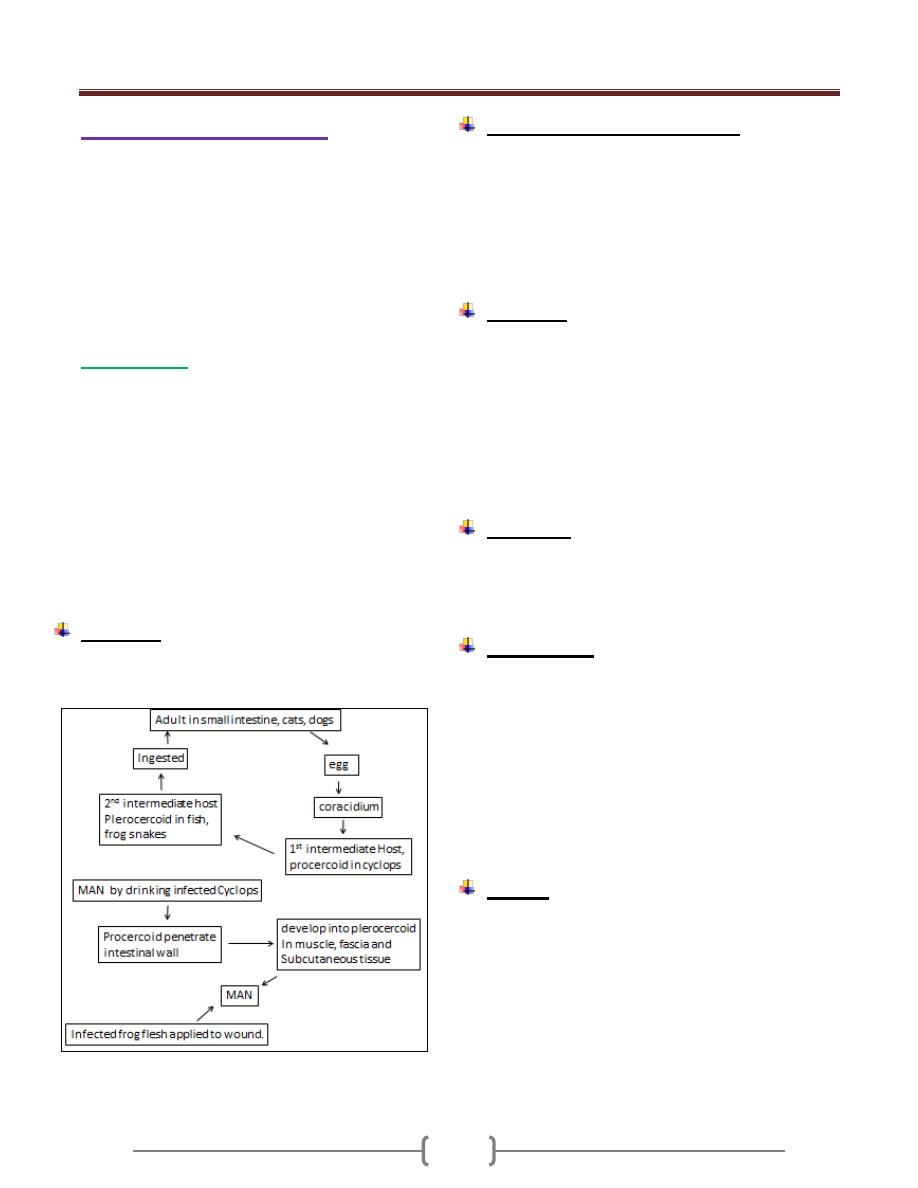
Unit 3: Helminthes (Cestodes)
62
Larval tape worm infection
The majority of adult tape worm parasitize the small
intestine of human .The larval stage (meta cestode) and
several spp.of tape worm develop in the extra intestinal
tissue of human (somatic tape worm) namely cysticercus
of T.solium ,coenurus of T.multiceps ,hydatid cyst of
Echinococcus granulosus and sparganum (plerocercoid)
of spirometra spp .
Sparganosis:
The plerocercoid larvae or spargana of spieces of
pseudophyllidea .tape worm may infect human & cause
sparganosis.
Most of spargana reported in human are believed to be "
spirometra mansoni " and other spieces.
Cats ,dogs and related wild animals are definitive host
Copepods Cyclops being the 1
st
intermediate host
Several spp.of vertebrates, fish, frog &snakes as 2
nd
intermediate host.
Human infection occurs by swallowing a procercoid in a
copepod or a plerocercoid in 2
nd
int.host.
Life cycle:
the life cycle of Spirometra species follows the same
pattern as that of Diphyllobothrium sp.
Pathogenesis & Symptomatology:
Little host tissue reaction occurs in the early stages later
in the infection, the area around the worm become
edematous & painful to the touch.
Death of the parasite result in marked inflammation with
local eosinophilia and charcot leyden crystal.
Ocular sparganosis: intense pain, irritation, excessive
lacrymation & edema.
Diagnosis:
1) Biopsy: typical worm structure can be seen by biopsy
(subcutaneous cyst).
2) Speices.diagnosis: can be made by feading a living
sparganum to cat or dog and subsequentelly examinating
the adult worm.
3) Serodiagnosis:
a. Indirect IF Ab test.
b. agar gel diffusion
c. Indirect haemagglutination test
Treatment:
Consist of surgical removal of the sparganum from the
tissue.
Infection about the eye treated with injection of 2-4 ml of
4% ethyl alcohol with procaine (epinephrine –free).
Epidemiology:
Man becomes infected by:
1) Swallowing infected Cyclops in drinking water from
pond, stream, lake).
2) Eating raw infected frog, snakes, small mammals.
3) Applying plerocercoid infected flesh of frog or snakes for
treatment of inflamed eye or finger
In such case, larvae migrate into human tissue and
encysted in various parts of the body.
4) human infection is also acquired from eating raw pork as
sparganum develop also in pigs
Control:
1) drinking only safe waer
2) Eating only well-cooked flesh of animals.
Unit 3: Helminthes (Cestodes)
63
Cysticercosis:
Is an infection by the larval stage of T.solium, the pork
tape worm refered to as cysticercous cellulosae
Man is the definitive host.
Pig is the intermediate host in which the hexacanth
embryo hatch from egg and develop into cysticercus
cellulosae or bladder worm.
Man is also satisfactory host for development of this
larvae .So man may serve as intermediate host when egg
is ingested by mouth, hatch in the small intestine,
liberated oncosphere burrow into the mucosal circulation
& carried to different organs & tissues producing (human
cysticercosis)
The fully developed cysticercus is a small ovoid ,smooth
bladder or cyst filled with fluid & measure 5 mm x 10
mm in size ,developed from the inner wall is single
invaginated scolex with 4 sucker & a double circular
crown of hooks (9-10weeks to develops)
Dead cysicercus possess a cloudy fluid and yellowish
color scolex.
Racemose Cysticercus:
This type is unencapsulated larva with numerous branches
reaching length of 15cm .It is only seen in CNS mainly in
the ventricular and subarachnoid spaces at the base of the
brain.
Pathogenesis:
The most common location of cysticerci inhuman body is
the CNS followed by muscle, subcutaneous tissues, eye,
lung, heart, liver and other visceral location.
Cysticerci survive in man for 4 to 5 years
The clinical feature depends on their location and the
number. Except in the brain and eye, live cysticerci are
surrounded by a tough adventitious.
Cysticerci in human are surrounded by a tough
adventitious capsule which allow them to be detached
easily from the surrounding tissue.
Cysticerci that develop in the subcutaneous and muscle
tissue cause no pain.
Symptom result from the death of larvae in the visceral
organs .With the death of the parasite cyst capsule
distended with fluid ,increase in size replaced by fibrous
tissue or undergo calcification and surrounded by capsule
of Connective tissues.
Cysticerci in brain cause:
Epileptic, seizures, hydrocephalus, stroke also severe
headaches, nausea, vomiting, dizziness, diplopia and
psychic changes.
Living cysticerci (race mose type) in the eye cause
damage to any tissue of the eye ball resulting in uveitis,
iritis, detachment of the retina, atrophy of the choroid.
Diagnosis:
1) Biopsy: surgical removal of the nodule and doing
histopathological examination.
2) Radiology: calcified larvae on x-ray film of muscle.
ocular cyst can be detached by ophthalmoscopy.
computed tomography of the brain for neurocysticercus .
3) Serological test: CFT (Complement Fixation Test), IHA
haemaglutination test, ELISA, immune electrophoresis
are used. using purified Ag and crude Ag ( extract of pig
cysticerci)
Treatment:
Syrgical removal of the cyst is useful in treating some
ocular or cerebral cases.
Chemotherapy: praziquantel following or accompanying
administration of corticosteroid is effective.
Epidemiology:
Human acquired infection of cysticercus by:
1) Accidental ingestion of eggs of T.solium in contaminated
food or drink (heteroinfection) .It is the usual mode of
transfer.
2) Anus to finger to mouth contact & that called external
autoinfection.
3) Internal autoinfection: gravid proglottids. in infected
person with T.solium detached from strobila and
regurgitated into stomach as a result of reverse peristalsis
then return to the duodenum.
Control:
1) Early detection & treatment of case of T .solium.
2) Improvement in sanitation.
3) Good personal hygiene.
4) Adequate cooking as prior freezing of pork to prevent
infection with the adult worm.
Unit 3: Helminthes (Cestodes)
64
Hydrated disease (hydatidosis),
Echinococcosis
The larval stage of species of the tape worm
Echinococcus is known as the hydatid cyst , several
species occur in human
Echinococcus granulosus
Common name: dog tape worm, hydatid tape worm.
Disease: unilocular hydatid disease.
Geographic distribution: Echinococcus granulosus
widely distributed throughout temperate and subtropical
regions ,commonly in sheep and cattle raising countries.
Human infection is common in south America ,parts of
Africa and Europe ,the middle east ,southern Australia,
New Zealand ,extensive area of Asia , south western
united states , Canada.
Habitat :
Man harbours the larval form (hydatid cyst ) specially in
liver and lungs
Adult worm is found in the small intestine of dog and
other canines.
Morphology:
Adult worms are small in size up to 6 mm long,
Scolex: pyriform in shape, has a rostellum with 28 to 50
hooks in 2 rows and 4 suckers.
Strobila: with neck ,one immature ,one mature and one or
two gravid proglottids
Mature segment: with male and female genital organ,
male with 45-65 testes.
Gravid segment: measures more than half the total length
of the whole tape worm with sac like uterus.
Eggs: spherical , 31-40 –m in diameter ,morphologically
similar to those of either taeniid species of dog
Outer shell surround ---- with radially striated
embryophore (inner shell) Hexacanth embryo
Morphology of larval stage (Hydatid cyst):
Larval stage found in organs and tissues of herbivorous
host such as sheep, cattle, hogs.
These animals act as Intermediate host
Man also becomes accidentally infected and act as
intermediate host.
The most common site for development of the cyst in man
is liver followed by lungs (about 70% in liver and 25%in
lungs)
Less frequently the spleen, kidneys, heart, bones,
peritoneum and CNS
These are 2 morphologic types in human tissue
1-Unilocular cyst 2-Osseous
1- Unilocular hydatid cyst:
Is a fluid –filled cyst that is spherical in shape
Cross-section of the cyst wall reveals .an external ,milky
white laminated membrane about 1 mm thick without
nuclei and an inner germinal layer
About 10-15 –m in thickness with nuclei. An outer layer
of fibrous connective tissue is formed as a result of host
reaction to the presence and growing of the cyst.
From the inner germinative layer small secondary cysts
develop ,They are known as broad capsules and as they
grow protoscolices develop from their inner wall (A
protoscolex is ovoid scolex with typical 4 sucker ,
rostellum , a double crown of hooklets deeply with drawn
into the post sucker region )
The brood capsules may detach to form daughter cysts,
which with free scoleces, form hydatid sand within the
cyst cavity.
The majority of human hydatids are unilocular ,with a
size depends on the site and on its age
After 12-20 years it may be 15 cm in diameter or more.
(Slowly growing) containing a liter or more of clear
sterile hydatid fluid.
Some cyst fail to develop broad capsules they become
sterile cyst
Multiple cysts in the liver may be the result of multiple
egg infections or the formation of exogenous daughter
cysts as a result of herniation of the germinative layer
before the host response has resulted in a fibrous
connective tissue wall.
2- Osseous hydatid:
This type of hydatid cyst form in bone of man particularly
the long bone—and pelvic arch.
Larval growth in bones is atypical the outer membranes
are not produced and the organism proceeds to grow as a
protoplasmic stream that erodies the cancellous tissues.

Unit 3: Helminthes (Cestodes)
65
Life cycle
Pathogenesis and symptomatology:
Most of the hydatid cysts of the unilocular type, develop
in the liver, infection of the lung is next in prevalence, the
other organs also invaded occasionally
An inflammatory reaction by the host results in the
enveloping of the cyst by a fibrous connective tissue wall.
After years .may die, shrink, calcify
As the cyst grows, pressure and necrosis may result in
the destruction of the normal liver tissue and impaired
liver function.
Leakage or rupture of the cyst causing the liberation of
hydatid sand into the pleural ,peritoneal or pericardial
cavities and the associated dissemination of scoleces
result in multiple secondary hydatid cyst formation .
The antigenic stimulus from the leakage may result in
anaphylactic shock marked allergic reactions with high
eosinophilia.
Rupture of pulmonary cyst cause chest pain, dyspnea,
cough.
Hydatid cyst in the brain produce increasing symptomatic
evidence of an intracranial tumor.
Osseous hydatid: there is minimal response on the part of
the tissue ,so fibrous outer layer is not produce .and there
is extensive bone erosion to a stage at which fracture or
crumbling suddenly occur
Most infection in human begins in childhood and
discovered in adult life.
Diagnosis:
1) Casoni ُ s test (Intradermal test)
Based on the principle of immediate hypersensitivity.
The antigen used is hydatid fluid collected from animal or
human cysts and sterilized by (seitz filter).
0.2 ml of the antigen injected intradermally on one arm---
--and 0.2 ml saline as a control on the other hand.
In positive cases a large wheal about 5 cm in diameter
with multiple pseudopodia appear within 1/2 hr at the test
side and fades away in an hour.
A delayed reaction appears after 18 to 48 hours -----------
Characterized by Oedema and indurations 5-6 cm
surrounding the site of injection .Anegative reaction does
not exclude echinococcal infection.
The test usually becomes positive 8-12 weeks after
infection and remains positive after surgical removal of
cyst from the patients .It is sensitive but not specific as
false +ve reaction occur in many other infection like
cysticercosis
2)
Radiology:
X-ray film demonstrate hydatid cyst in lung, bone, detect
uncalcified cysts.
Ultra sound, CT scan .Magnetic Resonance Imaging (MRI)
3)
Exploratory cyst puncture
:
Needle aspiration of cysts is dangerous because of
possible spillage of the contents causing secondary spread
or anaphylaxis.
4) Serological Tests:
Based on detection of antibodies and antigen in the patient
serum. .useful tests include ELISA , indirect
haemagglutination ,latex agglutination FIA -
immunoelectrophorensis test , the first 3 are highly
sensitive for initial screening of serum .
Specific confirmation of reactive serum can be obtained
with immunoelectrophoresis to detect the diagnostic (arc 5)
Cysticercosis gives cross reacting antibodies to
Echinococcus antigen 5
5)
Histological examination
of removed specimen.
Treatment:
Surgical removal of the cyst .is the most effective
treatment.
If surgical removed is not possible.
Oral therapy with mebendazole is useful ,in a dose of 400-
600 mg 3 times a day for a period of 21 to 30 days.

Unit 3: Helminthes (Cestodes)
66
Epidemiology:
Human infection with hydatid cyst occur in sheep or
(other herbivores) raising area.
Dogs harbor the adult worms,
Sheep or hogs serve as common reservoirs of the larval
stage
Hydatid in cattle is sterile,
Exposure commonly occur in childhood among boys
playing with infected dogs
Hydatid may grow for 5 to 20 years before diagnoses is
made.
Control:
To break the E.granulosus life cycle and subsequently
halt the spread of human disease several preventive
measure are essential.
All infected viscera should be buried or incinerated.
Stray dogs should be destroyed.
Domestic dogs should be periodically dewarmed
Personal hygiene to avoid ingestion of the eggs.
Hydatid of Echinococcus multilocularis
Disease: Alveolar hydatid diseases as multiloculae
hydatid disease.
Habitat: Adult worm E.multilocularis occur in small
intestine of foxes and other wild canines (definitive host)
Natural intermediate host wild mice, in which larval form
recoverd.
Alveolar hydatid in man occur in USSP, Jappan and -------
Morphology:
Adult: smaller than E.granulosus 1.2 to 3.3 mm long and
differ in the position of the genital pore with respect to the
genital organs also in the number of tests 16-29.
Egg: are like other taeniid egg but more resistant to cold
and other environment condition.
Larval form: Alveolar cyst or multiloculares cyst.
The cyst grow by exogenous budding into small irregular
cavities, each within a hyaline membrane, frequently
without fibrous encapsulation, thus there are resultant
metastases through the lymphaties and circulation.
Brood capsule scattered in the cyst
Scolices in the alveolar hydatid in man are few or none.
Most alveolar cyst occur in the liver.
Life cycle:
In general life cycle is similar to that of E.granulosus.
Pathogenesis and symptomatology:
Liver is the most common site to be affected in the
alveolar hydatid cyst .Rarely in lung.
It is a lethal disease.
In human .Intra hepatic portal hypertension results in
Jaundice, ascites, splenomegaly.
Diagnosis:
Biopsy. Ct scan, ultrasound helpful in ascertain the site &
shape. -Immunological test.
Treatment:
Alveolar cyst is not amenable to surgical removal.
Chemotherapy with mebendazole is useful.
Epidemiology:
Infection acquired from eating raw fruits and vegetable
picked off the ground and contaminated with the faeces
of infected foxes and other caniidae .
Control:
Personal hygiene
Good sanitation
Sacrifice of infected animals
Hydatid cyst of Echinococcus vogeli
Disease: Polycystic hydatid disease .
Habitat: in the small intestine of bush dog (definitive
host) in latin America. Rodent, paca (natural intermediate
host).
Morphology: Adult differs from E. granulosus in greater
length 3.9- 5.6 mm and more slender proglottids
Polycystic hydatid: is alveolar in characters but less so
than that of E. multilocularis, so it is intermediate between
cystic and alveolar hydatid disease, present like a mass of
tumor in the liver

67
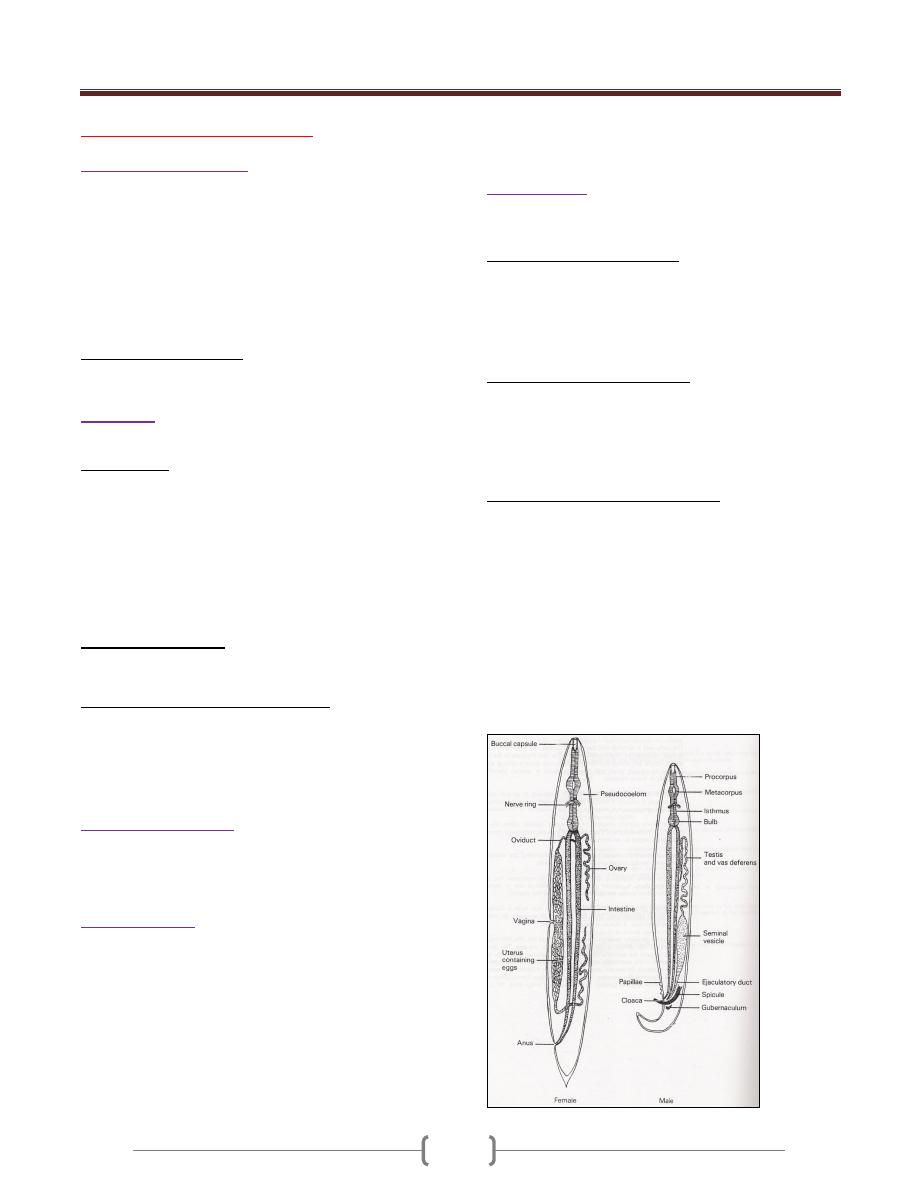
Unit 3: Helminthes (Nematodes)
86
Lecture 1 - Introduction
General characteristics
The word nematode comes from a Greek word Nema that
means thread.
Nematodes are unsegmented, elongate & cylindrical with
bilateral symmetry with a complete digestive tract which
started with mouth, esophagus, intestine & end in sub-
terminal anus. They have no circulatory system & nutrients
are transported through body via fluid in body cavity
(pseudocelom).The nematode has no respiratory system.
Pseudocelom (pseudocoel) Body cavity of nematode
which is filled with fluid in which internal organs float.
Body wall
The body wall is consists of an
Outer cuticle is tough and flexible, so does not allow
volume of the worm to increase and keep the hydrostatic
pressure inside the worm very high; this is why the round
worm appears round. Therefore, as the worm grows, it has
to molt and form new cuticle. This cuticle is periodically
shed during the life of nematode as it grows, usually four
times before reaching adult stage. The first molt and
occasionally the second molt may take place in the egg.
The remaining molts occur in the definitive host.
Inner muscular layer: aligned longitudinally along the
inside of the body, so the nematode can only bend from
side to side.
Intermediate thin syncytial hypodermis that secretes
the cuticula and binds it to the outer surface of the muscle
fiber. Arising from the hypodermis, cords project toward
the body cavity at the dorsal, ventral, and lateral lines,
dividing the muscles into distinct quadrents.
The excretory system
Consists of two tubes running inside the lateral chords. At
anterior end these tubes are interconnected and open in
the midventral region as an excretory sinus.
Nervous system
It is composed from a circumesophageal nerve ring and
short ventral nerve cord and small dorsal nerve cord.
Sensory structures at the anterior end called Amphid and
at the posterior end called phasmids.
Male is smaller than female and have a characteristic bent
tail for holding the female for copulation. In male, the
intestine narrows and turn ventrally to become the cloaca.
The cloaca opens to the exterior via the anus. There is no
cloaca in the female, so the gut and reproductive system
are independent of each other and they don’t share ducts
or openings, as they do in the male.
Reproduction
Nematodes are bisexual (dioecious) but in few instances
the female may be parthenogenetic
The male reproducti ve system consist of a single tubule
, beginning as a testis , then a seminal vesicle , a vas
deferens and an ejaculatory duct opening into the cloaca.
Accessory copulatory structures consist of 1 or 2 copulatory
spicules which move out of the cloaca & inserted into the
genital pore of the female during copulation.
The female reproductive system may be composed of a
single reproductive set as in Trichinella and Trichuris, but
in most nematodes the inner organs are paired. The
following regions can be recognized: ovary, oviduct,
seminal receptacle, uterus, vagina, ovejector and vulva
which is ventral in position.
The stages in the nematode life cycle are the egg, four
larval stages, and the adult. At the end of each larval stage
a new cuticula is secreted and the old one is molted.
The daily production of eggs per female varies in different
species. The stage of development at the time of
oviposition also varies, in some species the eggs are
unembryonated (Ascaris and Trichuris), in hookworms
are in the early stage of cleavage, and those of
Strongyloides frequently are in the morula or a more
advanced stage, in filariae, Trichinella and Dracunculus,
the eggs develop completely and the larvae hatch in utero
to be discharged as larvae or microfilariae.
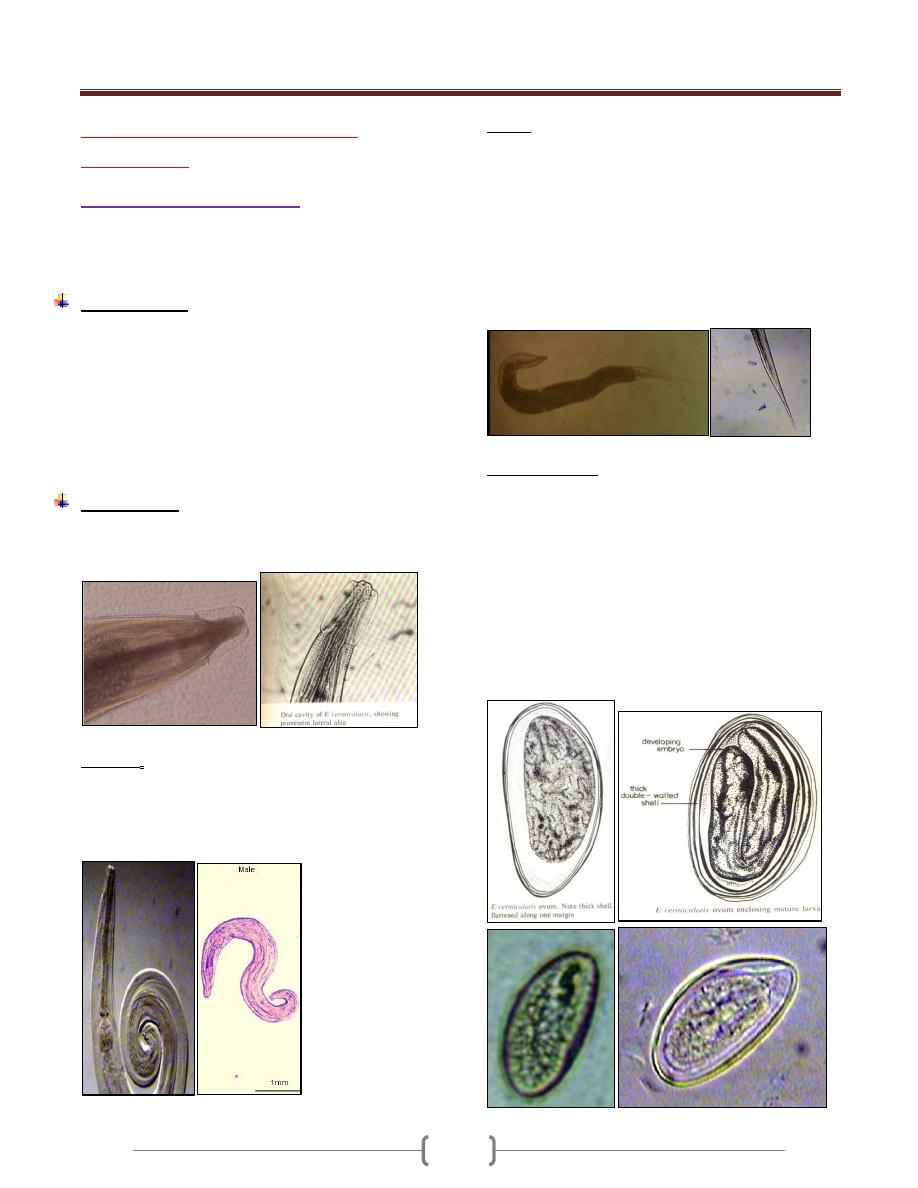
Unit 3: Helminthes (Nematodes)
86
Lecture 2+3+4+5 - Intestinal
nematodes
Enterobius vermicularis
Also called pinworm
It has a cosmopolitan distribution, but it is more common
in cool or temperate zone than in tropical areas.
Epidemiology:
Pinworm infection is prevalent in large family groups and
in schools and mental institution.
Contamination of clothing, bedding is a frequent cause of
familial outbreaks
The infection is more common in children than adults,
also prevalent where small children sleep together, and in
any population in which underclothing is worn day after
day and bathing is infrequent.
Morphology:
The adult male and female have an anterior expansion
from both side (ventral and dorsal) called (cervical alae).
The male measures up to 5 mm long with maximu m
width of 0.1- 0.2mm width.
The posterior end is strongly curved and the lateral view
of the worm forms an inverted question mark.
The male rarely seen as it die after female fertilization.
Female length 13 mm and 0.3-0.5 mm width. They are
light yellowish white.
The posterior end is sharply pointed and forming 1/3 of
the total length.
The male rarely seen as it die after female fertilization.
The adult worm inhabits the cecum and adjacent area of
the intestine.
Gravid female migrate down the bowel to the rectum and
during hosts sleep or relaxation, they crawl out of the anus
onto the perianal area and perineal skin.
Eggs morphology:
Eggs in utero are not fully embryonated when the female
worms migrate to the lower level of the colon.
Female discharge about 10,000 eggs. The eggs discharged
on the skin are essentially mature and within few hours
(about 6 hrs) contain a fully developed infective stage larva.
The eggs are flattened on one side and convex on the
other. It measures about 50-60µm by 20-30 µm. They
have a colorless inner double shell and an outer
albuminous layer that causes them to stick to each other
and to clothing and other objects.
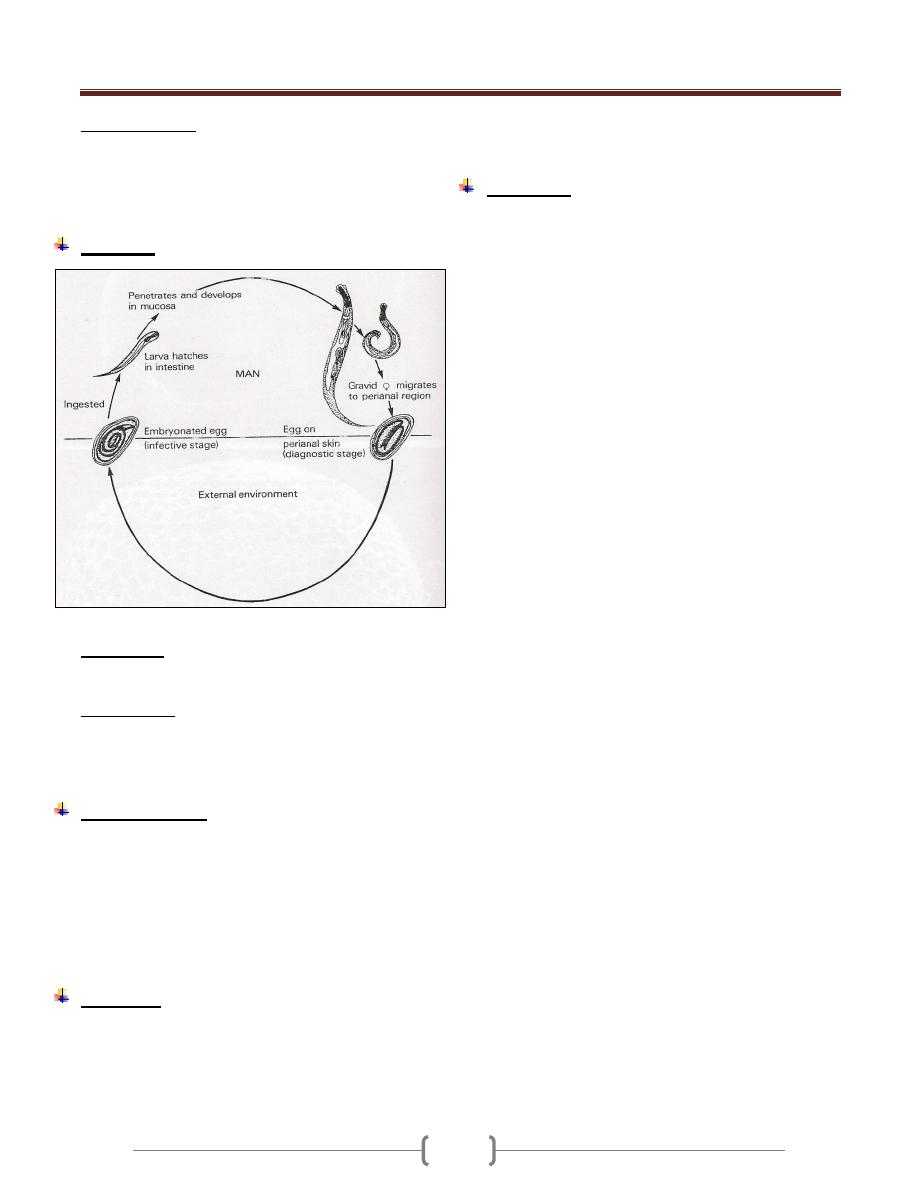
Unit 3: Helminthes (Nematodes)
07
Mode of infection:
1) By swallowing fully developed eggs with food or water.
2) Inhalation of eggs (light infection).
3) Autoinfection
4) Retroinfection.
Life cycle
Development of the adult worms require about 6 weeks.
Autoinfection
It develops when the eggs are carried to the mouth by
fingers after scratching the itching skin.
Retroinfection:
It involves hatching of the embryonarted eggs after their
deposition in the perianal area and subsequent migration
back into the rectum and large intestine.
Clinical features
The first recognizable symptom is pruritus as the worms
emerge from the anus to the perianal skin.
Itching followed by scratching which predispose to
secondary bacterial infection.
The infection also induce sleep disturbance especially in
children.
In some cases, no symptoms appear.
Diagnosis:
The eggs are recovered from perianal skin by using scotch
tape technique and examined microscopically.
The technique preferably done at night or in the early
morning before bathing.
The eggs can't be seen in the stool, although the adult
female can be seen in the stool or near the anus.
Treatment:
A Single dose of mebendazole 100mg or albendazole
400mg or pyrantel pamoate 10mg/Kg .These drugs kill
only the adult worm in the colon but not the eggs, so the
treatment should be repeated after 2 weeks to kill any
adult worms that might have hatched from eggs present at
the time of initial treatment.
All family members should be treated. During this period
all night clothes and bed linen are laundered .Finger nails
must be kept short and hands washed carefully before
meals.
Reinfection after treatment is very common.
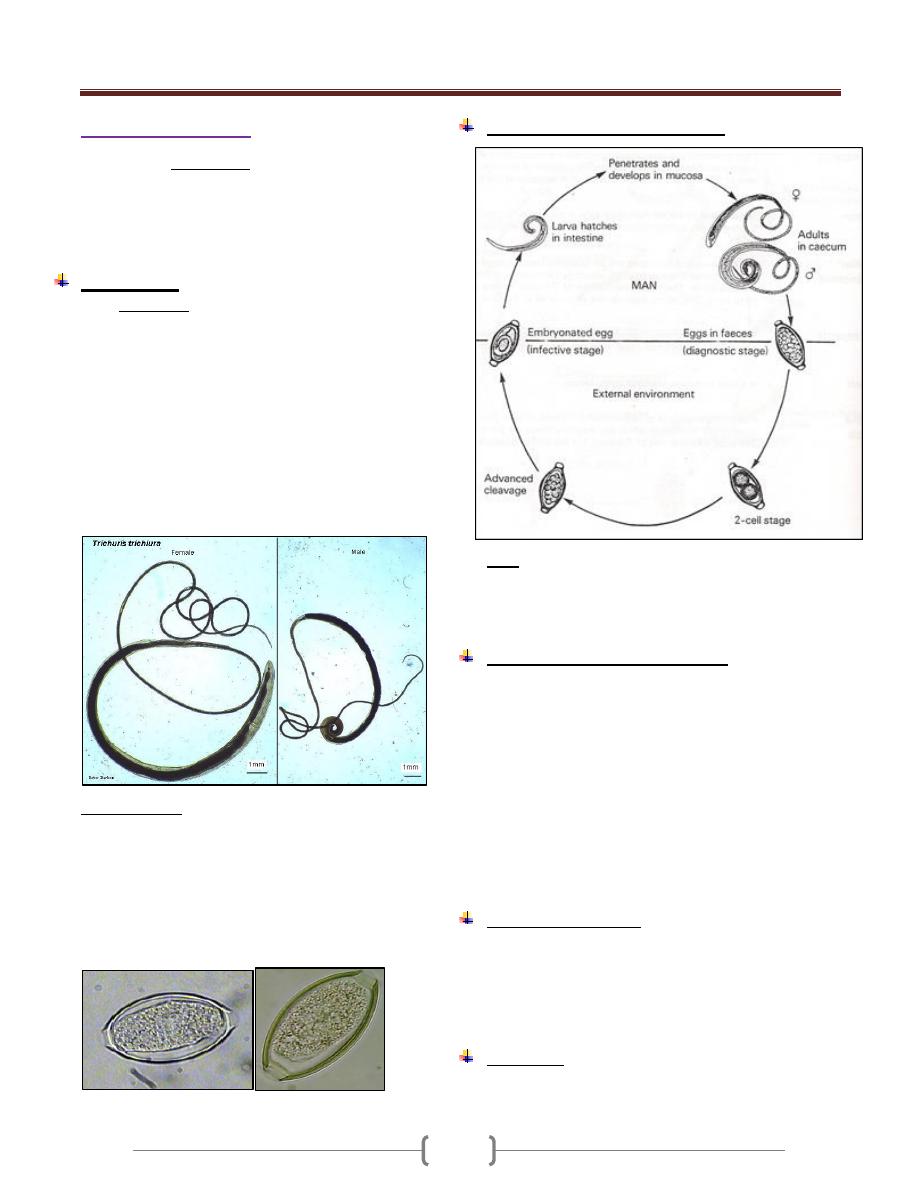
Unit 3: Helminthes (Nematodes)
07
Trichuris trichiura
Disease called Trichuriasis
It is a cosmopolitan and prevalent in worm or temperate
moist climates. In endemic area, the highest prevalence
occurs in children in of early school age, and adult also
have a high rates of infection.
Morphology:
Adult whipworms are thread like in their anterior 3/5 of
the body and wider in the posterior portion.
The anterior end is threaded into the mucosal epithelium
of the cecum or the appendix but when the worm present
in large number , the worms are distributed posteriorly
throughout the colon , even in the rectum . Whipworms
live for several years.
The Male measures 30 – 45 mm. Its posterior end is
curved ventrally into a coil of 360˚ or more. The Female
measures 35-50 mm. Its posterior end is club shape.
Egg/mature female = 3000-6000 daily.
Egg morphology: Narrow, barrel –shaped about 25*55
µm, laid in an unembryonated condition (one celled –
stage) and requires about 4weeks outside the body to
reach the infective stage. They have a thin, transparent
inner shell, a brownish outer shell and a transparent blister
like prominence at each pole.
Eggs remain infectious for few months under favorable
condition but shorter time under unfavorable condition.
In saline In iodine
Life cycle of Trichuris trichiura
Note:
1-All stages of development occur in the intestine
2-Development of Trichuris trichiura to the egg-laying
adult stage requires approximately 3 months.
Pathogenesis and clinical features
Light infections produce no symptoms.
Although adult worms burrow their hair like anterior end
into intestinal mucosa, they don’t cause significant anemia.
Heavy infection (tend to be between 1-5 years)
characterized by abdominal pain and distention, bloody or
mucoid chronic diarrhea, tenesmus, weight loss and
weakness and rectal prolapse which is due to increase
peristalsis that occur in an effort to expel the worms. The
whitish worms may be seen on prolapsed mucosa.
Entamoeba histolytica infection is frequently found in
association with trichuriasis.
Laboratory diagnosis
1) General stool examination to see the characteristic eggs.
2) Diarrheal or dysenteric stools contain eosinophils and
Charcot-Leyden crystals.
3) Adult or immature worms may be seen attached to the
prolapsed rectum or at sigmoidoscopy.
Treatment
Albendazole 400 mg/day for 3 days. Alternative is
Mebendazole 100 mg twice daily for 3 days or 500 mg once.

Unit 3: Helminthes (Nematodes)
07
Ascaris lumbricoides
The disease is called Ascariasis
It is the largest intestinal nematodes
With exception of Enterobius vermiculartis, Ascaris
lumbricoides is the most prevalent of all human
roundworms and occurs endemically in all parts of the
world except in cold, dry climates.
Morphology
Adult worm is elongated, cylindrical and tapers both
anteriorly and posteriorly to relatively blunt conical ends.
The heads is provided with three fleshy lips.
The mature male worm measures 12-31 cm in length by
2-4 mm in greatest diameter.
Its posterior end is somewhat curved ventrally.
The female measures 20-35cm in length by 3-6 mm in
greatest diameter but in certain cases, up to 45 cm may b e
observed.
Daily egg production per female averages about 200,000.
Egg morphology (Fig 1 &Fig 2)
The fertilized egg (figure 1) at the time of oviposition is
spherical or subspherical, measures 65-75 µ m by 35-50 µ
m and consists of the following:
1) a coarsely granular , spherical ovum that usually doesn’t
completely fill the shell
2) A thin innermost membrane that is highly impermeable.
3) A relatively thick, colorless middle layer that is smooth
on both inner and outer surfaces.
4) An outermost, coarsely mammilated, albuminoid layer
laid down in utero serving as a protective membrane.
Figure 1 fertilized egg Figure 2 unfertilized egg
Female worms without males produce infertile eggs that
are markedly subspherical.Interrnally they contain a mass
of disorganized granules and globules that completely fill
the shell (Figure 2).
Fertile eggs are passed in one cell stage. They survive
putrefaction and can withstand desiccation and cold. At
22 – 33C˚, development to the infective stage larva
usually occur s in 3-4 weeks. Eggs remain viable in soil
for more than a year.
Life cycle (Figure 3)
Ingestion of emryonated eggs in food or water
contaminated with human feces , eggs hatch in the small
intestine , larva migrate through the gut wall into the
blood stream and then to the lungs. On about the 9
th
day
of infection, they, pass up the bronchi and trachea and are
swallowed. Within small intestine, they become adults 8-
12 weeks after exposure. They live in the lumen don’t
attach to the wall, and derive their substance from
ingested food.
Pathogenesis and clinical features
1) In the stage of larval migration, passage through the liver
and lungs in the initial infection provokes no remarkable
pathologic changes unless hundreds of larvae are
migrating simultaneously.
2) Large No. of larvae in subsequent infection lead to intense
tissue reaction in the liver and lungs even if few larvae are
involved.
Liver:
In the early stage, focal eosinophilic infiltration and
granuloma formation around and in the paths of migrating
larvae and general inflammation around the portal tract.
Later, fibrosis of the periportal and interlobular space.
The cardinal symptoms associated with Ascaris
pneumonitis consist of fever ( moderate – 40C),
productive cough, dyspnoea and high transient peripheral
eosinophila and Chest x-rays shows scattered, s hifting
mottling of the lungs (these picture is variable from day to
Unit 3: Helminthes (Nematodes)
07
day and spontaneously clears after a few days to 2 weeks).
Pulmonary infiltration and peripheral eosinophilia is
called Loffler's syndrome. Migrating larvae may also be
seen in the sputum. Pulmonary Ascariasis appears to be
most common and severe in endemic area and sometime
fatal, even in adults.
When adult worms present in the small intestine in small
No., usually cause no symptoms.
Average infection in children cause intermittent colic, loss
of appetite, and nervous symptoms.
The nutritional demands and space requirements of
massive infection may be great and the total mass of
worms may reach to 1 liter and may lead to nutritional
impairment especially in children.
From time to time worms are passed spontaneously,
unassociated with illness and these events are of no
special significance. If there is an febrile illness, the
worms migrate outward in both directions or aggregate in
closely packed masses that will obstruct the bowel.
Sometimes the worms may enter and block the biliary and
pancreatic ducts or sometimes reach the liver and form
liver abscess which is more common than that of
E.histolytica sometimes the worm may reach the lung
from the intestine or may reach the nasopharynx and
emerge from the nares.
NOTE:
1- The survival time of mature A.lumbricoides in human
intestine is short and not exceeds a year.
2- Ascariasis is frequently associated with whipworm
infection as well as diseases due to other causes.
Diagnosis:
In the early stage of disease, diagnosis is difficult unless
there is an immature worm passed.
Once the worm in the intestine and eggs are passed by the
mature worms, the diagnosis can be made with ease by
detecting eggs in the stool. Sometime the patient sees
adult worms in the stool.
Treatment:
Albendazole 400mg on1ce
Mebendazole 100 mg twice daily for 3 days.
Pyrantel pamoate.
Epidemiology:
1) Endemicity is maintained by fecal contamination of soil.
2) Eggs are relatively resistant to desiccation, and
embryonation takes place in clay soil as well as in loam.
3) Infective stages eggs remain viable for weeks, months, or
even years.
4) Dirt eating is responsible for heavy infection in small
children.
5) Hogs infected with Ascaris sum probably an occasional
source of intestinal ascariasis in man and the larva make
lung migration and sometimes develop to adult stage in
man.
6) Ascaris of human origin may develop to adult stage in
pigs.
Control
1) Anthelmintic drugs.
2) Home and community sanitation
3) Mass treatment repeated at intervals of 2 months – 2 years.
Unit 3: Helminthes (Nematodes)
07
Hook worms
Belong into 2 genera: Necator and Ancylostoma
Ancylostoma duodenale (Ancylostomiasis)
"Old world hookworm"
Morphology
Adult worm are grayish white or pinkish. The body is
narrowed anteriorly.The head has a slight bend dorsally.
The mouth (figure 1) is well developed, with a pair of
teeth on either side of the median line and a smaller pair
in the depth of the buccal capsule.
Figure 1: mouth part of adult worm (male and female)
Male measures 8-11 * 0.4-0.5 mm in width.
The posterior end (figure 2) is provided with a prominent
copulatory bursa that is broader than it is long and is
supported by rays having the following pattern for each
half: a dorsal, single at its root but bifurcated at the tip,
externodorsal ,arising from the root of the dorsal; three
laterals separated from one another and two ventral close
to each other.
Female measures 120-13*0.6mm in width and tapered at
the posterior end (figure 3). The anus lies ventrally near the
caudal tip and the vulvar opening is situated mid-ventrally
at the beginning of the posterior third of the body.
Female lies around 20,000 eggs daily. Man is probably
the only normal host of
Ancylostoma duodenale.
Eggs morphology (Fig 4)
The egg is broadly ovoid measures 60 by 40 m , have a
thin , transparent shell , and are in the 2-8 cell stage of
cleavage when evacuated.
Embryonation to the first rhabditiform larval stage takes
place in 24-48 hr on moist sandy loam in a shaded
environment and 25 c .The rhabditiform larva measures
0.25-0.3 µm in length by 17 µµm in maximu m diameter.
It feeds on bacteria and organic debris, grows, sheds its
cuticle and continues to feed and increase in size.
After 5-8 days the rhabditiform larva stop feeding,
becomes inactive, and transforms into a more slender
filariform larva which has closed mouth, an elongated
esophagus and a sharply pointed tail.
Life cycle (Fig 5)
In Ancylostoma duodenale, the filariform larva is adapted to
enter the body by the oral as well as by the skin. After skin
penetration , the larva carried by blood to the lungs ,
migrate into the alveoli and up the bronchi and trachea and
then swallowed to reach the small intestine and develop
into adults and attached to the wall of the intestine with the
aid of the teeth. They feed on blood from capillaries of the
intestinal villi.6 weeks is required from the time filariform
larva enter the skin until the worms become mature in the
intestine , copulate and begin to lay eggs which passed with
feces to repeat the cycle.
Figure 3 posterior
e nd of female
Figure 2 Bursa
(posterior end) of male
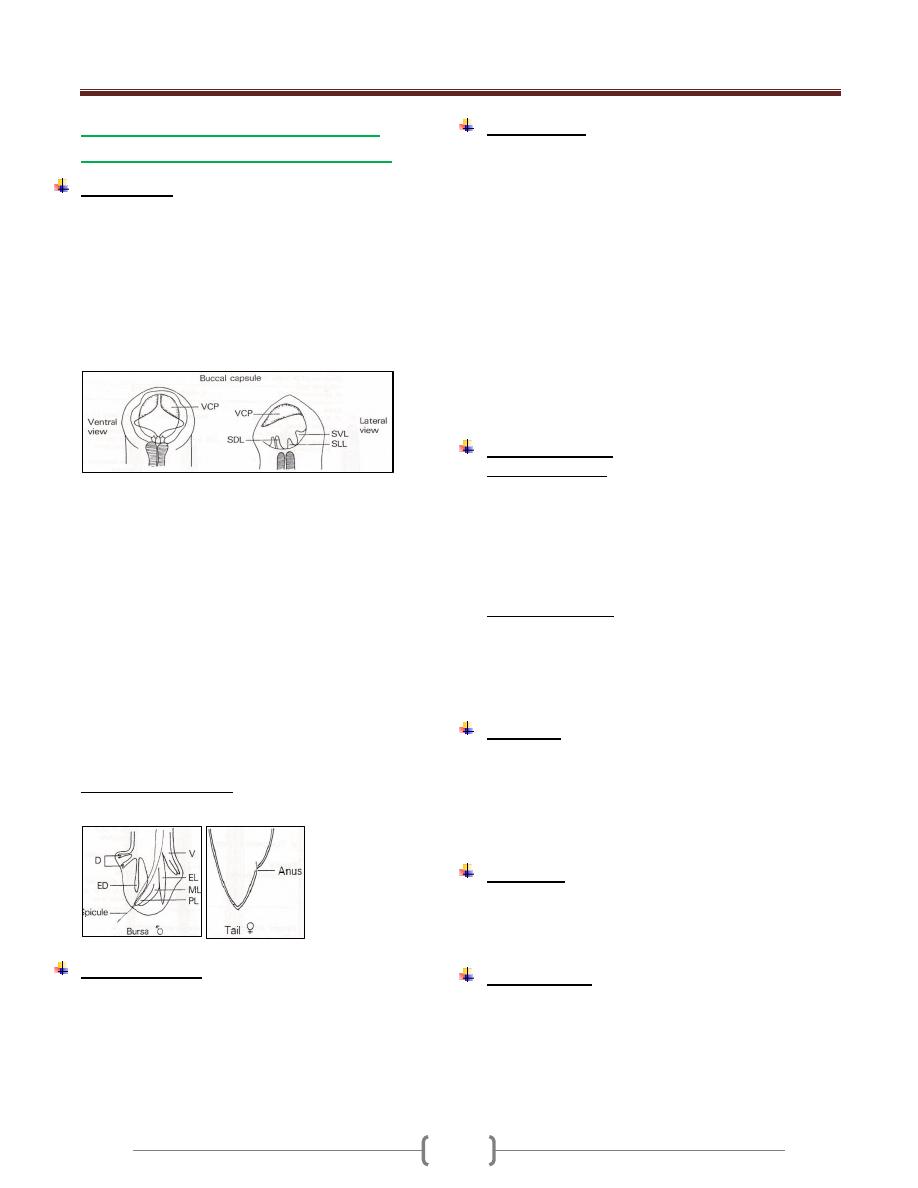
Unit 3: Helminthes (Nematodes)
07
Necatror americanus (Uncinariasis,
necatoriasis), New world hook worm.
Morphology
The adult worm is strongly flexed dorsally at the ant. end.
The small buccal (figure 6) capsule is provided with 2
ventral cutting plates,2 poorly developed dorsal
plates((median dorsal )and in the depth of the mouth
cavity a pair of short triangular lancets. A pair of cephalic
glands opens into the buccal capsule that secretes an
anticoagulant. The esophagus bears a pair of ventrolateral
glands and dorsal gland which secrete proteolytic enzyme.
Figure 6: mouth part of adult worm (male and female)
The male measures 7-9 mm in length by 0.3 mm in width.
The copulatory bursa is symmetrical. The supporting rays
for each half consist of a small separated dorsal,
bifurcated at the tip; a slender, unbranched externodorsal;
three larterals arising from a large fleshy trunk; 2 ventral
fused half way or more to the tip; and short prebursal ray.
The two copulatory spicules are long that are fused at
their outer ends terminating in a barb [figure 7: Bursa
(posterior end) of male].
The female measures 9-11 mm in length by 0.4 mm in
width. The vulvar opening is midventral anterior to the
midline (figure 8: posterior end of female). the female
lay 5000 eggs /day
Egg morphology (fig. 4)
Similar to that of Ancylostoma duodenale
Life cycle (Fig 5)
Similar to that of Ancylostoma duodenale but the
filatiform larva is adapted to enter the body only through
skin penetration of the epidermis , where they remain
relatively inactive for 1 or 2 days before moving deeper to
the cutaneous blood vessels.
Pathogenesis
1) Larva may cause allergic inflammation at the site of entry
through the skin and in heavy infection, passage through
the lungs may cause pulmonary eosinophilia.
Anemia as the worm suck blood from the intestine. blood
loss of Ancylostoma duodenale =0.15 ml /worm/day while
in Necatror americanus = 0.03 ml / worm/day. It is of
hypochromic microcytic anemia. In light and moderate
infection with good nutrition, blood loss can be
compensated .In severe infection anemia is inevitable.
2) A complete normal mucosal pattern of the small intestine.
3) Malabsorption is uncommon and it is not a characteristic
of pure hook worm infection.
4) Necatror americanus may survive as long as 18 years and
Ancylostoma duodenale lives for 1-5 years.
Clinical features
A. In acute infection :
1) Skin penetration by the larva cause allergic reaction
known as ground itch which is more severe in
Necatror americanus than Ancylostoma duodenale.
2) Pneumonia in case of heavy infection.
3) When the worm reach the small intestine, vomiting,
epigastric pain and sometime diarrhea.
B. In chronic infection.
1) Sign and symptoms due to iron deficiency anemia.
2) Hypoprotenemia due to long term complication of
IDA manifested by facial and peripheral edema.
3) Pica, ingestion of nonfood constituent as a result of IDA.
Diagnosis
General Stool Examination for eggs demonstration.
In a stool sample that is not fresh, the eggs may have
hatched to release rhabditiform larvae, which need to be
differentiated from those of Strongyloides stecolaris.
Test for occult blood in the stool in case of heavey infection.
Treatment
Albendazole single dose of 400 mg for both n and a
Mebendazole 100 mg twice daily for 3 days.
Correction of anemia.
Epidemiology.
Hook worms infection is widespread in tropics & subtropics.
Ancylostoma duodenale is endemic in the Far East and
Mediterranean coastal and in Africa
Necatror americanus endemic in west, east and central
Africa and central & south America as well as in Far East.
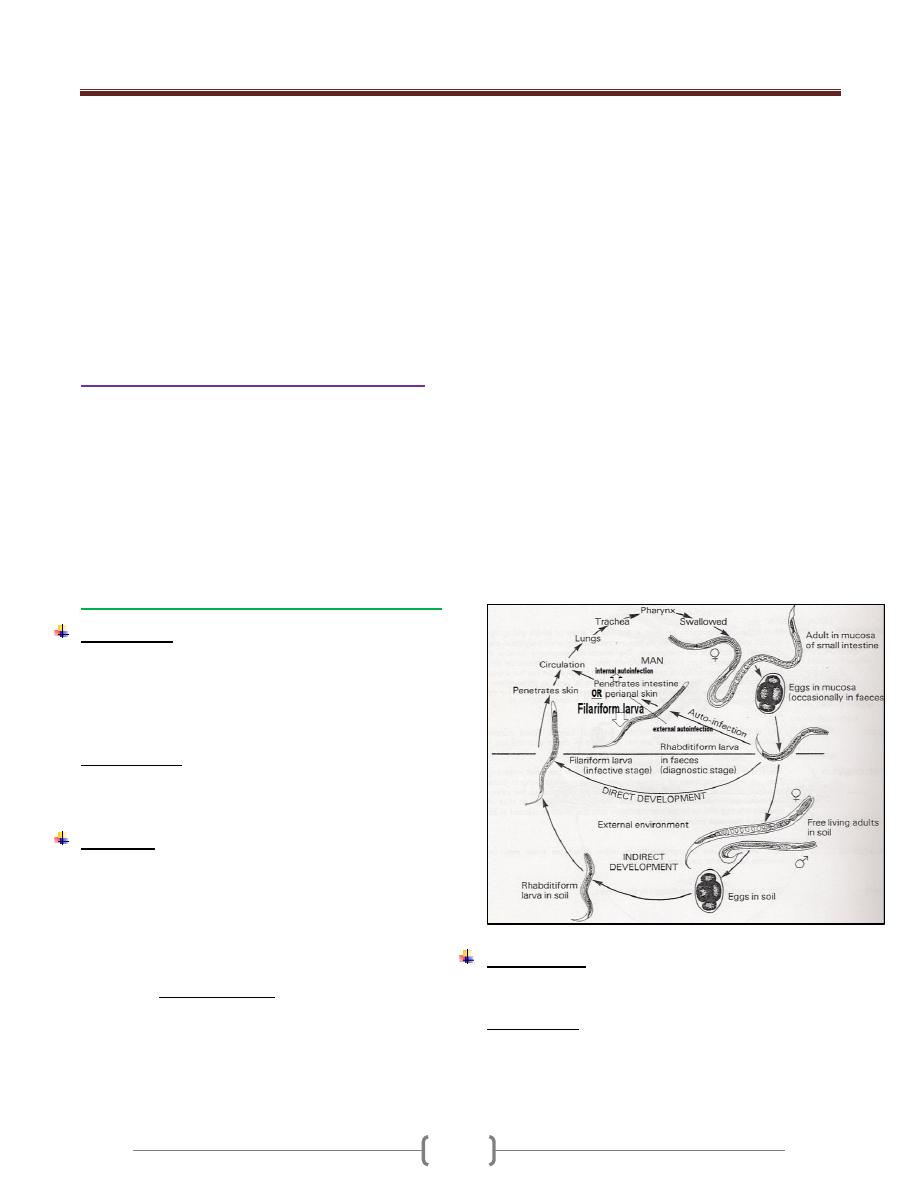
Unit 3: Helminthes (Nematodes)
08
A Human Hookworm Vaccine is currently being
developed by the Sabin Vaccine Institute & is in phase 1
clinical testing.
The candidate vaccine is comprised of two recombinant
antigens known as Na-GST-1 and Na-APR-1, each of
which is an important parasite enzyme required for
hookworms to successfully utilize host blood as a source
of energy. Na-GST-1 is a 24 kDa recombinant N.
americanus glutathione- S-transferase , while Na-APR-1
is a 45 kDa recombinant N. americanus aspartic protease.
Strongyloides and other Rhabditoidea
The rhabditoidea is a large group containing mostly,
small, free-living forms, some of which may be
encountered as pseudoparasites in human feces, urine,
gastric washing, or sputum. The parasitic members of
rhabditoidea are unique in that they have an alternation of
free-living and parasitic generations. Adults of the free
living generation are dioecious, while in the parasitic
generation the adult is parthenogenetic.
Strongyloides stercoralis (Strongyloidiasis)
Morphology
The slender parasitic female measures up to 2.7 mm in
length by 30- 40 µm in diameter.
The normal habitat is the mucosal epithelium of the upper
small intestine
Reproduction is parthenogenetic.
Egg morphology
Thin- shelled ovoid eggs, 50-58 µm by 30-34 µm are laid
in the epithelium .Each female producing < 50 eggs /day.
Life cycle
There are direct and indirect developments.
The eggs after being laid by the female, they undergo
development and are sloughed into the lumen of crypts of
lieberkuhn, where the first stage rhabditoid larva hatches.
Larvae then migrate into the intestinal lumen and are
evacuated in the stool. If deposited in a warm, moist,
shaded, site, direct development to the third stage
filariform larva may occur 24-36 hrs in the fecal mass or
in the soil. This larva initiates infection by skin
penetration.
Under certain condition, the first stage larva develops to
the infective stage within the intestinal tract , and the larva
can reinfect the host by penetrating the mucosa of the
colon without leaving the body (internal autoinfection) or
by penetrating the perianal or perineal skin after being
passed in the stool (external autoinfection). Autoinfection
occurs at low level in healthy individual and at high level
in immunodeficient patient resulting in a disseminated,
fatal infection.
After the first stage larva reaches the external
environment in feces, it will develop to free living
rhabditoid adults in 2-4 days (indirect development). The
male which is about 0.9 mm long with caudal end curved
ventrally. The female is about 1.2 mm long and contains
up to 28 eggs in its two uteri. After the eggs are deposited
by the female, they rapidly develop to the infective
filariform larval stage.
On contact with skin, the filariform larvae (in direct or
indirect development ) penetrate the epidermis to the
small blood vessels and are carried to the lungs, then they
reach the pulmonary capillaries , break out into the
alveoli, then via trachea, to the intestine, where they
invade the epithelium of the glands , molt twice, and
reach maturity in about 2 weeks. The most common level
of the intestine parasitized by Strongyloides stercoralis is
the duodenum, followed by the jejunum.
Pathogenesis
Minor local lesions occur on penetration of the larv a into
the skin.
Larva currens: a transient itchy linear urticarial wheels
across abdomen and buttocks.
The pulmonary response to larval migration is not so
severe but in heavy infection, pneumonitis may occur and

Unit 3: Helminthes (Nematodes)
00
the larva may be seen in sputum and even s ometimes the
eggs or adult form may be seen in sputum.
The adult female produces extensive ulceration and
sloughing of the mucosa and fibrosis and inflammatory
infiltration of the submucosal layers and granulomas
surrounding the larvae and these pathological changes
may be found in other affected organs.
Eosinophilia of 10-40%.
Total serum IgE is usually elevated.
Clinical features
Entry of the larva through the skin produces mild needling
sensation.
Pneumonitis may be produced by the larvae but it is less
severe than that of ascariasis.
The adult in the small intestine may give rise to no
demonstrable symptom or to moderate to severe diarrhea.
Malabsorption syndrome with steatorrhoea may occur.
Heavy infection may give rise to symptoms similar to
duodenal ulcer.
If the GIT and lungs are involved, the condition is
referred to as the hyperinfection syndrome which
present with dyspnoea, fever, GIT symptom, wheezing,
hemoptysis and cough.
When the numbers of migrating larvae are so great as to
injure other organ such as the liver , heart , adrenals,
pancreas , kidneys or central nervous system , this
referred as disseminated strongyloidiasis , seen
primarily in patients whose normal defenses have been
compromised e.g. HIV, chemotherapy, corticosteroid. It is
fatal unless diagnosed and treated early.
Diagnosis
Finding larvae in the stool. Repeated examination is
required as the excretion of the larvae is intermittent.
Treatment
- Ivermectin 200 µgm /Kg single dose.
- Albendazole 15 mg/Kg twice daily for 3 days. In
hyperinfection syndrome, 400 mg for 15 days.
S trongyloides
Hookworm
2
nd
stage larva
a) Rhabditiform esophagus
with a small buccal capsule.
a) Rhabditiform esophagus
with a large buccal capsule
b)Genital premordium large
b) Genital premordium small.
3
rd
stage larva
a) Esophagus 40% of the
length
a) Esophagus 25% of the
length
b) No sheath.
b) Sheath.
c) Tail forked
C) Tail pointed
Trichostrongylus sp.
The adults are 5-10 mm in length and are located in the
Small intestine.
They are mostly parasites of ruminants and infect humans
living in close proximity to these animals
Infection may occur by ingestion of the infective larvae or
by penetration of the skin.
The eggs are similar to that of hookworms but the ends
are more pointed and the ovum is passed in an advanced
cleavage stage.
Clinical features
Light infections are a symptomatic. In heavy infections
there may be anemia, abdominal pain and diarrhea.
Diagnosis
Eggs in the stool.
Trichinella spiralis (Trichinosis)
Trichinella spiralis is a parasite of rats and pigs and is
transmitted to humans by eating partially cooked pork
meat. The main tissue invaded is the striated muscle.
Morphology
The adult is minute, barely visible to the unaided eye. The
male measures 1.4-1.6 mm in length by 30-40 µ m in
diameter. The cloacal opening is terminal and is guarded
by a pair of conical papillae.
The female is viviparous measures 2.2 -3.5 mm by 50-60
µm in diameter. The vulva lies midventrally approximately
one fifth the body lengths from the anterior end.
Clinical features
A few days after eating undercooked meat, the patient
experiences diarrhea followed 1-2 weeks later by fever,
muscle pain, periorbital edema and
eosinophilia.Subconjunctival hemorrhage. Signs of
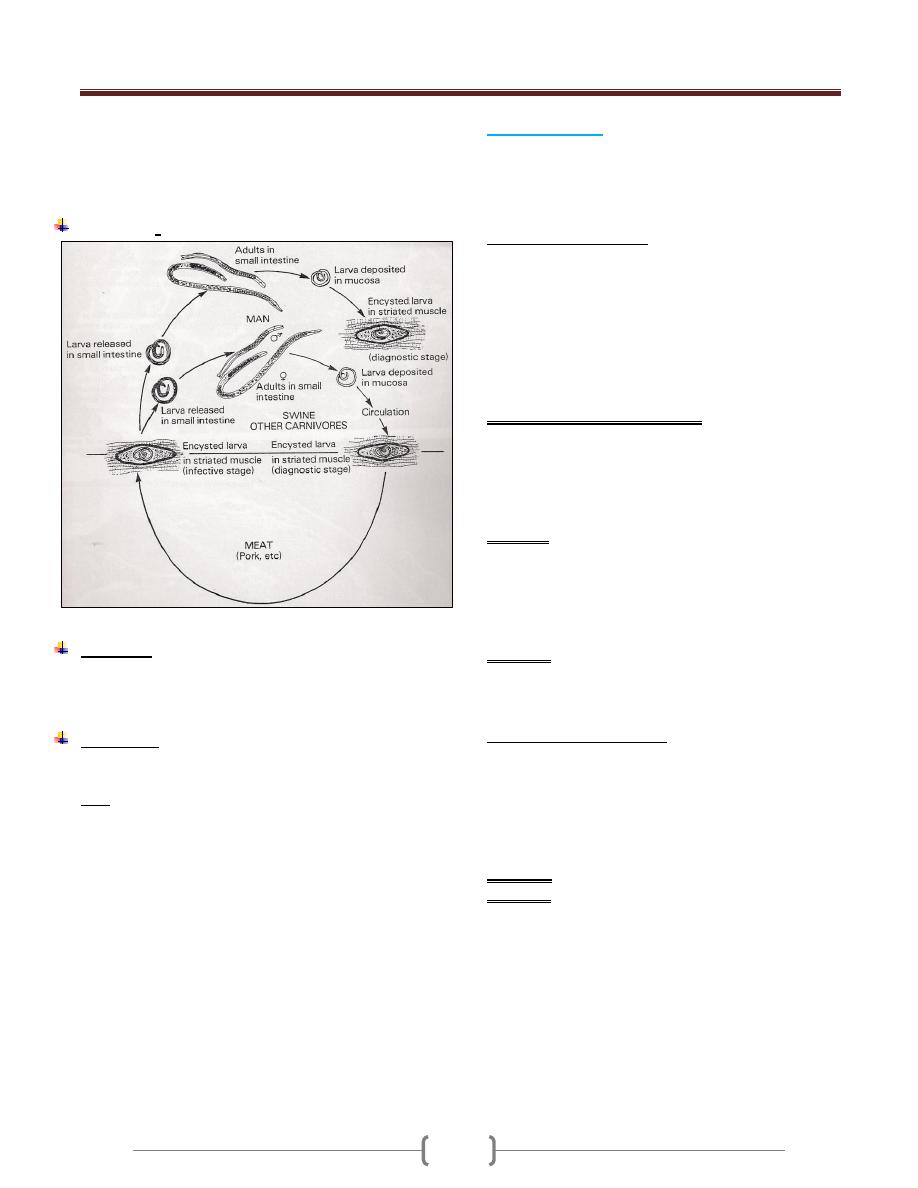
Unit 3: Helminthes (Nematodes)
06
cardiac and central nervous system disease are frequent
because larvae migrate to these tissues as well. Death
which is rare is due to congestive heart failure (CHF) or
respiratory paralysis.
Life cycle:
Diagnosis
1) muscle biopsy reveals larvae within striated muscle
2) Serological tests, (bentonine flocculation test), become
positive 3 weeks after infection.
Treatment
- Albendazole 20 mg/Kg for 7 days
- Steroid to control the effects of acute inflammation.
Note: there is no established specific therapy for tissue
phase of Trichinosis.
Larva Migrans
It is a term applied to the migration of larval helminths in
hosts that are suitable for long survival but are unsuitable
for their development to the mature adult stage. There are
2 types of larva migrans:
1) Visceral larva migrans
Toxocara canis is the major cause of visceral larva
migrans
The definitive host for T.canis is the dog .Humans ingest
soil containing the eggs, which hatch into larvae in the
small intestine. The larvae migrate to many organs
especially, the liver, brain, and eyes. The larvae
encapsulated and die. The life cycle is not completed in
humans; humans are therefore accidental dead –end host.
Pathogenesis and clinical features
- Granuloma around the larvae as a result of delay type
hypersensitivity reaction (DTH).
- The most serious clinical finding is blindness associated
with retinal involvement, fever, splenomegaly and
eosinophilia.
Diagnosis
- Definitive diagnosis depends on visualization of the
larvae in tissue.
- Serologic tests are commonly used
- The presence of hypergammaglobulinemia and
eosinophilia support the diagnosis.
Treatment
- Albendazole or mebendazole.
- Many patients recover without treatment.
2) Cutaneous larva migrans
It is caused by the filariform larvae of Ancylostoma
caninum (dog hookworm) and Ancylostoma braziliense
(cat hookworm).
The larvae penetrate the skin and migrate through the
subcutaneous tissue causing an inflammatory response.
The lesions (creeping eruption) are extremely pruritic.
Diagnosis: clinically.
Treatment: Oral or topical thiabendazole is effective.
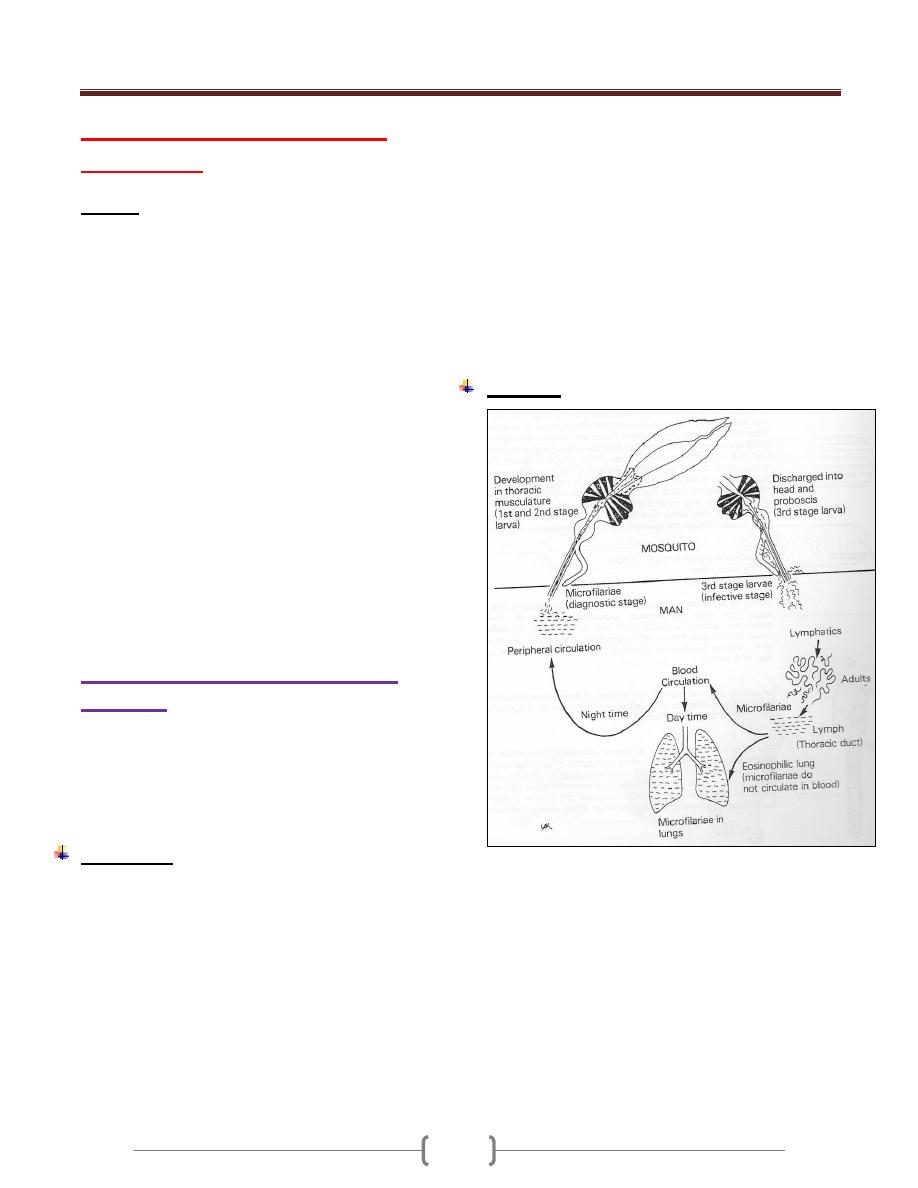
Unit 3: Helminthes (Nematodes)
06
Lecture 6 - Blood and tissue
nematodes
Filariae
The filariae, members of the superfamily filariodea .These
nematodes have a unique stage in their life cycle, the
microfilaria, which distinguishes them as a group.
The adult worm lives in various tissues of the definitive
host including the body cavities, subcutaneous tissues,
and the lymphatic and vascular systems.
The microfilariae produced by the female worm are
motile, and those of some species migrate into the blood
stream; others accumulate in the skin.
Host to host transmission is accomplished when the
microfilaria is ingested by a blood sucking arthropod
intermediate host, in whose tissues it develops to the
infective stage; when the infected arthropod again takes a
blood meal, the larva invades the tissues of the definitive
host through the bite site and develops to the sexually
mature adult stage.
Eight species of filariae are endemic in various parts of
the world, including, Wuchereria bancrofti, Brugia
malayi, Brugia temori, Loa loa, Onchocerca volvulus,
Mansonella ozzardi, Mansonella perstans, and
Mansonella streptocerca
Wuchereria bancrofti (Bancroftian
filariasis)
The disease is widely distributed through the tropical area
of Africa, Asia and Latin America.
Man is the only natural definitive host.
Intermediate host is the female mosquitoes especially
anopheles and culex species.
Morphology
Adult worms found in the lymphatic vessels .They are
small, thread like and have a smooth cuticle.
Females are viviparous. Young embryo fills the uterine
tubes and is confined in thin, hyaline, ovoid shells .The
female discharge motile microfilaria.
Microfilaria from peripheral blood measures 244-246 µm
in length by 75-100 µm in diameter. The anterior end is
bluntly rounded, the posterior end is tapered and the body
is enclosed in large sheath. In stained film the body
appears to be composed of no more than a column of dark
–staining nuclei that do not extend to the end of the tail.
The motile microfilaria make their way from lymphatic to
the blood stream and in most regions of the world,
Wuchereria bancrofti
microfilaria circulate in the peripheral; blood with a
marked nocturnal periodicity and the number is high
between 10 p.m-2 am and are scant , usually absent
during day light hours when they tend to accumulate in
the viscera and particularly in lungs.
Diurnal subperiodic in pacific region, microfilaria show a
marked periodicity but tend to circulate continuously with
some increase in number during the afternoon and
evening hours.
Life cycle
Microfilaria ingested by an appropriate mosquito vector
.within the mosquitoes the microfilaria produce infective
(third-stage) larvae that are transferred to human with the
next bite. After they enter the skin, the larvae move to the
afferent lymph vessels and subcapsular sinuses of the
regional lymph nodes. The time necessary for the worms
to grow to sexual maturity is several months. The mature
adult produces microfilaria which circulates in the blood,
chiefly at night, and are ingested by bitting mosquitoes to
repeat the cycle
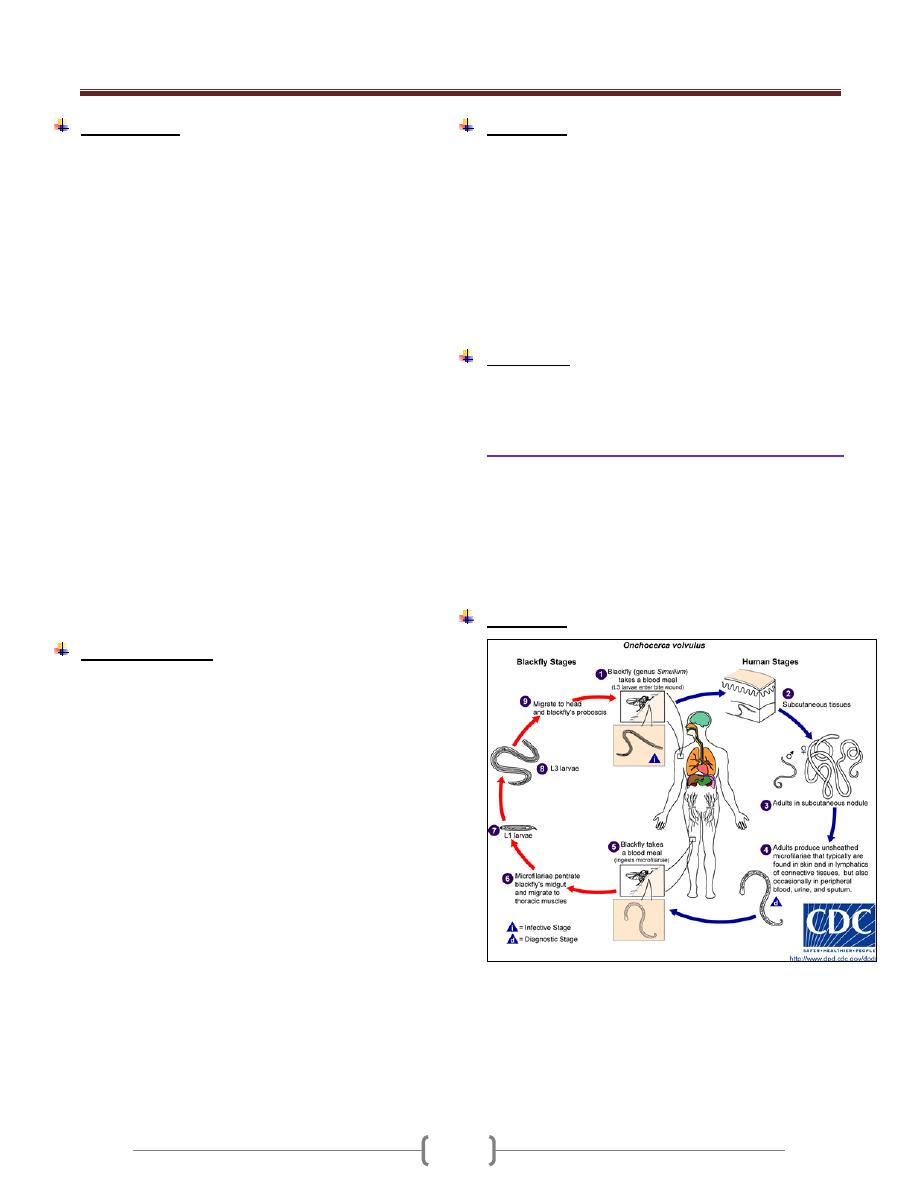
Unit 3: Helminthes (Nematodes)
67
Pathogenesis
Adult worms are found in lymph vessels throughout the
body but principally in or around axillary, epitrochlear,
inguinal and pelvic nodes and the lymphatic distal to
them, as well as, in testis, epdidymus, and cord.
In acute stage: symptoms results from infection with
wucheraria bancrofti are caused primarily by locally and
systemic sensitization and tissue reaction to the parasite.
There is an accumulation of histeocyte, epitheloid cells,
lymphocytes, plasma cell, giant cell and eosinophills in
the lumen of the vessel around the worms.
Attacks of lymphangitis and lymphadenitis as a result of
reactions to the products of developing adult or dying
worms or from bacterial or fungal infection. These attacks
are marked by retrograde lymphadenitis, funiculitis,
epidydimytis or orchitis with fever.
Repeated attacks if lymphangitis lead to thickening of the
affected lymphatic vessels which may become
incompetent leading to lymphodema and the lymphatic
vessels tend to become fibrosed after the repeated attacks.
In chronic lymphodema, there will be hyperplasia of the
connective tissue and infiltration of plasma cells,
macrophage, and eosinophils. Finally woody indurations
of the tissues may take place with thick and verrucous
change in the skin leading to elephantiasis.
Clinical features:
1) Asymptomatic: patient is heavily infected without
showing any signs of the disease other than large number
of microfilariae in the circulating blood.
In some patient, the presence of even few numbers of
worms provokes severe reaction and this is character of
immunologically naïve patient rather than those who are
native to the endemic area.
2) Acute phase: characterized by fever, lymphangitis,
lymphodema, and fever. These attacks remain high for 1-
2 days and gradually subside over 2-5 days period.
3) Chronic phase: repeated acute attacks lead to Odem and
fibrosis of the leg and genitalia (scrotum) lymphatic
vessels. Elephantiasis occurs mainly in those with
repeated attacks over long period of time.
4) Tropical pulmonary eosinophilia is characterized by
coughing and wheezing especially at night .These
symptoms are caused by microfilariae in the lung that
elicit immediate hypersensitivity reaction characterized by
high IgE concentration and eosinophilis .Peripheral blood
don’t show any parasites.
Diagnosis:
1) Thick blood smears taken from the patient at night reveal
the microfilariae.
2) Antigen detection using an immunoassay for circulating
filarial antigens constitutes a useful diagnostic approach,
because microfilaremia can be low and variable. A rapid-
format immunochromatographic test, applicable to
Wuchereria bancrofti antigens, has been recently
evaluated in the field.
3) Molecular diagnosis using polymerase chain reaction is
available for W. bancrofti
Treatment:
Diethylcarbamazine is effective only against microfilariae
No drug therapy for adult worms is available.
Onchocerca volvulus (Onchocerchiasis)
It is endemic in Africa, Central America and small area in
Yemen.
The disease is a major cause of blindness and called (river
blindness), because the black flies develop in rivers and
people who live along those rivers are affected. Infection rate
are often greater than 80% in areas of endemic infection.
Life cycle:
Human are infected when the female blackfly ,simulium,
deposits infective larvae while bitting .The larvae enter
the wound and migrate into the subcutaneous tissue where
they differentiate into adult ,usually within dermal
nodules. The female produces microfilaria that is ingested
when another blackfly bites. The microfilaria develops
into infective larvae to complete the cycle.
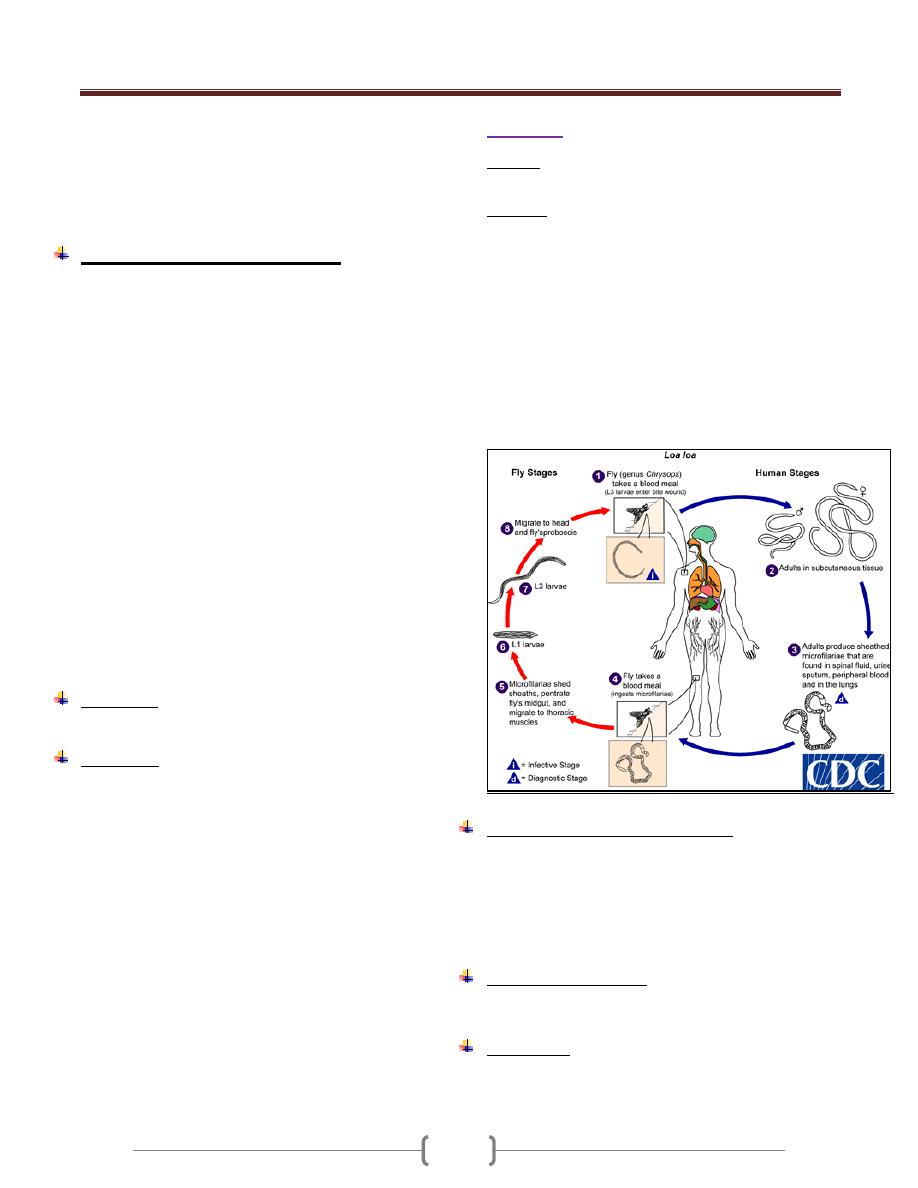
Unit 3: Helminthes (Nematodes)
67
The microfilaria lacks a sheath; the column of the nuclei
doesn't extend to the end of the tail.
The microfilaria typically found in the upper layers of the
dermis, frequently in the urine, in cytology smear and
rarely in blood.
Pathogenesis and symptomatology
The presence of the adult worm causes a local cellular
reaction of a fibrotic nature; results in encapsulation of the
worms. There may be only a single nodule or many.
In Africa the nodules are common about the pelvis (iliac
crest), trochanters and the lateral chest wall.
In America, nodules are more common on the head.
The dispersal of microfilaria into the dermal layers of the
skin throughout the body causes various types of acute
and chronic skin lesions
Sowda: localized onchocerchiasis in Yemen
Lymphadenopathy is commonly associated with
onchocerchiasis.
In some areas of Africa, hanging groin: a sac like
projection of loose, atrophied skin containing a mass of
large, fibrotic lymph gland.
Ocular complications are the most serious consequences
in some area of Africa and central America in which the
microfilaria invade all tissues of the eye, producing
congestion, hemorrhage and degeneration of the tissues as
well as degenerative changes in the optic nerve.
Diagnosis:
Biopsy of the affected skin reveals microfilaria.
Treatment
Ivermectin against microfilaria but not the adult.
Suramin kills adult worm but it is toxic and is used
particularly in those with eye disease.
Loa Loa
Disease: Loiasis.
The disease is endemic in tropical central and West Africa.
Life cycle
Humans are infected by the bite of the deer fly (mango
fly), Chrysops), which deposits infective larvae on the
skin. The larva enters the bite wound, wanders in the body
and develops into adults. The females release
microfilariae that enter the blood, particularly during the
day. The microfilariae are taken up by the fly during a
blood meal and differentiate into infective larvae, which
continue the cycle when the fly bites the next person.
The microfilaria is sheathed and exhibits a diurnal
periodicity in the peripheral blood .The column cells
(nuclei) extend to the tip of the tail.
Pathogenesis and clinical features:
There is no inflammatory response to the micofilariae or
the adults, but a hypersensitivity reaction causes transient
localized, non-erythematous, subcutaneous edema
(Calabar swelling) .The most dramatic finding is an adult
worm crawling across the conjunctiva of the eye, a
harmless but disconcerting event.
Laboratory diagnosis:
Visualization of the microfilariae in a blood smear
No serological test.
Treatment:
Diethylcarbamazine eliminates the microfilariae and may
kill the adults.Worms in the eye require surgical excision.
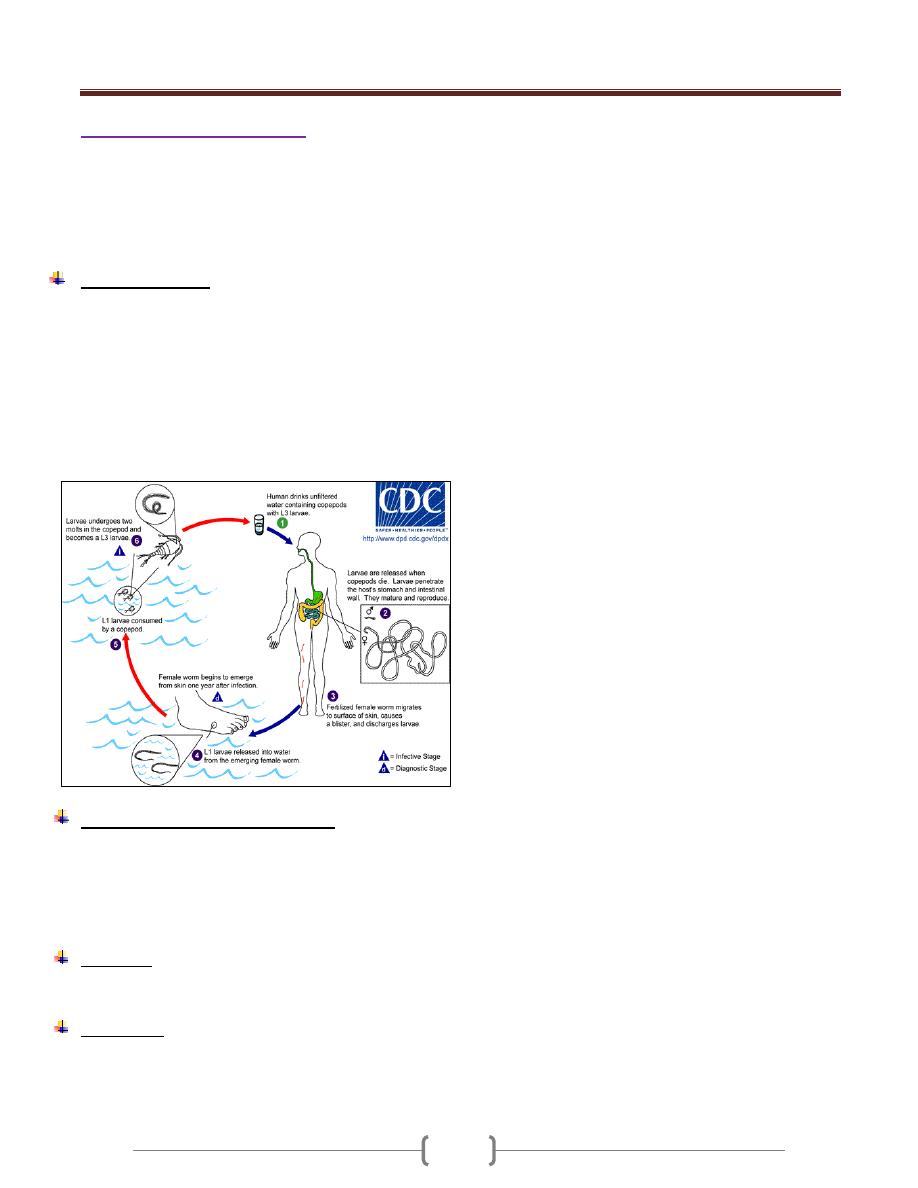
Unit 3: Helminthes (Nematodes)
67
Dracunculus medinensis.
Guinea fire worm
Dracunculiasis
The disease occurs over large areas of tropical Africa, the
Middle East, and India. Though it is sometimes classed
with filarial worms, Dracunculus is not a true filaria.
Life cycle:(Fig.1)
Humans are infected when tiny crustaceans (copepods)
containing infective larvae are swallowed in drinking
water. The larvae are released in the small intestine and
migrate through the intestinal wall into deep somatic
tissue, where they develop into adults. Meter –long adult
females cause the skin to ulcerate and then release motile
larvae into fresh water. Copepods eat larvae, which molt
to form infective larvae .The cycle is completed when
these are ingested in the water.
Pathogenesis and clinical finding:
The adult female produces a substance that causes an
inflammation, blistering, and ulceration of the skin,
usually of the lower extremities. The inflamed papule
burns and itches, and the ulcer can become secondarily
infected.
Diagnosis
Is usually made clinically by finding the head of the worm
in the skin ulcer.
Treatment:
Gradually extracting the worm by winding it up on a stick
over a period of days.Thiabendazole or metronidazole
makes the worm easier to extract.

83
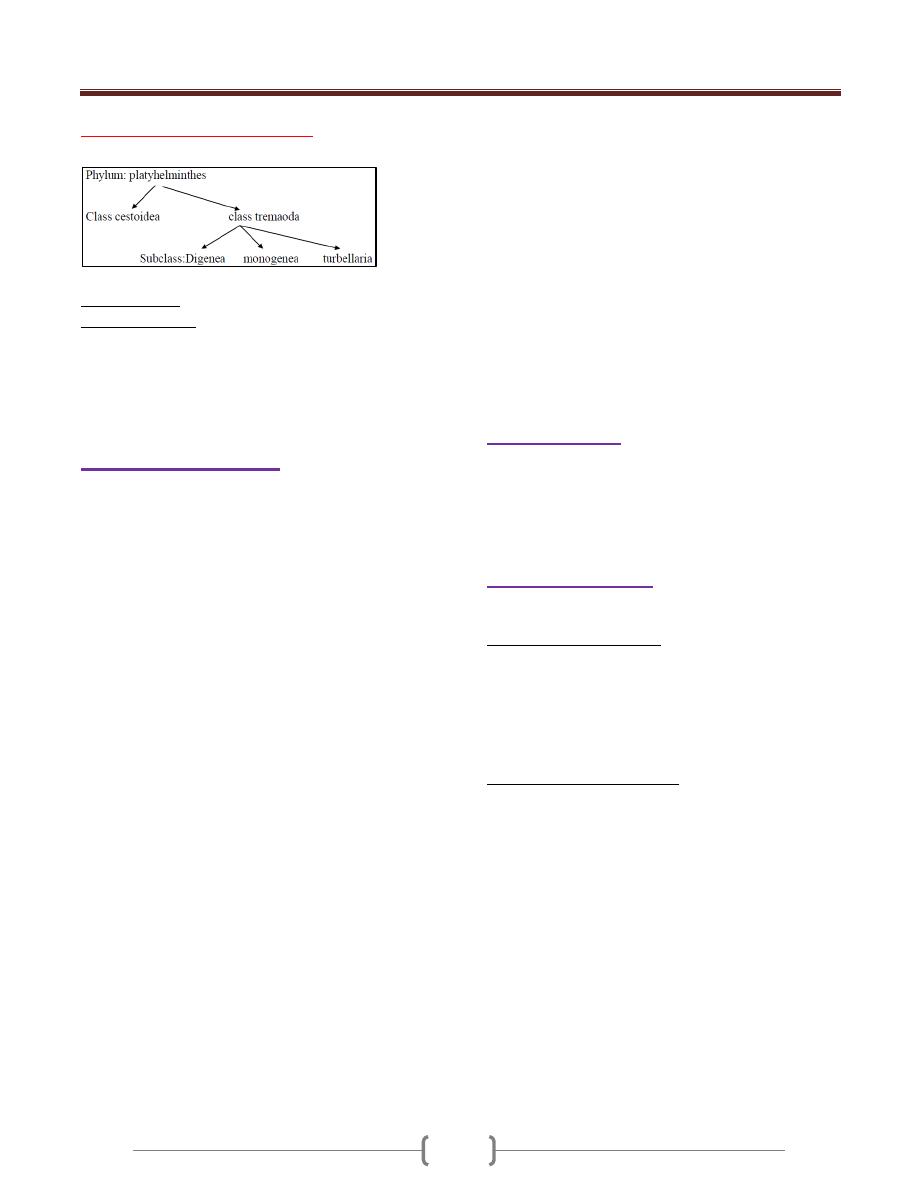
Unit 3: Helminthes (Trematodes)
48
Lecture 1 - Introduction
Class: tremaoda
Subclass: Digenea
Only Digenetic trematoda produce infection in man.
Adult trematodes are parasites of vertebrates also called
Flukes.
Trematades are hermaphrodites or monocious ,but
schistosomes are unisexual(diecious)
Life cycle of Trematodes :
Adult worm liberting fertilized egg in water hatch into
ciliated miracidium (1
st
larval stage) which is infective to
molluscan hos. In the mollusk hemolymph spaces,
transform to elongated sac ,1
st
generation sporocyst
(second larval stage )
Germ cell developed from the inner wall give either to
number of 2
nd
generation sporocyst in some species or to
radia (3
rd
larval stage) in other spp.
Asexual multiplication occurs in this stage & 2
nd
generation of organisms escape from the first & grow &
internally produce cercaria (4
th
larval stage)
The cercaria when mature emerge from the mollusk &
temporarily become free living
According to the nature of the tail ,different name are
given :
1) furcocercus :fork-tailed cercaria
2) Microcercus :short , stumpy ,rudimatory tail
e.g.paragonimus
3) lophocercus : large , floode e.g. clonarchis
4) pleurophocercus : long ,powerful tail & apair of
finfolds e.g.opisthorchis
Metacercaria: a stage of development between the
cercarial & adult stages which is an encysted cercaria
without a tail .so depending on a particular group the free
cercaria:
a) Becomes attached to the skin of the definitive host,
discards it ۥs tail & penetrate he mucous membr.
b) crawls onto an aquatic plant ,drop it ۥs tail ,round up &
encysts by covering itself with material secreated by
cystogenous glands e.g.:fasciola
c) or shed it ۥs tail & penetrate he tissues of aquatic
animal like fish e.g.clonorchis ,Heterophys or crab as
crayfish e.g.paragonimus or terrestrial animal
e.g.Dicrocoelium in which it become encysted. In the
later two types of development, the diffini.host
become infected after ingestion of the encysted
cercaria in uncooked veretable or animal tissues.
The mature worm: vary in size & shape .Some are large
& fleshy ;other are thin & flabby ;other are small but
blood inhabing fluks are delicately cylindrical .
There are 2 sucker :the oral sucker surrounding the moth
opening on the ant.end of the worm & vertral sucker
(acetabulum) (which is a cup shaped ,muscular by which
the worm attach to the host on the veritral surface
Digestive system:
Consist of a mouth then an esophagus that is provided by
with spherical or pyriform ,muscular pharynx then two
intestinal caeca which run parallel & blindly near the
post. End of the body .They may be simple or brunched or
they reunite to form a single tube.
Reproductive system:
All digenetic trematodes except schistosomes are
hermaphroditic
Male Reproducti ve system:
Consist of 2 testes from each one vas defferens arise ,join
to form vas deferens ,pass forward ,a swollen seminal
vesicle , prostate gland , muscular cirrus or penial organ ,
the cirrus sac surround these terminal male genital organ
which open into the commone genital atrium which
provided with genial pore.
Female Reproducti ve system:
Consist of single ovary ,oviduct seminal receptacle ,laurer ۥ
scanal (open on the dorsal surface ) ootype (which is the
chamber where the eggs fertilized)surrounded by Mehlis
gland (associated with egg shell).
Vitelliaria that contain yolk cells & shell gland lie along
the margin of the body & ducts from the gland open into
the ootype .Coiled uterus originate on the ant. face of the
ootype &proceed to the genial atrium.
Eggs are operculated ,except schistosome egg non -
operculated.
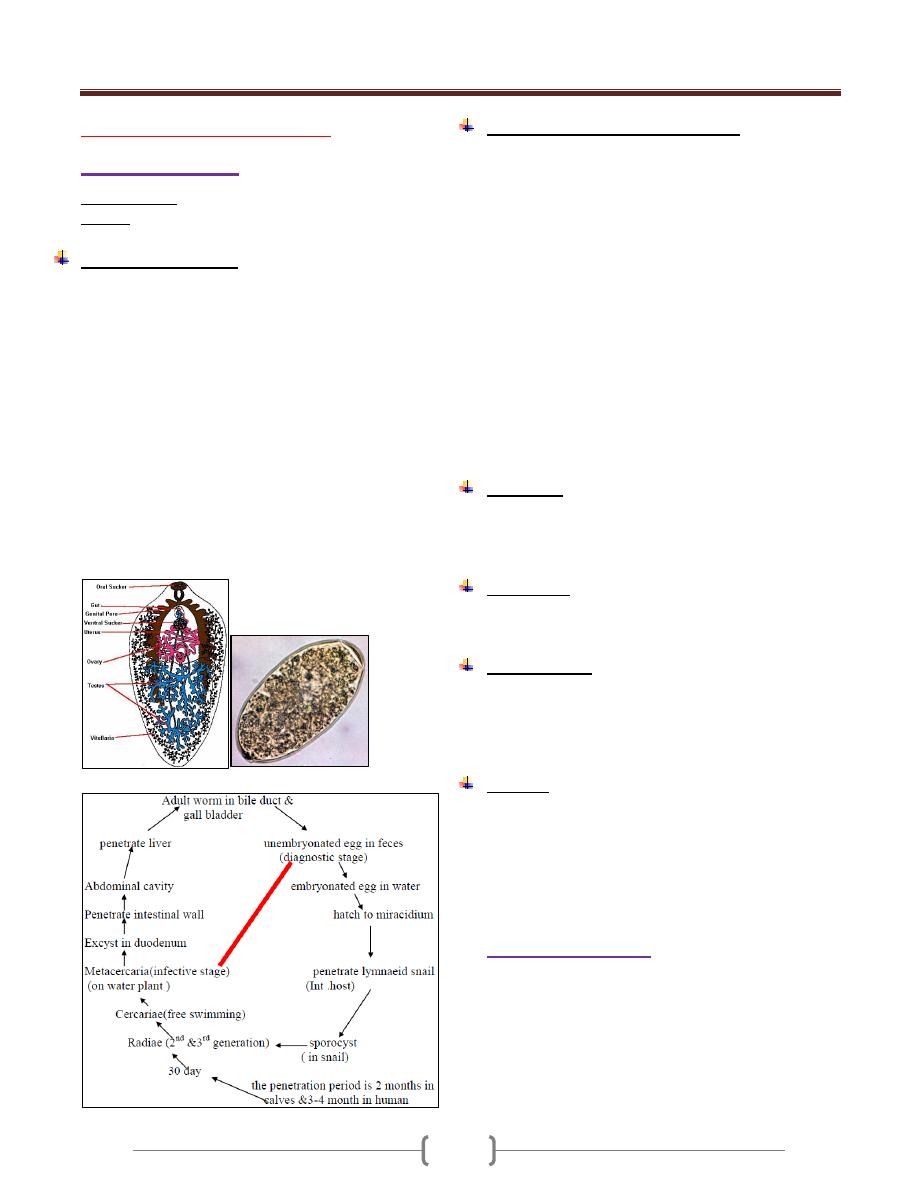
Unit 3: Helminthes (Trematodes)
48
Lecture 2 - Hepatic Flukes
Fasciola hepatica
Common name: sheep liver fluke.
Disease: fascioliasis hepatica
Biology &Life cycle:
The hermophordite adult fluks is leaf shaped & large 30
mm by 13 mm.
It is flattened, the ant.end forms aconical projection that
broadens at the sholders , than gradually narrowers
towards the post. End.
The intestinal caeca, testes, vitelline follicle are highly
branching.
There is short convoluted uterus.
The habitat of F.hepatica adult worm is the larger bile
ducts & gall bladder of sheep, goats & occasionally man.
Occasionally found ectopically in the peritoneal cavity or
other sites.
Eggs pass from the bile duct into the intestinal tract &
evacuated in the feces .It is large ,thin shell with
operculum.
Pathogenesis & Symptomatology:
Pass of the young worms through the hepatic parenchyma
to the bile ducts cause traumatic damage & intense
eosinophilic inflammation. In the larger bile passages they
produce hyperplasia of the biliary epithelium with
leukocytic infilteration & fibrosis of the ducts.
Early symptoms of human infections are:
Right upper quadrant abdominal pain, fever,
hepatomegaly, biliary colic, coughing, vomiting, Jaundice
also abdominal rigidity, diarrhea, fever, profuse sweating,
urticaria, eosinophilia ,macrocytic anemia ,empyema of
gall bladder, cholecystitis or choleithiasis , obstructive
Jaundice. Ectopic worm found in abscess pockets in
blood vs., lung subcutaneous tissues, brain, eye.
False fascioliasis: can occur when F.hepatica egg found
in feces after ingestion of infected liver of sheep, goat or
cattle, raw or cooked.
Diagnosis:
By recovery of eggs in feces .In case of false fascioliasis ,
egg ceased to appear in feces a few days after placing the
patient on a liver-free diet.
Treatment:
-Bihionol in oral dose is the drug of choise.
-Praziquantel is also effective.
Epidemiology:
Human infection is aquired by ingestion of raw aquatic
vegetation contaminated with metacercaria such as lettuce
& green salad.
Reservoir host is primarily the sheep
Control:
Health education of general public regarding the mode of
transmission of parasite & the danger of eating uncooked
or partially cooked & contaminated vegetables is the most
important measure.
Other measures are treated of sheep & other herbivorous
mammals.
Fasciola gigantica
Common name: giant liver fluke.
Disease: Fascioliasis gigantica.
1) is longer & more attenuated
2) with larger acetabulum
3) More ant. Position of the testes.
4) larger size of the egg 160-190
–
m by 70-9
---
m
5) The natural host is cattle, sheep.
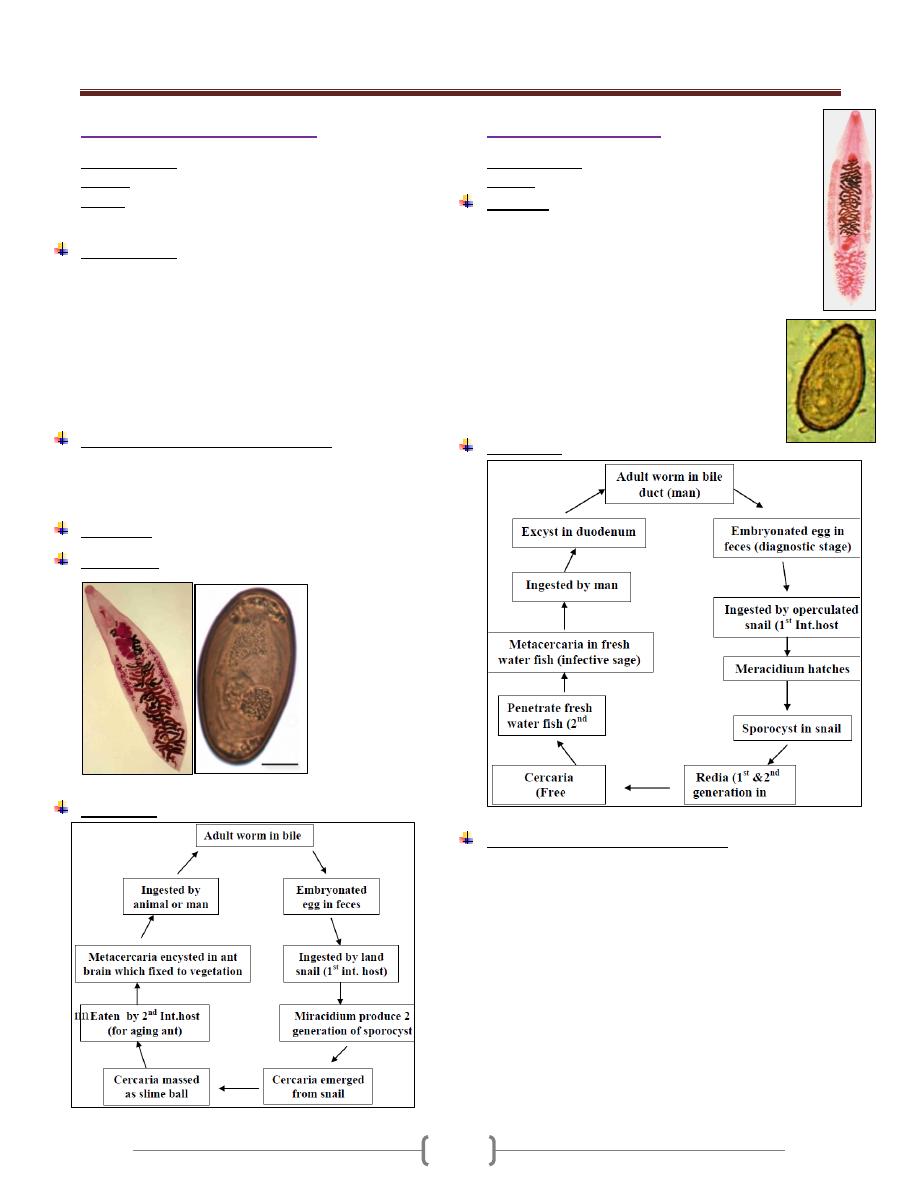
Unit 3: Helminthes (Trematodes)
48
Dicrocoelium dendriticum
Common name: Lancet fluke
Disease: Dicrocoeliasis
Habitat: It inhabits the biliary passage of man, sheep &
herbivorous animals .
Morphology:
Adult worm is lancet –shaped, flat, thin, transparent,
small (5-15) mm by (1.5-2.5) mm with smooth tegument.
There are 2 testes anterior to the ovary in the anterior half
of the body .Uterus coiled in the median field of the
posterior part of the body.
Egg: ovoid operculated, thick shelled, dark brown in
color, measuring (35-45) by (22-30) mm embryonated
when laid
Pathogenesis and symptomatology:
Similar to Fasciola hepatica but less marked
Symptoms: biliary colic, hepatitis, abdominal distress,
diarrhea, vomiting, chronic constipation.
Diagnosis: Examination of stool
Treatment: Praziquantel.
Life cycle:
Clonorchis sinensis
Common name: Chinese liver fluke.
Disease: clonorchiasis
Biology:
It lives in bile passage
Lanceolate, flate, transparent, pink in color (10-
25)mm long by (3-5) mm
Broad, smooth tegument, globose oral sucker, small
acetabulum.
Egg: Broadly, ovoid with thick, light,
yellowish, brown shell with distinct operculum
opposite a small knob, measure (27-35) µm in
length by (12-20) µm---- in diameter. fully
embryonated when discharge in faeces.
Life cycle:
Pathogenesis & Symptomatology:
Hyperplasia of biliary epithelium with dense fibrous
envelopment of the duct.
In heavy infection, there is fibrous thickening of the wall
with pressure necrosis of adjacent hepatic parenchyma.
The clinical onset was gradual or sudden with chills &
fever up to 40c˚
Liver is large & tender ,sometime there is congestive
spleenomegaly, eosinophilia counts ranged from 10 to 40%
Some weeks later the picture was one of cholecystitis
& hepatitis.

Unit 3: Helminthes (Trematodes)
48
Diagnosis:
Detection of eggs in direct fecal smear
by
concentration technique.
Duodenal or biliary drainage.
Treatment:
praziquantel is effective.
Epidemiology:
Infection is acquired by eating fresh water fish that is raw
,pickled in brine or rice wine ,
smoked or dried fish containing the encysted metacercaria
Control:
Cooking all fish that intended to be eaten.
Protection of fish ponds from contamination with night
soil.

Unit 3: Helminthes (Trematodes)
44
Lecture 3 - Intestinal Flukes
Fasciolopsis buski
Common name: Giant intestinal fluke.
Disease: Fasciolopiasis
In several conturies of south east Asia
Biology:
Large ,fleshy worm (20-75)mm long, (8-20)mm in width,
(0.5-3) mm thick
Tegument is spinose, oral sucker is smaller than the
nearby acetabulum. Small branched ovary, uterus is short,
convoluted .highly branched tests .Simple intestinal caeca.
Habitat: the worm lives attached to the wall of the
duodenum &jejunum of the man & pig.
Egg: large ,shaped-like hen ُ s egg (130-140 )--µ-m by
(80-85)
µm—, thin transparent shell , small operculum at
one end & are unembryonated when evacuated in the
host ُ s feces . .yellowish – brown in color
Life cycle:
Symptoms:
Include diarrhea and abdominal pain.
Heavier infection causes edema of legs, face, ascites,
eosinophilia. Some patients die of intestinal obstruction
and malnutrition.
Diagnosis
: by demonstration of eggs in stool.
Treatment
: Praziquantel is the drug of choice
Epidemiology:
Human beings acquire the infection by eating
contaminated raw water plants, especially
When peeling off the outer layers with their teeth. Rate of
infection is more in children.
Using of human excreta containing eggs to fertilize fields
of aquatic plants provide a major source of inoculum for
the molluscan stage of the life cycle.
Several animals including rabbit, pigs and dogs serve as
reservoir hosts.
Control:
Human excreta are treated before use as fertilizer.
Adequate washing of water plants with hot
Heterophyes heterophyes
Disease: Heterophyiasis
Biology:
Minute pyriform worm, rounded posteriorly,
It measures 1.o _ 1.7 mm in length by o.3 _ o.4 mm in
breadth covered by minute spines . Oral sucker very small,
but ventral sucker is large .There is a genital sucker which
lies on the lateral posterior border of the ventral sucker.
Seminal vesicle lacks the cirrus sac & cirrus organ.
Egg:
small 28_ 3o µm by 15 _ 17 µm, with operculum.
Life cycle
Pathogenesis and symptomatology
Superficial irritation of the intestinal mucosa with excess
secretion of mucous.
In heavy infection, colicky pain and mucous diarrhea.
At times the worms encysted in the tissue, Eggs get into
mesenteric venules or lymphatic, carried to heart, brain or
spinal cord where they stimulate granulomatous reaction.
Diagnosis
: Recovery of eggs in faeces .
Treatment
: Praziquantel and Niclosamide are effective
Epidemiology
: Heterophyes is found in variety of wild
and domestic
Mammals especially fish eating mammals which acquired
the infection by eating fish in a raw, salted or dried.
Control
: The easiest possible measure is the avoidance
of consuming under cooked fish.
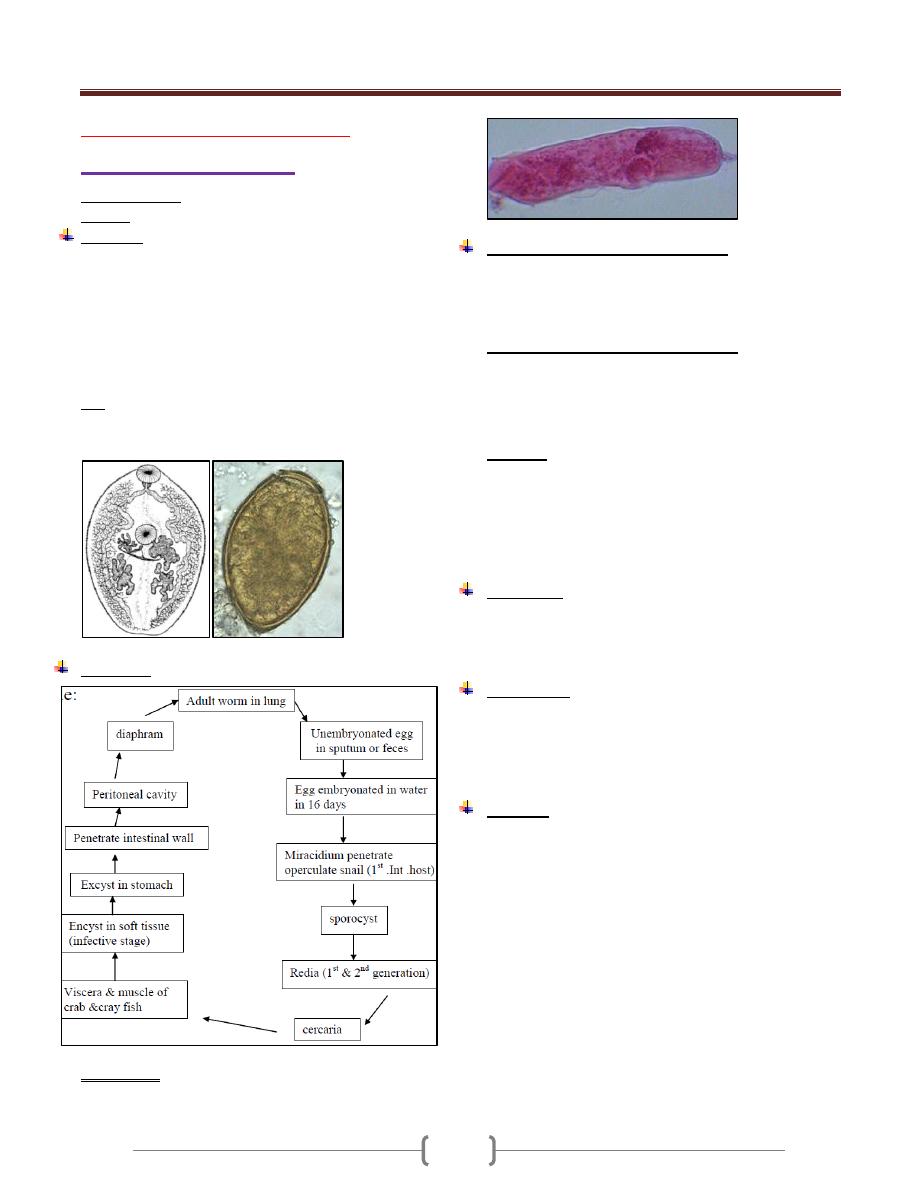
Unit 3: Helminthes (Trematodes)
48
Lecture 4 - Pulmonary fluke
Paragonimus westermani
Common name: oriental lung fluke.
Disease: Paragonimiasis.
Biology:
Adult worm lives in (fibrous capsule in the lung & other
tissue of the body.
The worm stout & reddish-brown in the living state.
7.5-12 mm in length , 4-6 mm in breadth, 3.5-5 mm in
thickness.
Tegument with scalelike spines
The oral & ventral suckers are subequal.
Egg: Ovoid, thick-shelled, golden-brown in color
&flattened operculum (80-118)-µ--m by (40-60)µ—m.
They are unembryonated when laid
Life cycle
Microcercus: Minute 200-µ-- m in length with large oral
sucker, dorsal stylet & delicate knob like tail.
Pathogenesis & Symptomatology:
In the lung there is host –tissue reaction consist of
eosinophilic &neutrophilic infilteration around the worm ,
followed by development of thick fibrous capsule which
cause cough with blood in sputum .
Ectopic location of the worm including:
Liver, intestinal wall, mesenteric lymph nodes,
peritoneum, muscles, myocardium, testes, pleura, brain &
subcutaneous tissue .In these abnormal sites, there is
tendency for development of abscesses &
pseudotubercules, or the lesion may be ulcerative
Symptom:
Occasional cough with rusty sputum, dyspnea, fever,
malaise, anorexia.
In ectopic location cause leukocytosis with fever,
purulent effusion
In the brain will cause epilepsy
Diagnosis:
recovery of eggs in feces , sputum or pleural aspirate
by intradermal & serodiagnostic test
x –ray to see fibrous capsule
Treatment:
Bithionol in 10 to 15 doses on alternate days.
Niclofolan is effective in a single oral dose but
produce side effect
Praziquantel in 3-day course.
Control:
No eating fresh water crabs & crayfish only when well cooked
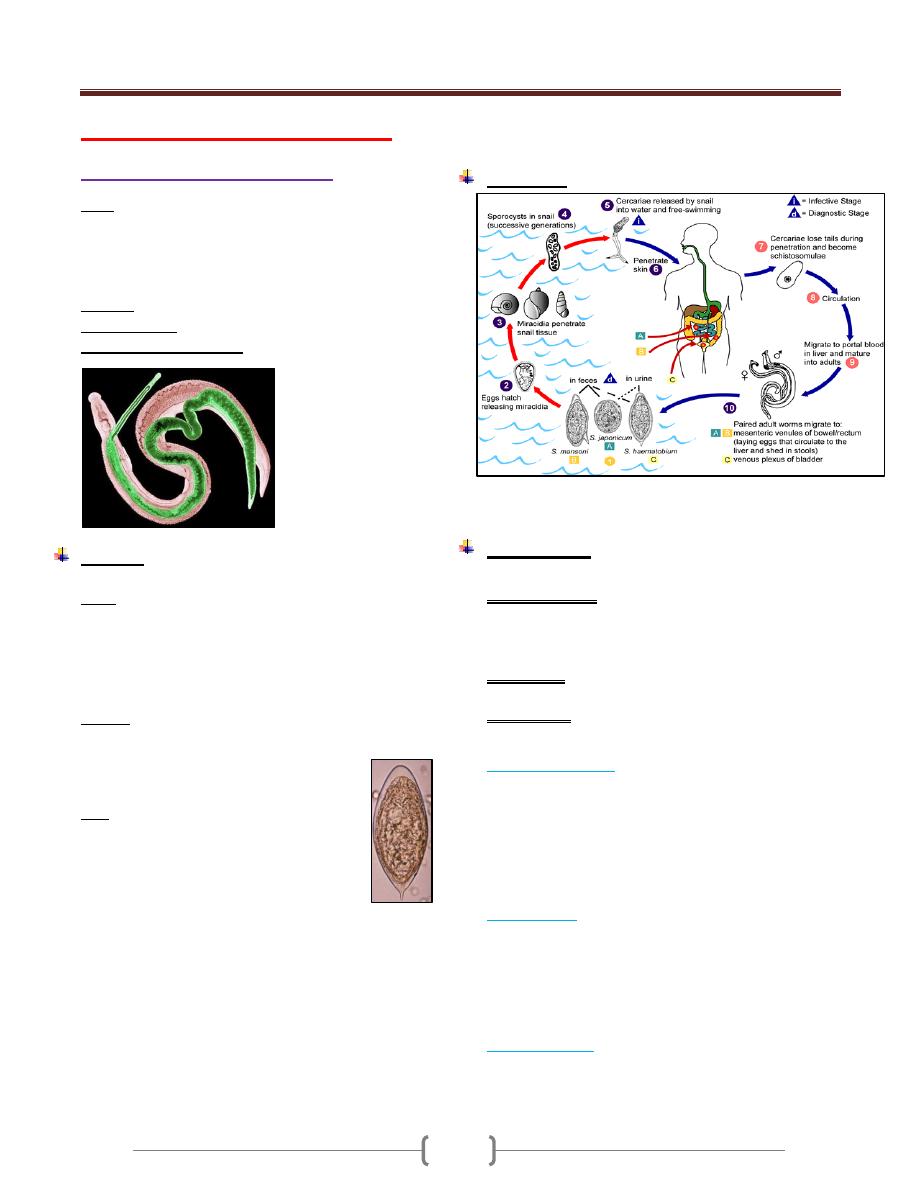
Unit 3: Helminthes (Trematodes)
89
Lecture 5+6+7 - Blood Fluke
Schistosoma haematobium:
Schistosomes are digenetic trematodes that inhabit the
blood stream of vertebrate host. So referred as blood
flukes .Schistosome so called because of the (split body)
on the ventral side of the male in which the female is held
during insemination & egg laying.
Disease: Shistosomiasis haemotobia (urinary schistosomiasis
Natural habitat: vesical venules.
Geographical distribution: In Africa ,middle east
Biology:
Sexes are separated .adult worm are delicate & cylindrical
Male: about 10-15 mm in length &1 mm in diameter ,the
tegument is provided with fine tubercle .There is 4-5
small sub globose (testes) behind the acetabulum .There is
a gynecophoric canal on the ventral side of the body .The
muscular male is attached by its sucker to the wall of the
vessels holding the thread-like female in its sex canal.
Female: about 20mm in length &0.25 mm in width .The
genital organ exclusive of the vitellaria occupy the
median longitudinal field.
ovary in the posterior .half of the body.
Uterus –long containing 20-30 eggs
Egg: the mature egg is rounded at one pole &
has a terminal spine at the other .It measure
upto 170-µ--m in length by 70—µm in width
.Straw coloured & relatively transparent.
Holding the female in the sex canal of the
male enable the female to extend its anterior
.extremity into the smaller venules to deposite it ُs eggs.
obstruction of the venules
pressure exerted by the worm
Increase in size of the eggs
hypermotility of the parasitized organ cause the blood
vessel .to repture & discharge the egg into the
surrounding tissue .On oviposition ,the eggs are immature
but when shed from the tissue & excreated .they become
fully embryonated .(maturation takes about one week)
Then the egg is sloughed into the lumen of the bladder &
excreated in the urine.
Life cycle:
Egg appears in urine 10-12 weeks after skin penetration.
Sch.haematobium may live 20-30 years
Pathogenesis:
The pathologic changes are divided into 3 consecutive periods
1) Prepatent period: From the skin penetration to the
appearance of eggs in urine .In this period petechial
haemorrhage, popular pruritic rash ,small foci of
eosinophilic & inflammatory changes in the lung & liver
2) Acute stage: when we have active egg deposition &
extrausion
3) chronic stage: which consist of stable egg output, tissue
proliferation & repair
Prepatent period:
Schistosoma dermatitis , resulting from contact with
cercaria of Schistosomes. The lesion composed of initial
Prickling sensation accompanied by erythema and local or
general urticaria then irritation subside leaving a macules ,
but in few hours there is intense itching and papules
formation. The reaction reaches its maximu m between the
2
nd
and 3
rd
day, then gradually decrease.
Acute stage:
Egg deposition and extrusion cause local damage to the
tissue of the rectum or urinary bladder. Ulceration and
irritation of the epithelium of the bladder lead to
formation of Polyps which may undergo malignant
changes. Numerous eggs calcified giving the inner surface
a "sandy appearance" and Calculi may form in the lumen
Chronic stage:
Extensive fibrosis of the bladder wall leads to contraction
of the organ. Fibrosis of the bladder neck obstructs the
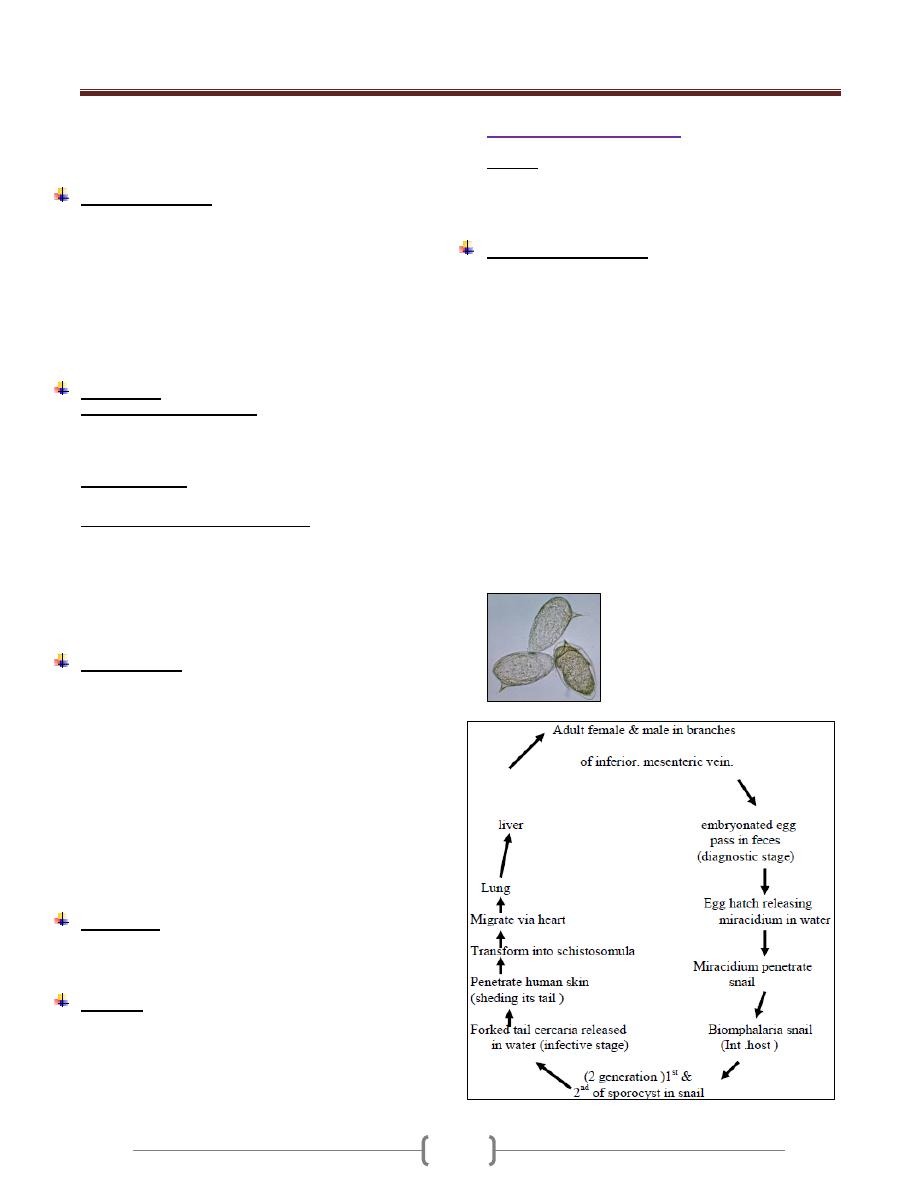
Unit 3: Helminthes (Trematodes)
89
flow of urine with the development of hydro ureter and
hydronephrosis which may be associated with bacteriuria.
In Female the vulva is hyper plastic and indurated.
Symptomatology:
During the prepatent period the patients may be
symptomless or may have malaise with late afternoon
fever, moderate hepatic pain or epigastric distress .
If worms mature in the rectal veins there is severe
tenesmus with dysentery.
The first evidence of infection is the painless passage of
blood at the end of micturition, then discharge of pus
cells and necrotic tissue debris , decrease in the interval
between urination and eventually incontinence or Anuria
.
Diagnosis:
1) Urine examination for eggs
* Simple sedimentation (centrifugation)
* Nucleopore filtration method
* Miracidial hatching.
2) Intradermal test:
By using purified extracts of adult worm
3) Serological and Immunological test:
* Indirect immunofluorescence
* Elisa
* Circumoval precipitin
* Cercarien _ hullen reaction
* Radiologic test
* Rectal and Bladder mucosal Biobsy and Cystoscopy.
Epidemiology
Three factors must coincide for transmission to occur:
Presence of the vector snail Bulinus. Man is the only
important definitive host of Sch.
Presence of human infected with the parasite.
Human habit, that lead to urine contamination of fresh
water and to water contact and expos ure to cercaria.
Schistosomiasis haematobia is endemic in Africa, Middle
east,. In Iraq it is distributed in southern parts . After the
extension of irrigation system other areas are included.
Prevalence increase with age peaking at age 15_2o years
then start to decline.
Treatment
Praziquantel (Biltricide) single oral dose 4o mg/ kg.
Side effect: Abdominal discomfort, headache, drowsiness,
backache, fever, sweating, giddiness.
Control:
Effective measures includes
Chemotherapy
Snail control: Biological & chemical
Environmental sanitation.
Health Education
Schistosoma mansoni
Disease: Schistosomiasis mansoni
Manson ُ s blood fluke is a parasite of man occur widely
in Africa, several foci of the Arabian in south America .It
is hyper endemic in the Nile delta (Egypt )
Biology & Life cycle:
Male of S.mansoni measure (6.4-9.9) mm in length &
female (7.2-14)mm
The tegument of the male is provided with numerous
warty excrescences
The number of testes (6-9) form a grape like cluster a
short distance behind the acetabulum.
The most striking internal feature of female is a short
uterus containing very few eggs
The adult worm usually reside in tributaries of the inferior
mesenteric vein adjacent to the lower colon ,they may be
found at higher level of the intestine , in intra hepatic
portal veins ,vesical venules , pulmonary arterioles.
Fully developed egg of Sch. mansoni are large ,rounded
at both ends &provided with a conspicuous lateral spine
near one pole .The egg range in size from (120 x 45)-µ--m
when laid to (170 x 65 )—µm when ready to hatch .
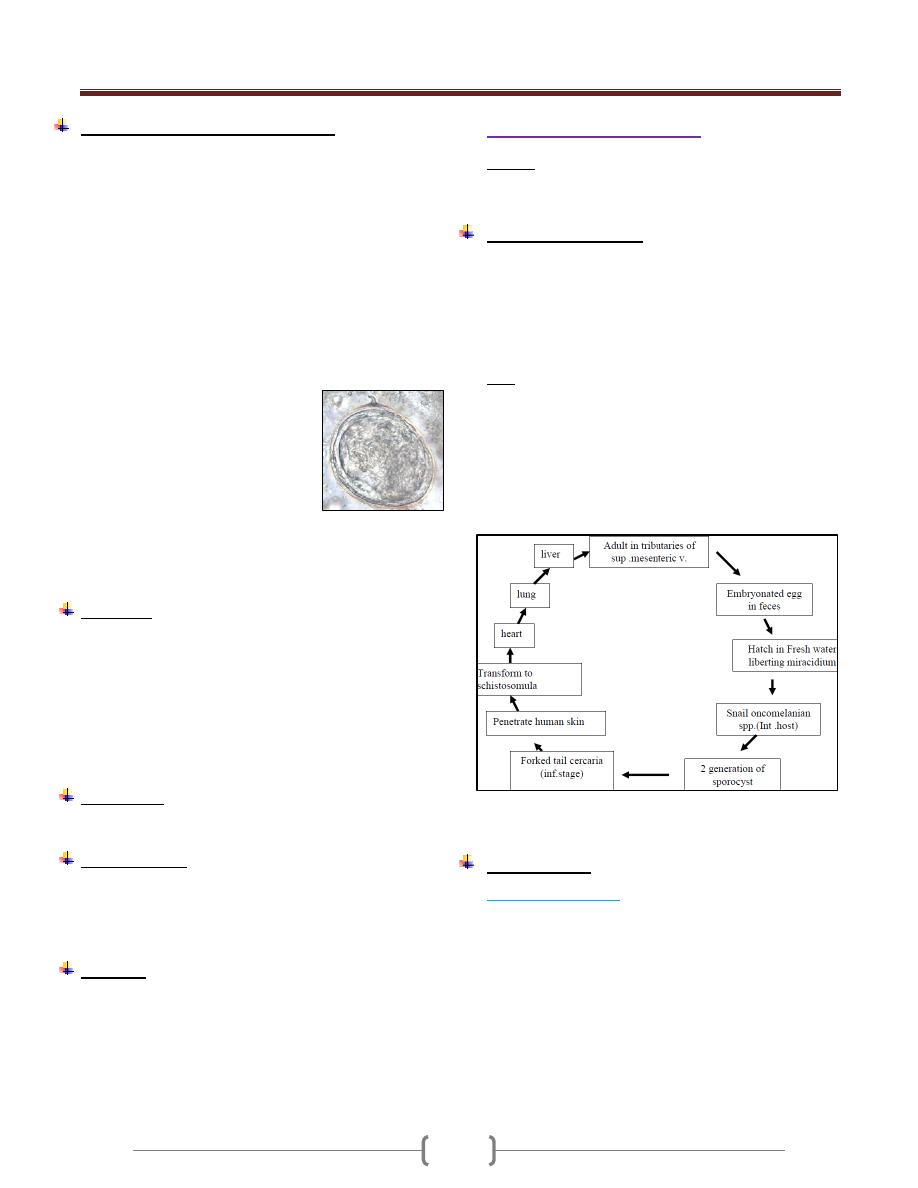
Unit 3: Helminthes (Trematodes)
89
Pathogenesis & Symptomatology:
Humeral & tissue changes caused by S. mansoni resemble
those of infection with other intestinal schistosome spp.but:
1) The incubation period is about 2 weeks longer.
2) The early intestinal lesions typically develop in the
colon rather than the small intestine.
3) The number of eggs produced by each female is less ,
there for fewer eggs extruded from the intestinal
wall& fewer that later become trapped in the
perivascular tissue of the intestine & liver
Thus intestinal & hepatic fibrosis develop more slowly in
Manson ۥs schistosomiasis.
The common manifestation with S. mansoni are:
Fever, chills, weakness, weight loss,
headache, nausea, vomiting, diarrhea
(at times bloody)
There may be evidence suggestive of
peptic ulcer, malabsorption
syndrome, gastrointestinal bleeding,
marked eosinophilia, rectal polyps,
thrombophlebitis . hepatic fibrosis
which may lead to portal hypertension & splenomegaly.
Frank ascitis is less frequent.
pulmonary lesion & symptoms are relatively common
Diagnosis
1) Fecal examination.
2) Rectal biopsy.
3) Immuno diagnostic test.
a) circumoval precipitin test
b) cercarien-Hüllen reaction
c) Immuno diffusion
d) Immuno electrophoresis.
e) Fluorescent antibody test.
Treatment:
-Praziquantel (drug of choice) 40 mg/kg .once
-Oxamniquine.15mg/kg .once
Epidemiology:
S. mansoni is also a parasite of several spp. of mammals.
Ex: Rodent, monkey in Africa & brazil
Man is only definitive host.
Exposure result from contact with cercaria- infected water.
Control :
1) Chemotherapy
2) Snail control.
a) chemical (Niclosamide)
b) Biology Predators (Thiara spp)
Competitor (Marisa spp.)
3) Enviromental sanitation
4) Health education.
Schistosoma japonicum
Disease: Schistosomiasis japonicum
It occurs in the Far East mainly in china, Japan &
Philippines.
Biology &Life cycle:
adult male measure 12-20 mm by 0,5 mm
The tegument is smooth
Oral & ventral sucker are subequal.
There are 7 relatively large testes behind the ventral sucker
Female are delicate with a length (15-30) mm &width
(0.1-0.3)mm
Egg: are round measuring 70-100—m each contains
ciliated miracidium. Aminute blunt projection may be
seen on the outer surface.
The earliest habitat of the young adult is tributaries of the
superior .mesenteric vein. of small Intestine.
Later some worm migrate into the inferior. mesenteric vein.
In these location, female continue to lay eggs daily over a
period of years.
Intramolluscan phase 6-7 weeks. -4-5 weeks from
cercaria penetration & adult maturation & laying eggs.
Pathogenesis:
The prepatent period
is short 4-5 weeks
Female Schistosoma japanicum produce large
number.of eggs.
most of damage is born by the small intestine & liver
Damage is produced as each egg escape from the
venule, filter through the tissue & extruded into the
lumen of the intestine through the ruptured mucosa
with accompanying hemorrhage.
The worm metabolites causes systemic sensitization,
resulting in eosinophilic leukocytosis .over a period of
5 years in heavy infection there is evidence of fibrosis,

Unit 3: Helminthes (Trematodes)
89
papillomas, stenosis of small intestine, hepatic fibrosis
with ascitis, splenomegaly &pulmonary fibrosis.
Towards the end of prepatent period: late
afternoon,fever ,night sweat &diarrhea ,enlarged
tender ,liver ,epigastric distress , pain in the back, groin
or legs .also sometime urticaria develop.
Acute stage:
characterized by :
Diarrhea, eggs in feces, fever, epigastric pain,
enlargement of liver, loses of appetite & weight.
After few weeks ,he may feel better & return to work,
but symptoms return on physical exertion
the blood picture is one of anemia ,increase in serum
globulin level with high eosinophilia
Chronic stage
:
Liver become increasingly fibrosed with multiple minute
granulomas in the parenchyma &on the surface.
The mesentery & omentum may be thickened so as to
bind down the colon & separate the abdomen into an
upper & lower portion .Then increasing ascitis &
emaciation develop dyspnea on slight exertion ,dilatation
of the superficial abdominal veins ,myocarditis due to
infiltaration of eggs into the cardiac wall .The patient
gradually goes into a decline & may die of exhaustion or
supervening infection.
Diagnosis:
Is similar to that of Sch .mansoni
Sedimentation & Kato-thick smear technique may be
required to discover the eggs.
Notes about human blood fluke:
Schistosomiasis japonicum :
most pathogenic - least responsive to treatment.
Schistosomiasis haematobia
Least pathogenic - most responsive to treatment.
Treatment:
Praziquantel 30 mg/kg in one day.
Epidemiology:
Most mammals are susceptible to infection .Exposure
occur when cercariae come in contact with the skin during
washing .The principal source of infection in the snail is
faecal contamination by man.
Control:
Is very difficult because other mammals is susceptible to
infection but it is similar to the control of Sch.haematobium
S.
haematobium
S. mansoni
S.
japonicum
length
10-15 mm
20 mm/adult
6.1-9.9 mm
7.2-14 mm
12-20 mm
15-30 mm
Male
tegument
Slightly
tuberculated
Warty
exerescence
smooth
Reproducti ve system
In male /
testes
4-5
6-9 grape
like cluster
7
Location
of uterus
Anterior two
third
Anterior
half
Anterior
half
Position of
ovary
Posterior half
Anterior
half
Middle half
Location
of caecal
junction
middle
Anterior
half
Posterior
fourth
Schistosoma.dermatitis
Cercarial dermatitis (swimmerۥs itch)
Is the skin reaction that produced by the cutaneous
penetration of cercaria of non-human schistosoma.
They are cercaria of schistosome of aquatic birds
&mammals & may affects person exposing skin to fresh
or salt water, all over the world.
Avian schistosomes that are responsible for Cercarial
dermatitis belong to the genera Trichobilharzia,
ornithobilharzia, Gegantobilharizia, Microbilharzia,
Austrobilharzia
When human are attacked by bird or non-human
mammalian schistosome cercaria, the parasite become
trapped in the skin & fail to complete their circulatory
migration, initiate cercarial dermatitis & then die in
several day.
Clinical Aspect:
Consist of prickling sensation, erythema, urticaria &
macules & papules .The reaction reached into maximu m
within 48-72 hr after exposure then gradually decreases .
Treatment:
Application of palliative (calamine lotion), antihistamine
cream or orally to relieve itching or trimeprazine

94

Unit 4: Medical Entomology
95
Lecture 1+2+3 - Arthropods Vectors
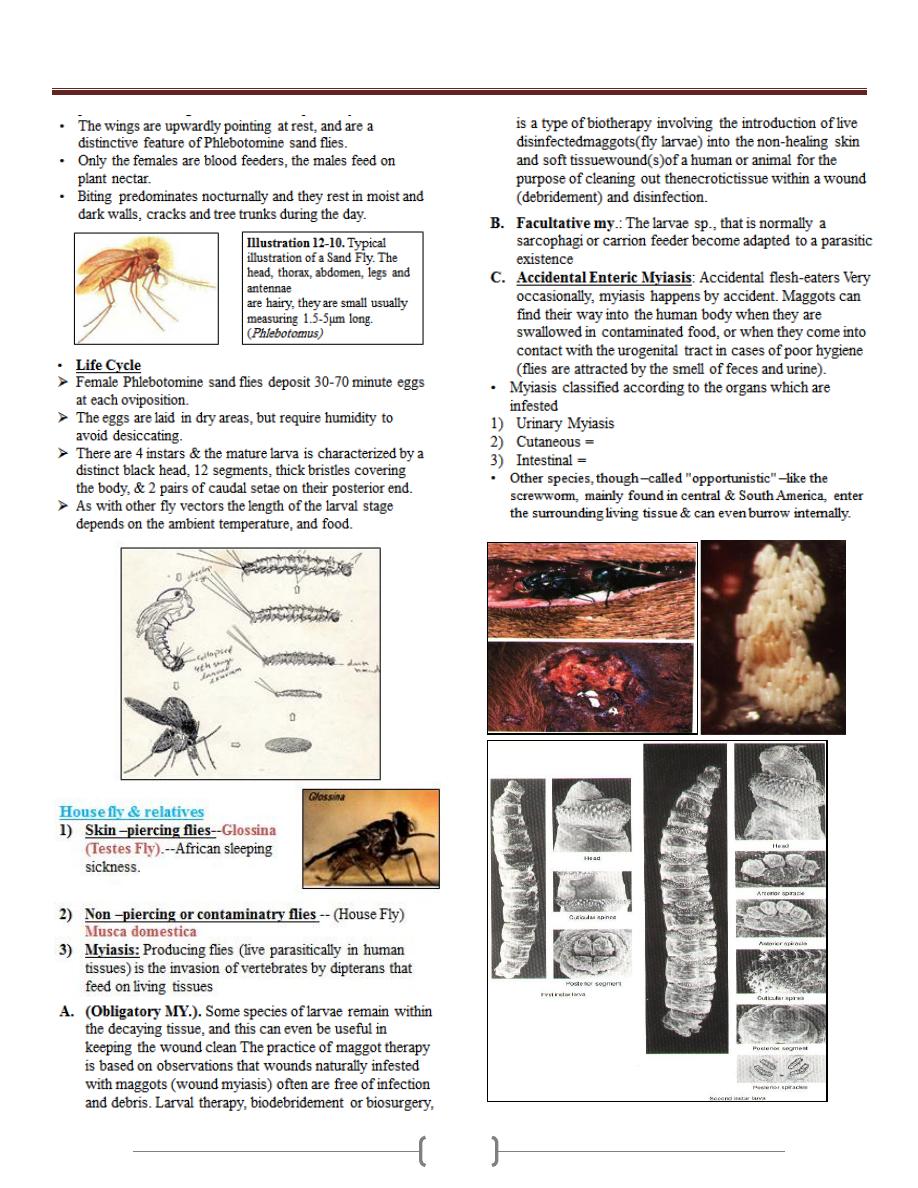
Unit 4: Medical Entomology
96
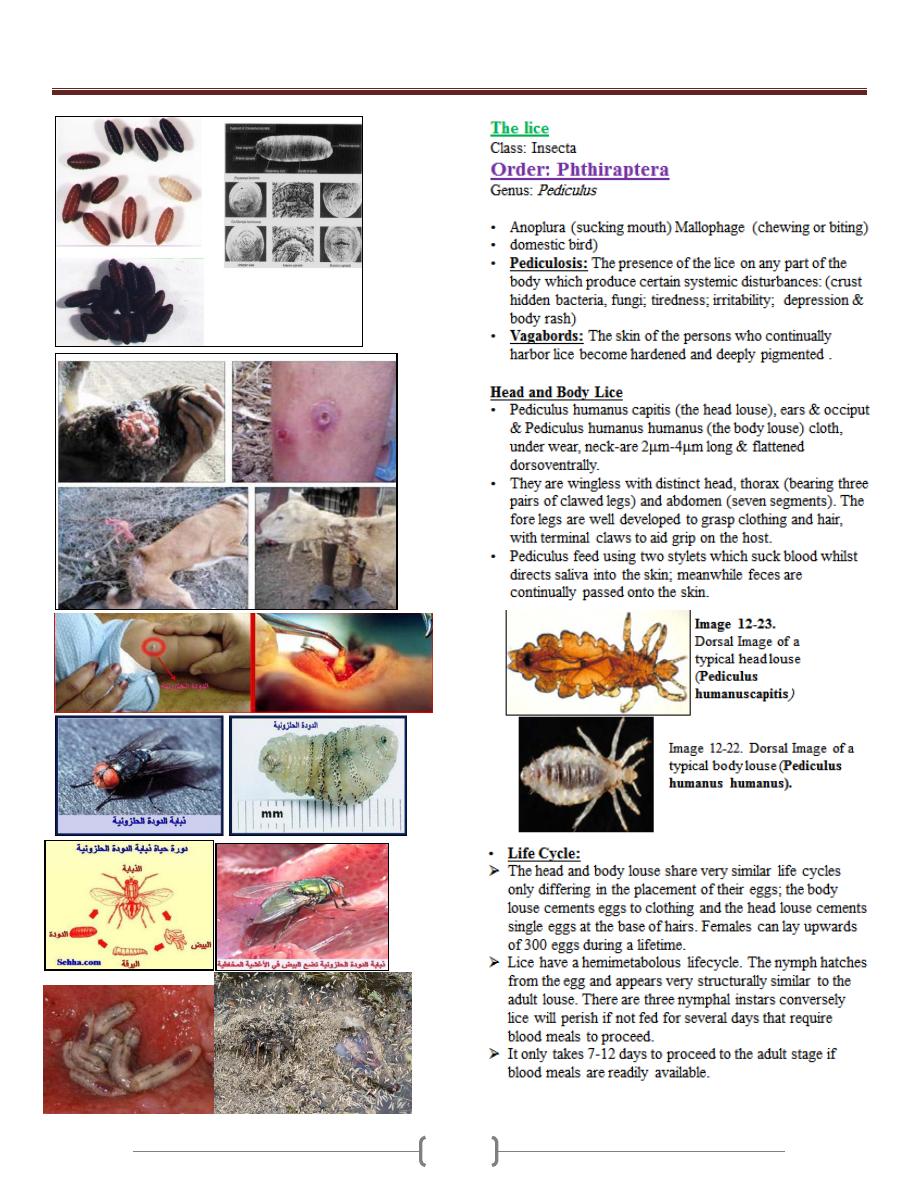
Unit 4: Medical Entomology
97

Unit 4: Medical Entomology
98

Unit 4: Medical Entomology
99

Unit 4: Medical Entomology
100
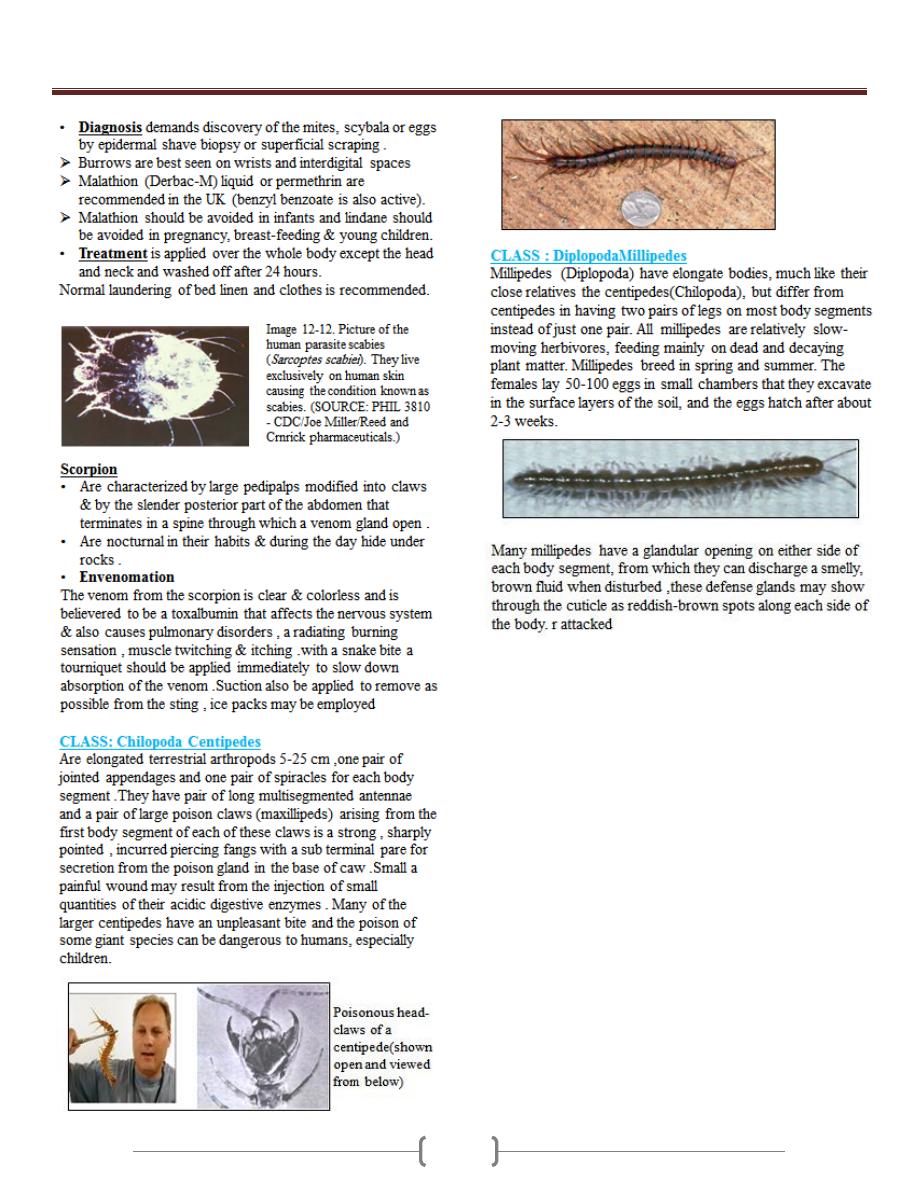
Unit 4: Medical Entomology
101
