
Bone Development
9
th
lecture
December 17, 2015

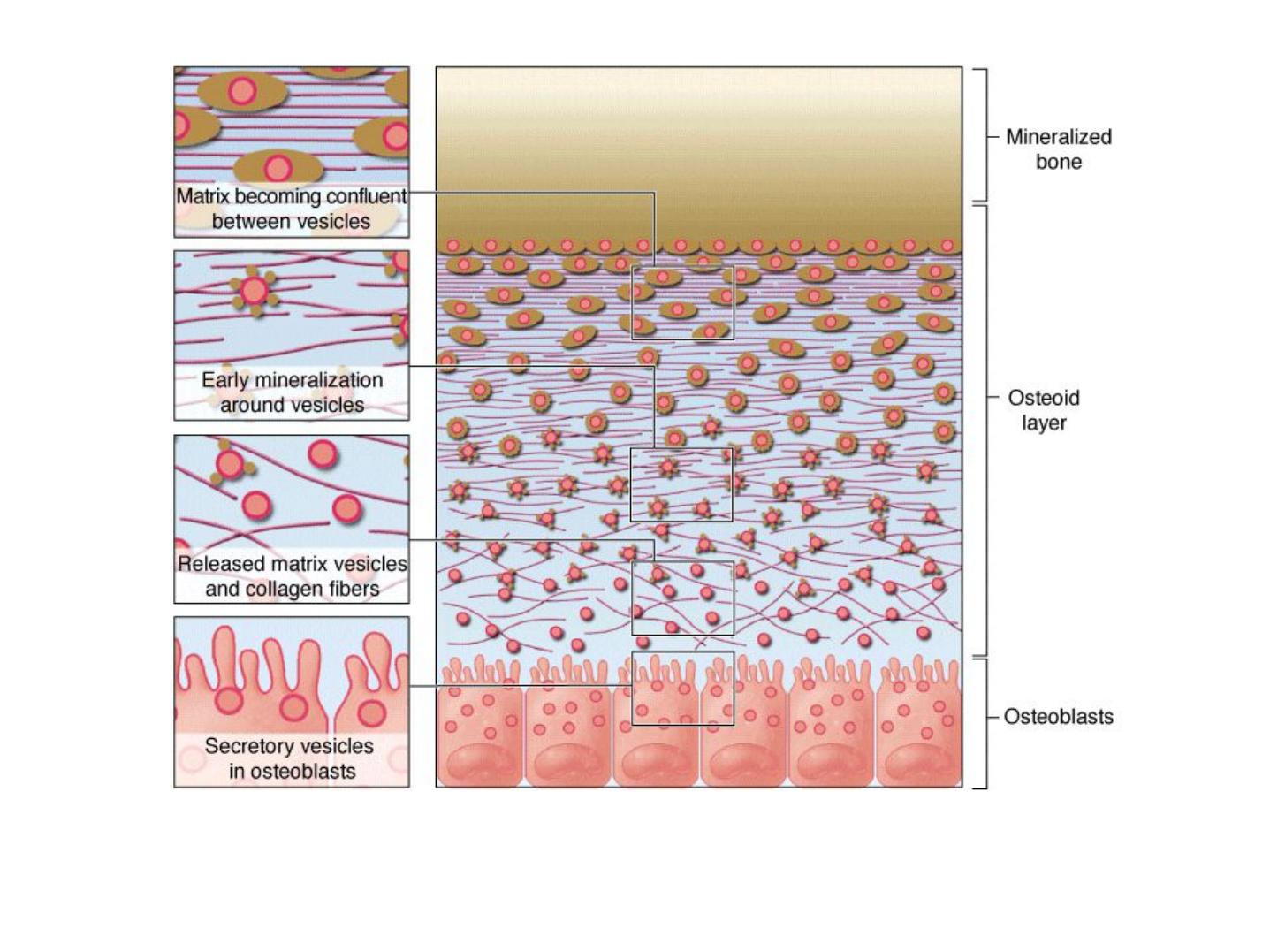
Mineralization in bone matrix (calcification)
• Osteoblasts secrete type I collagen, several glycoproteins, and proteoglycans.
• Some of these factors, notably osteocalcin and certain glycoproteins, bind Ca
2+
with high
affinity, thus raising the local concentration of these ions.
• Osteoblasts also release very small membrane-enclosed matrix vesicles with which
alkaline phosphatase and other enzymes are associated.
• These enzymes hydrolyze PO
4
ions from various macromolecules, creating a high
concentration of these ions locally.
• The high ion concentrations cause crystals of CaPO
4
to form on the matrix vesicles.
• The crystals grow and mineralize further with formation of small growing masses of
hydroxyapatite [Ca
10
(PO
4
)
6
(OH)
2
] which surround the collagen fibers and all other
macromolecules.
• Eventually the masses of hydroxyapatite merge as a confluent solid bony matrix as
calcification of the matrix is completed.

Osteogenesis
Bone can be formed initially by either of two ways:
• Endochondral ossification, in which the matrix of preexisting hyaline cartilage is eroded
and replaced by osteoblasts producing osteoid.
• Intramembranous ossification, in which osteoblasts differentiate directly from
mesenchyme and begin secreting osteoid
In both processes, the bone tissue that appears first is primary or woven. Primary bone is a
temporary and is soon replaced by the definitive secondary lamellar bone. During bone
growth, areas of primary bone, areas of resorption, and areas of secondary bone all appear
side by side.

Stages of Endochondral Ossification
The process takes many weeks and major developmental stages include:
• (1), formation of a bone collar around the middle of the cartilage model and degeneration
of the underlying cartilage
• (2), followed by invasion of the resulting ossification center by capillaries and
osteoprogenitor cells from the periosteum
• (3), osteoid deposition by the new osteoblasts, calcification of woven bone, and its
remodeling as compact bone
• (4), This primary ossification center develops in the diaphysis, along the middle of each
developing bone. Secondary ossification centers develop somewhat later by a similar
process in the epiphyses. The primary and secondary ossification centers are separated by
the epiphyseal plate
• (5), which provides for continued bone elongation. The two ossification centers do not
merge until the epiphyseal plate disappears
• (6), when full stature is achieved.
Endochondral ossification, forms most bones of the skeleton and occurs in
the fetus in models made of hyaline cartilage
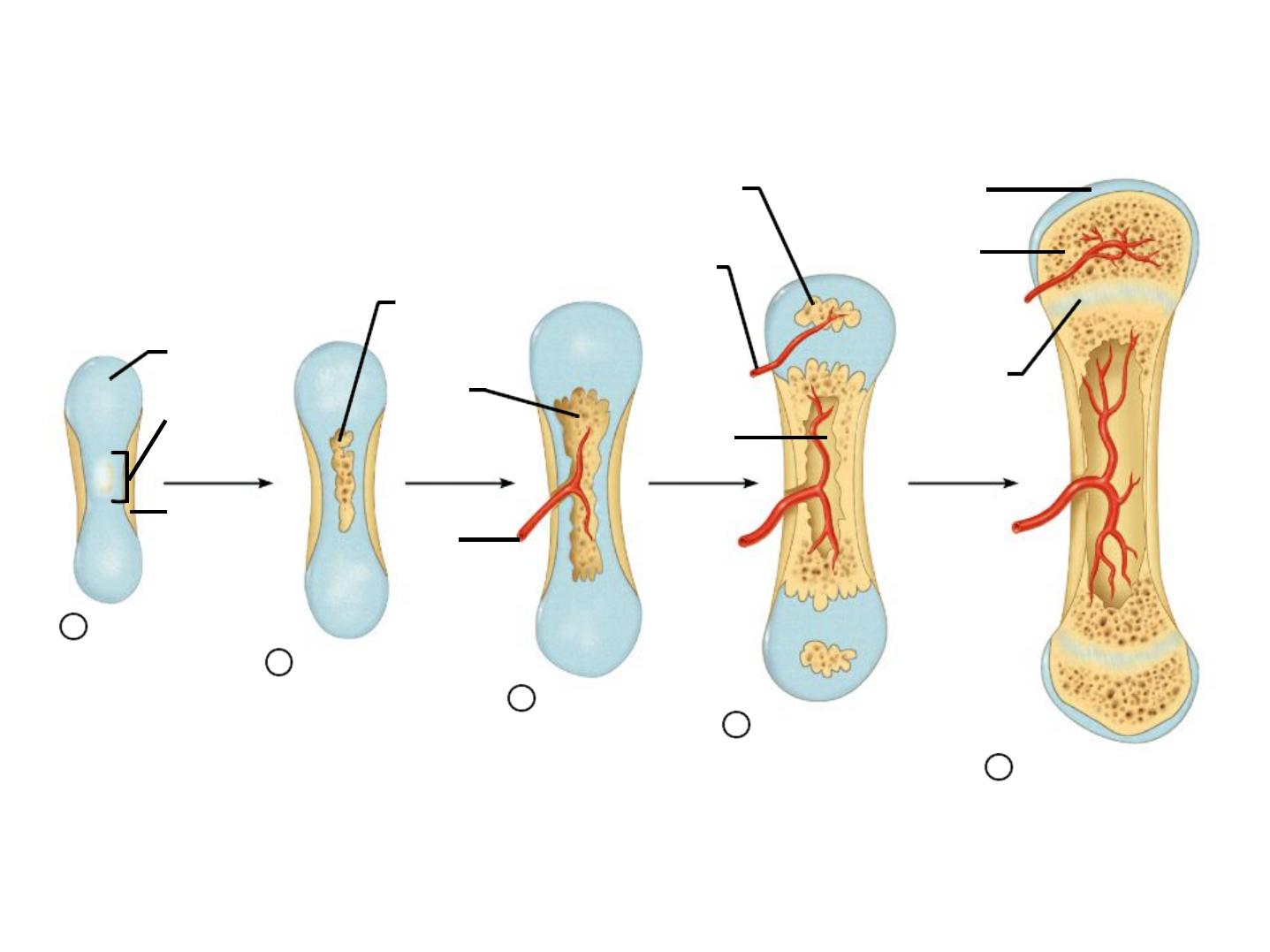
Stages of Endochondral Ossification
Formation of
bone collar
around hyaline
cartilage model.
Hyaline
cartilage
Cavitation of
the hyaline carti-
lage within the
cartilage model.
Invasion of
internal cavities
by the periosteal
bud and spongy
bone formation.
Formation of the
medullary cavity as
ossification continues;
appearance of sec-
ondary ossification
centers in the epiphy-
ses in preparation
for stage 5.
Ossification of the
epiphyses; when
completed, hyaline
cartilage remains only
in the epiphyseal plates
and articular cartilages.
Deteriorating
cartilage
matrix
Epiphyseal
blood vessel
Spongy
bone
formation
Epiphyseal
plate
cartilage
Secondary
ossificaton
center
Blood
vessel of
periosteal
bud
Medullary
cavity
Articular
cartilage
Spongy
bone
Primary
ossification
center
Bone collar
1
2
3
4
5
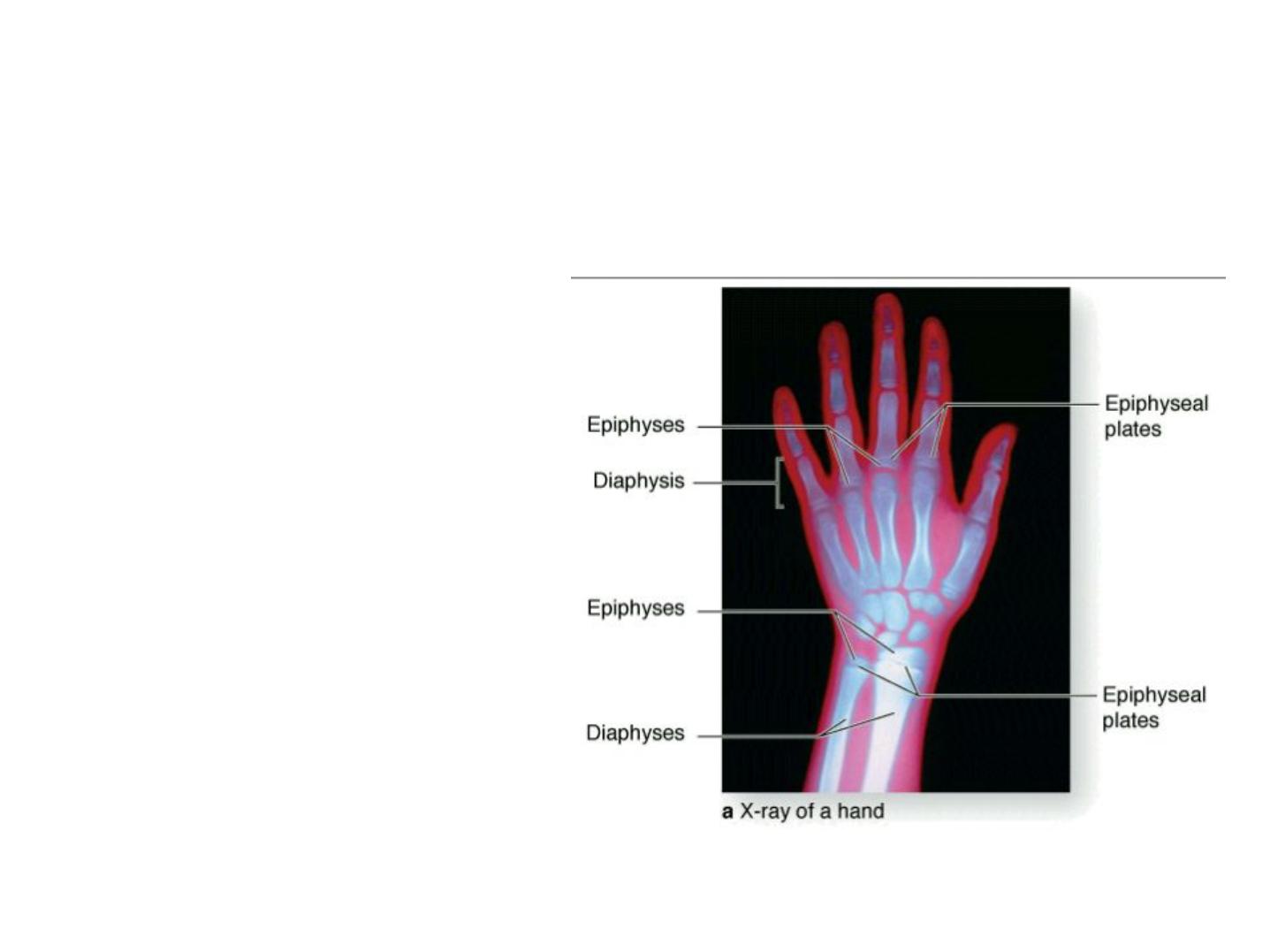
(a): Epiphyseal plates can be
identified in an x-ray of a
child's
hand
as
marrow
regions
of
lower
density
between
the
denser
ossification centers. Cells in
epiphyseal growth plates are
responsible
for
continued
elongation of bones until the
body's full size is reached.
Epiphyseal growth plate: locations and zones of activity.
The large and growing primary ossification center in long bone diaphyses and the
secondary ossification centers in epiphyses are separated in each developing bone by a
plate of cartilage called the epiphyseal plate.
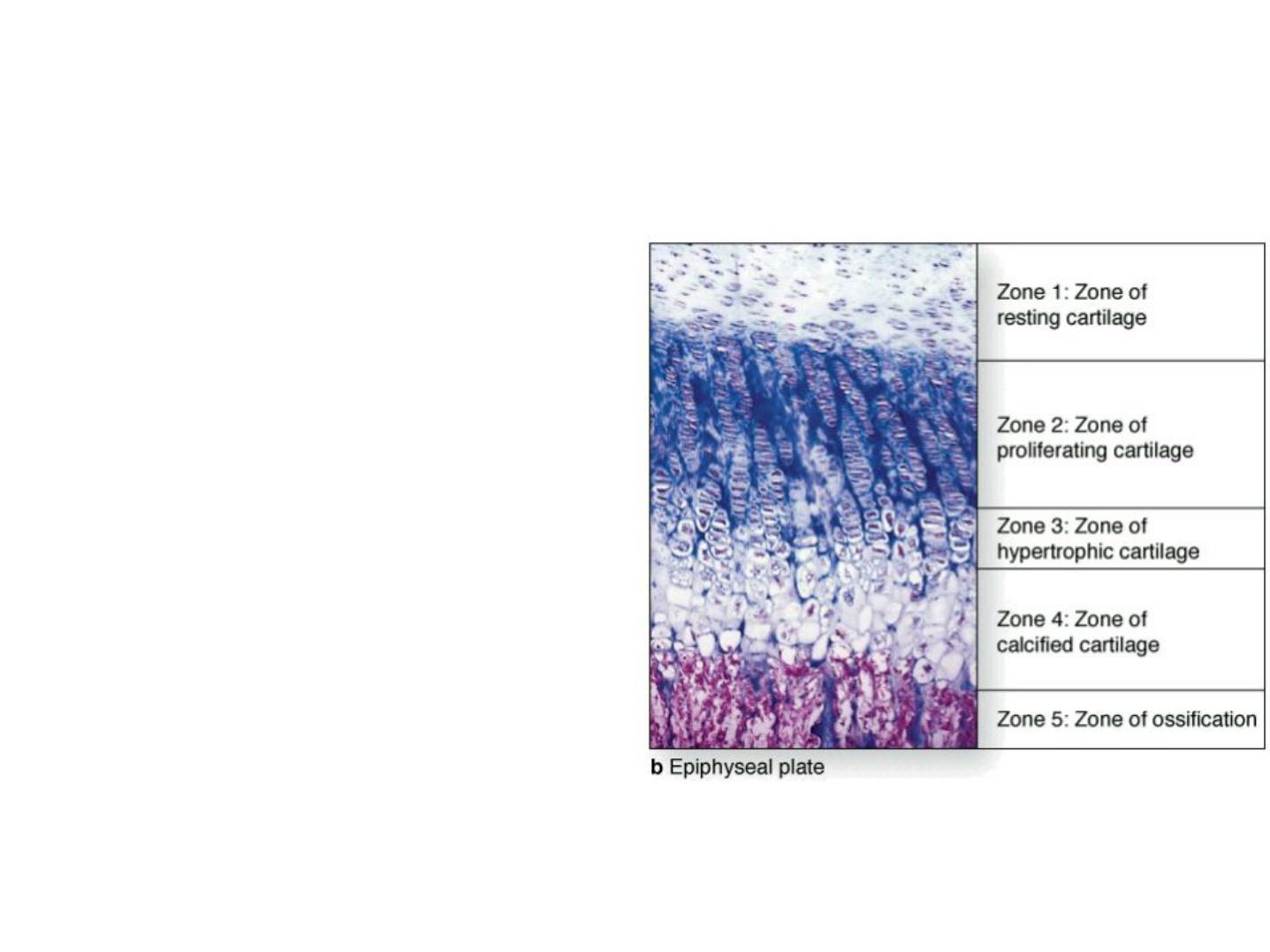
A plate of epiphyseal cartilage is divided into five zones, starting from the epiphyseal side
of cartilage:
1.The resting zone consists of hyaline cartilage
with typical chondrocytes.
2.In the proliferative zone, chondrocytes begin
to divide rapidly and form columns of stacked
cells parallel to the long axis of the bone.
3.The hypertrophic cartilage zone contains
swollen chondrocytes whose cytoplasm has
accumulated glycogen. Hypertrophy compresses
the matrix into thin septa between the
chondrocytes.
4.In the calcified cartilage zone, loss of the
chondrocytes by apoptosis is accompanied by
calcification of the septa of cartilage matrix by
the formation of hydroxyapatite crystals.
5.In the ossification zone, bone tissue first
appears. Capillaries and osteoprogenitor cells
originating from the periosteum invade the
cavities left by the chondrocytes. Many of these
cavities will be merged and become the marrow
cavity.

Intramembranous Ossification
• Intramembranous ossification, by which most flat bones are produced
• is so called because it takes place within condensations of embryonic mesenchymal
tissue.
• The frontal and parietal bones of the skull—as well as parts of the occipital and
temporal bones and the mandible and maxilla—are formed by intramembranous
ossification.
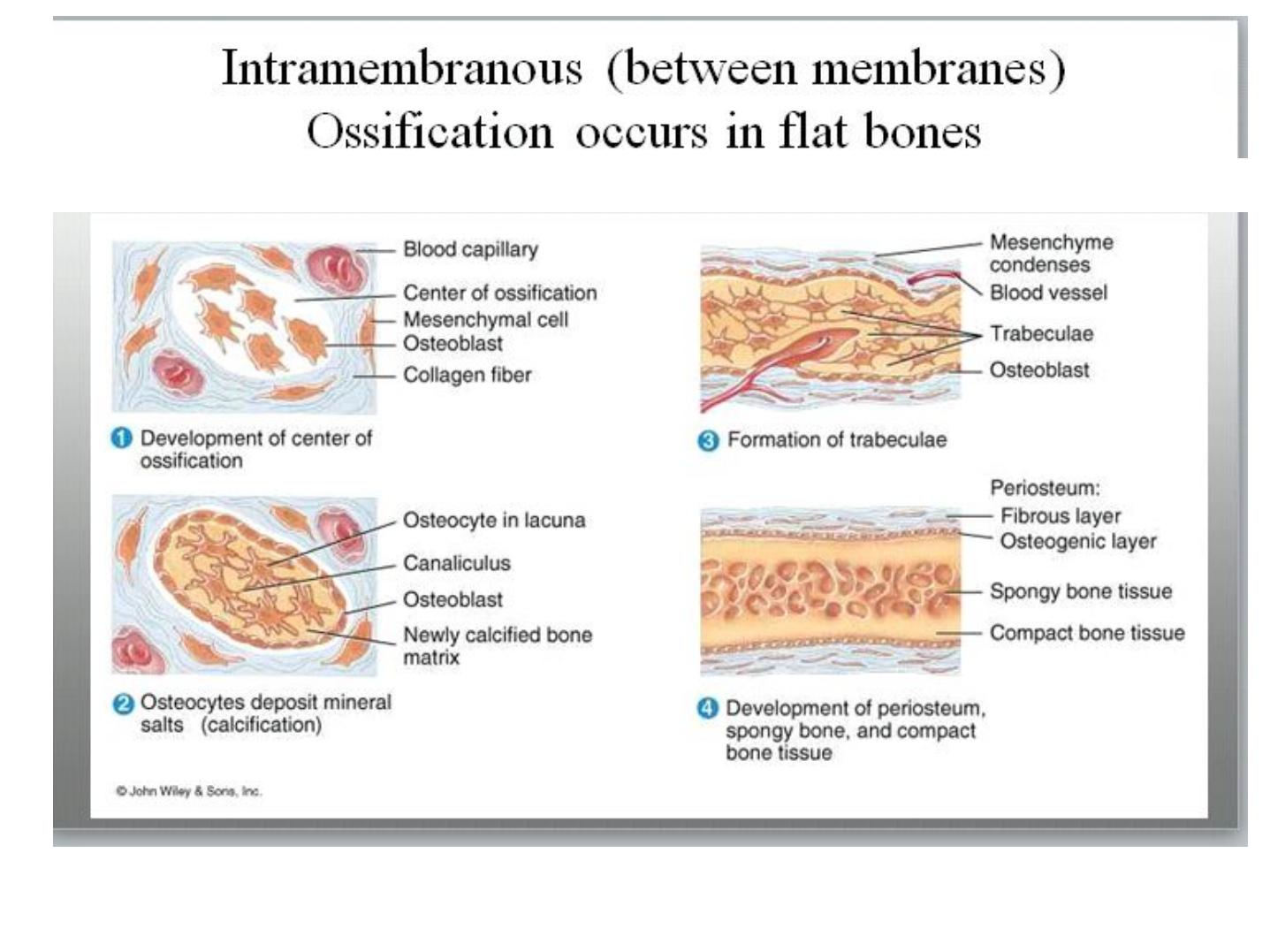
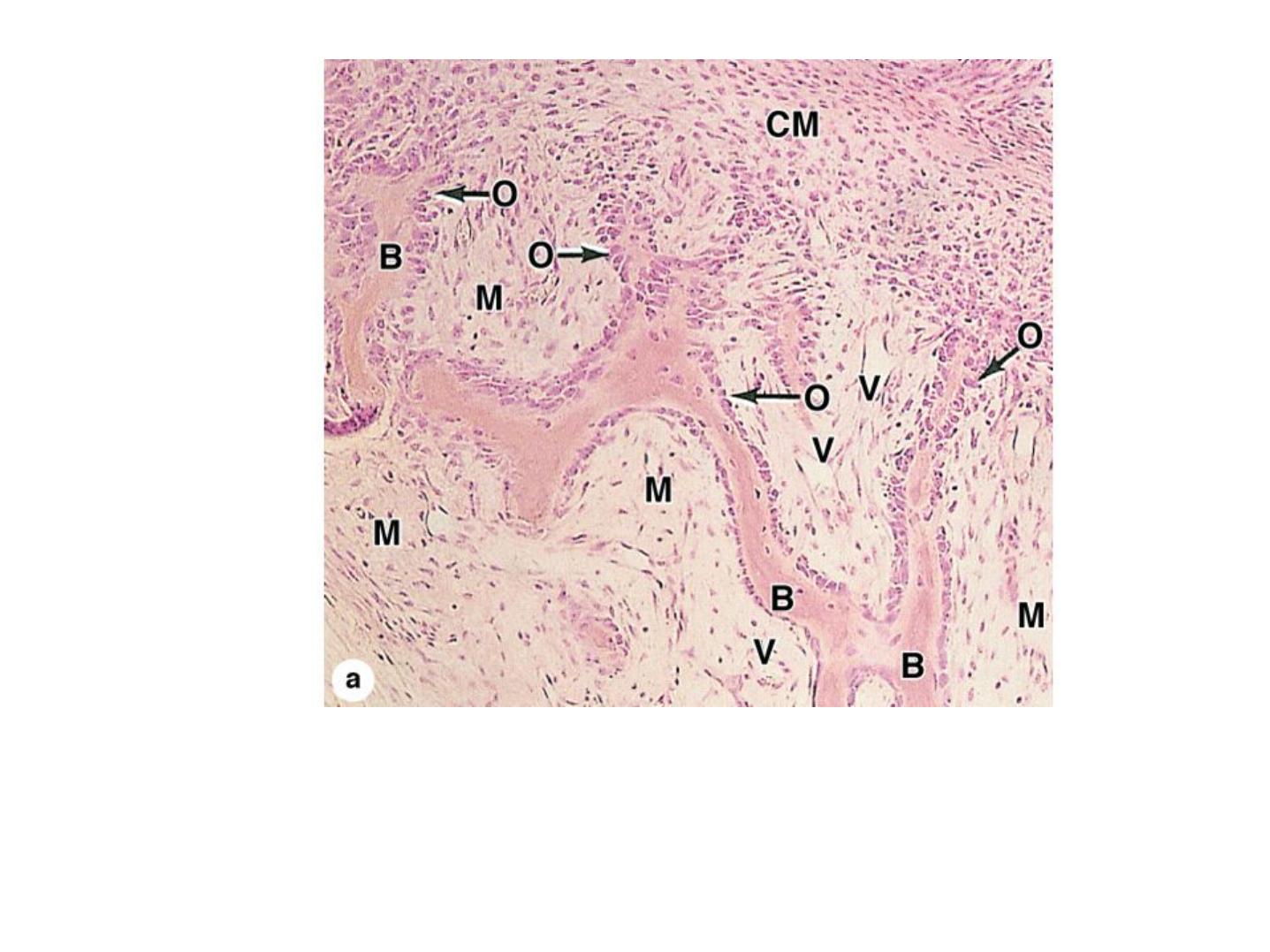
Intramembranous ossification.
A section of jaw from a fetal pig undergoing intramembranous ossification. (a): Areas of typical
mesenchyme (M), condensed mesenchyme (CM) adjacent to aggregates of new osteoblasts (O).
Some osteoblasts have secreted matrices of bone (B) which remain covered by osteoblasts.
Between these trabeculae of newly formed primary bone are vascularized areas (V) that will
form marrow cavities. X40. H&E.
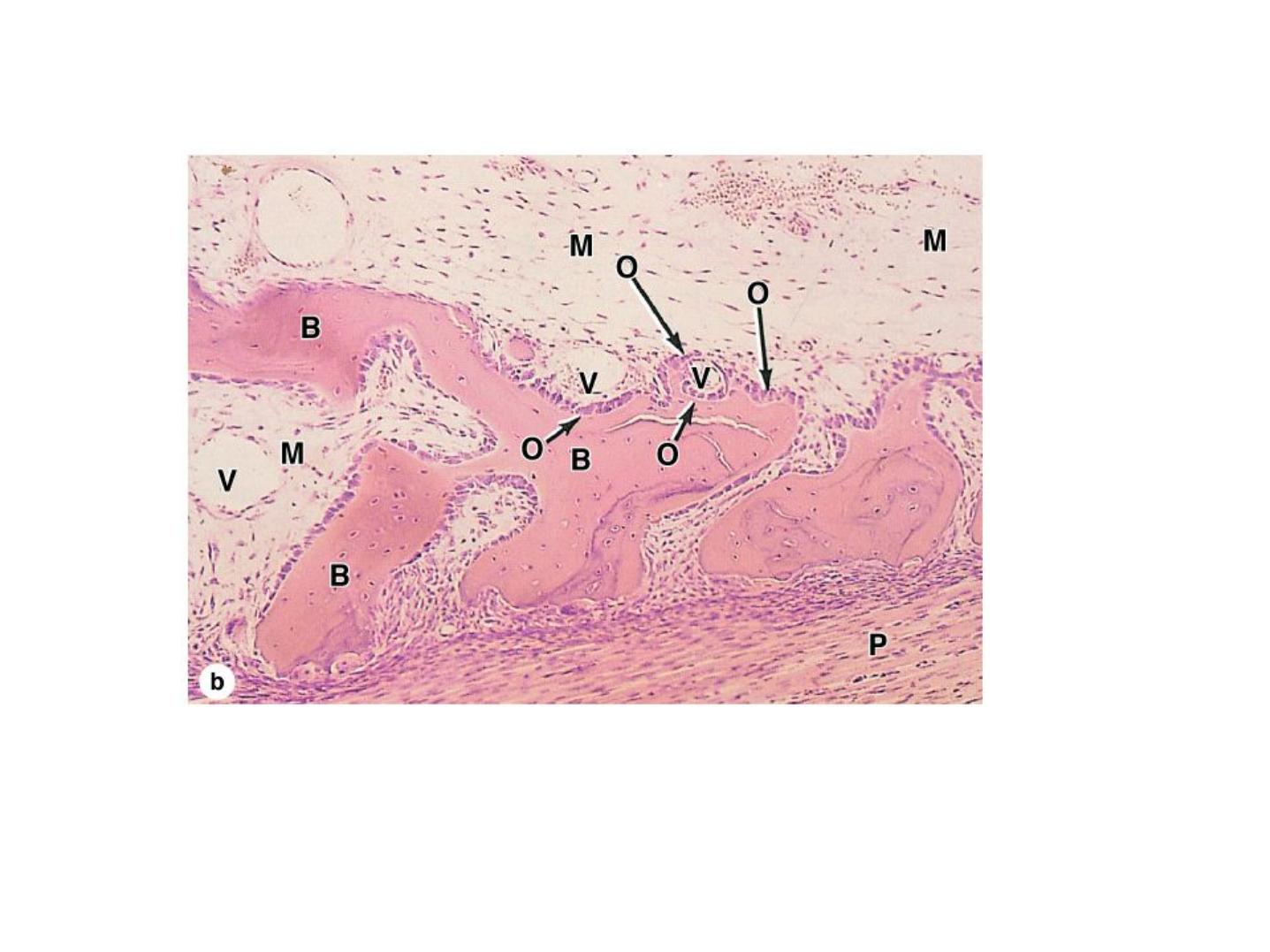
(b): Higher magnification shows the developing periosteum (P) that covers masses primary
bone that will soon merge to form a continuous plate of bone. The larger mesenchyme-
filled region at the top is the developing marrow cavity. X100. H&E.

Long Bone Growth and Remodeling
• Growth in length –cartilage continually grows
and is replaced by bone as shown
• Remodeling –bone is resorbed and added by
appositional growth as shown
- compact bone thickens and strengthens
long bones with layers of circumferential
lamellae
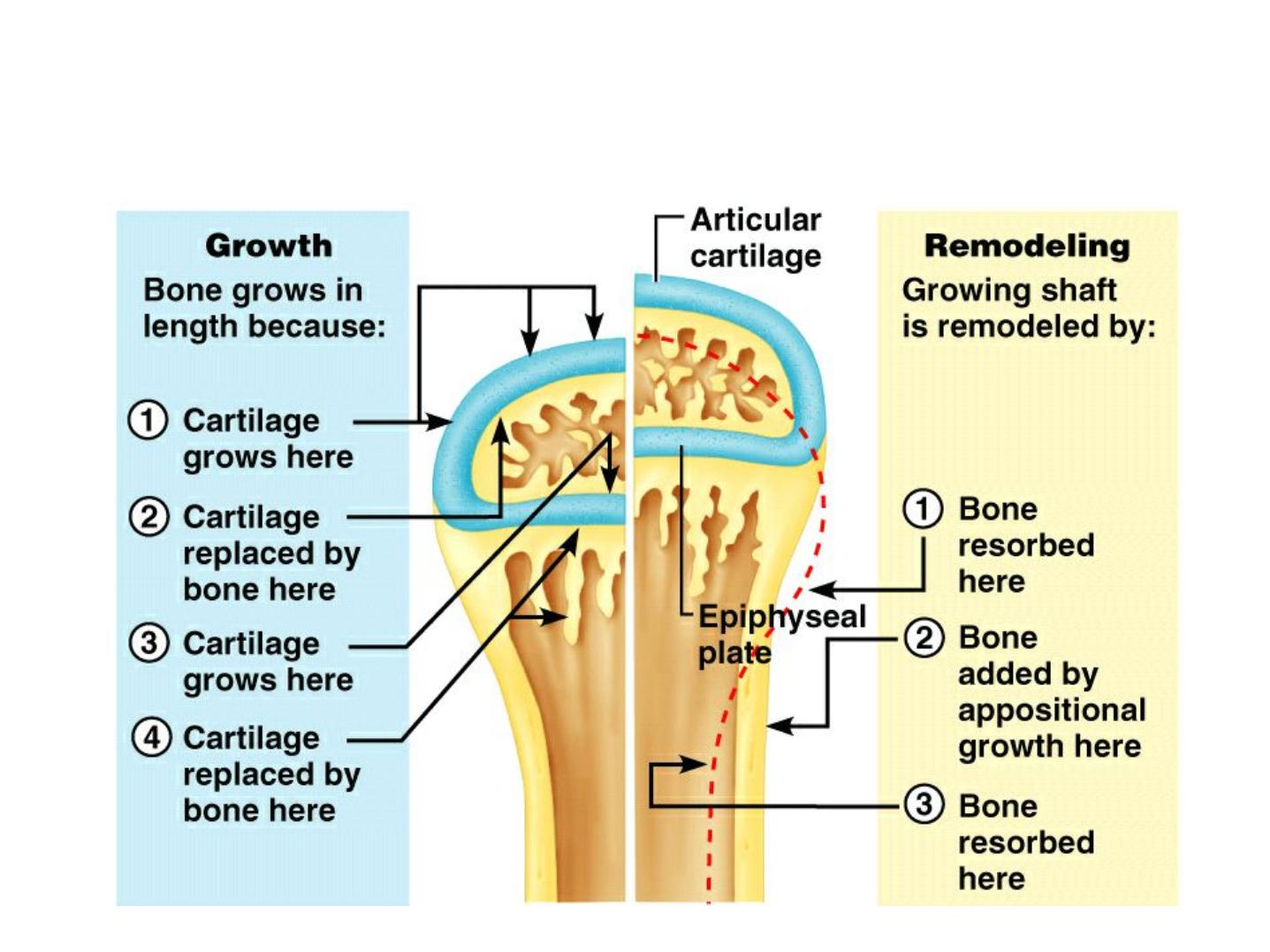
Long Bone Growth and Remodeling
Figure 6.10
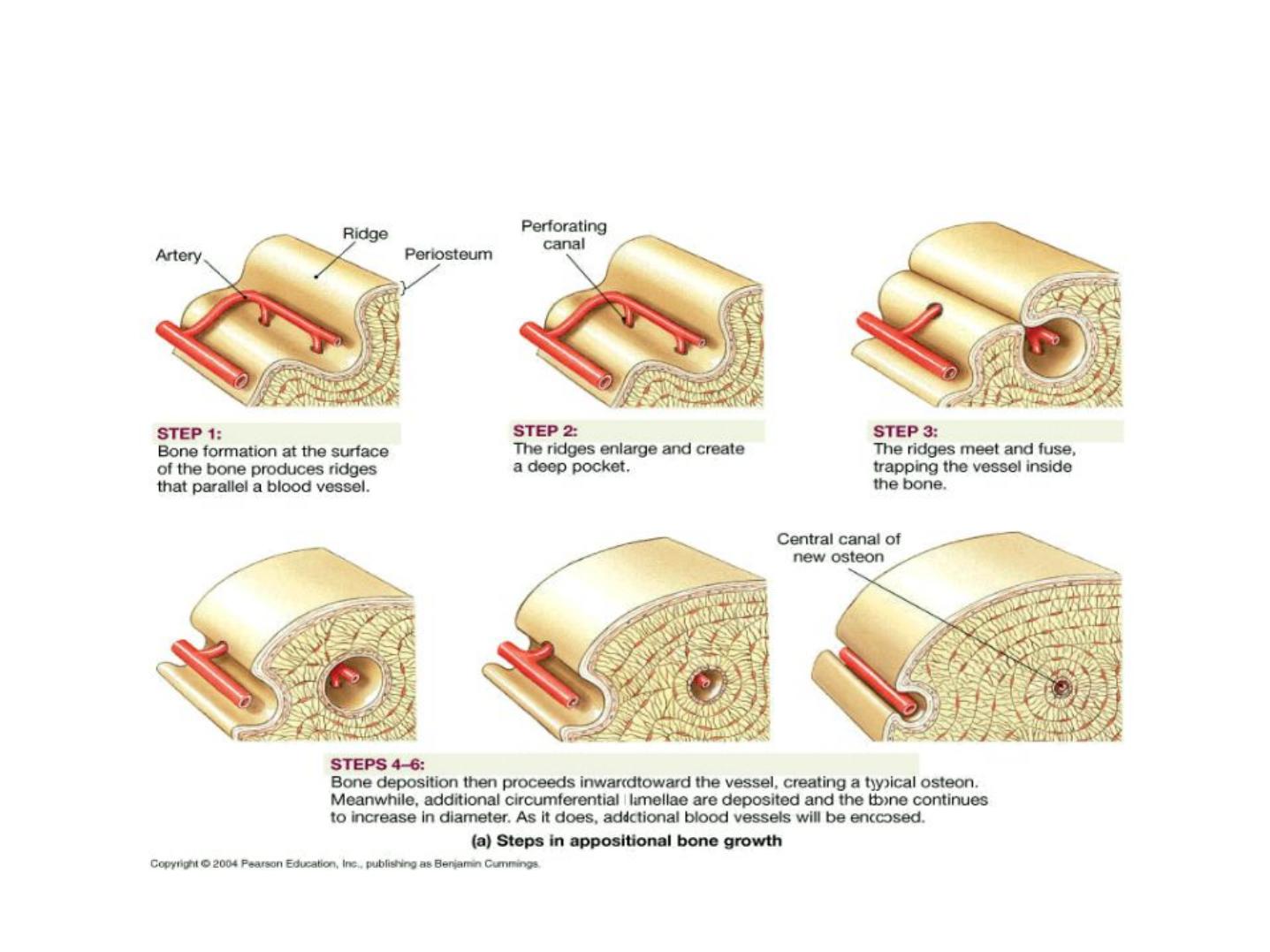
Appositional Growth

Fractures
• Fractures:
- cracks or breaks in bones
- caused by physical stress
• Fractures are repaired in 4 steps
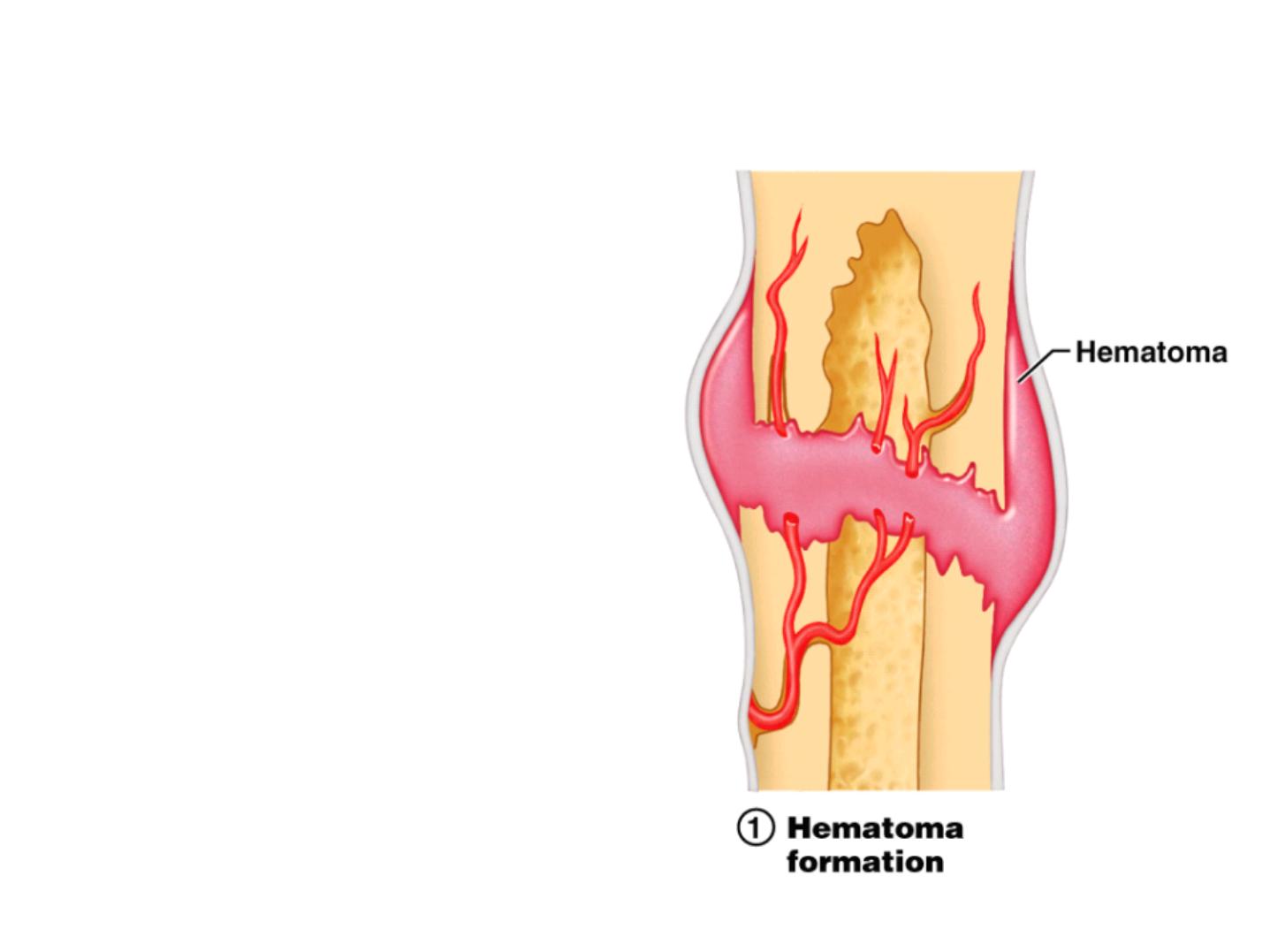
Fracture Repair Step 1: Hematoma
• Hematoma formation
- Torn blood vessels
hemorrhage
- A mass of clotted blood
(hematoma) forms at the
fracture site
- Site becomes swollen,
painful, and inflamed
• Bone cells in the area die
Figure 6.13.1
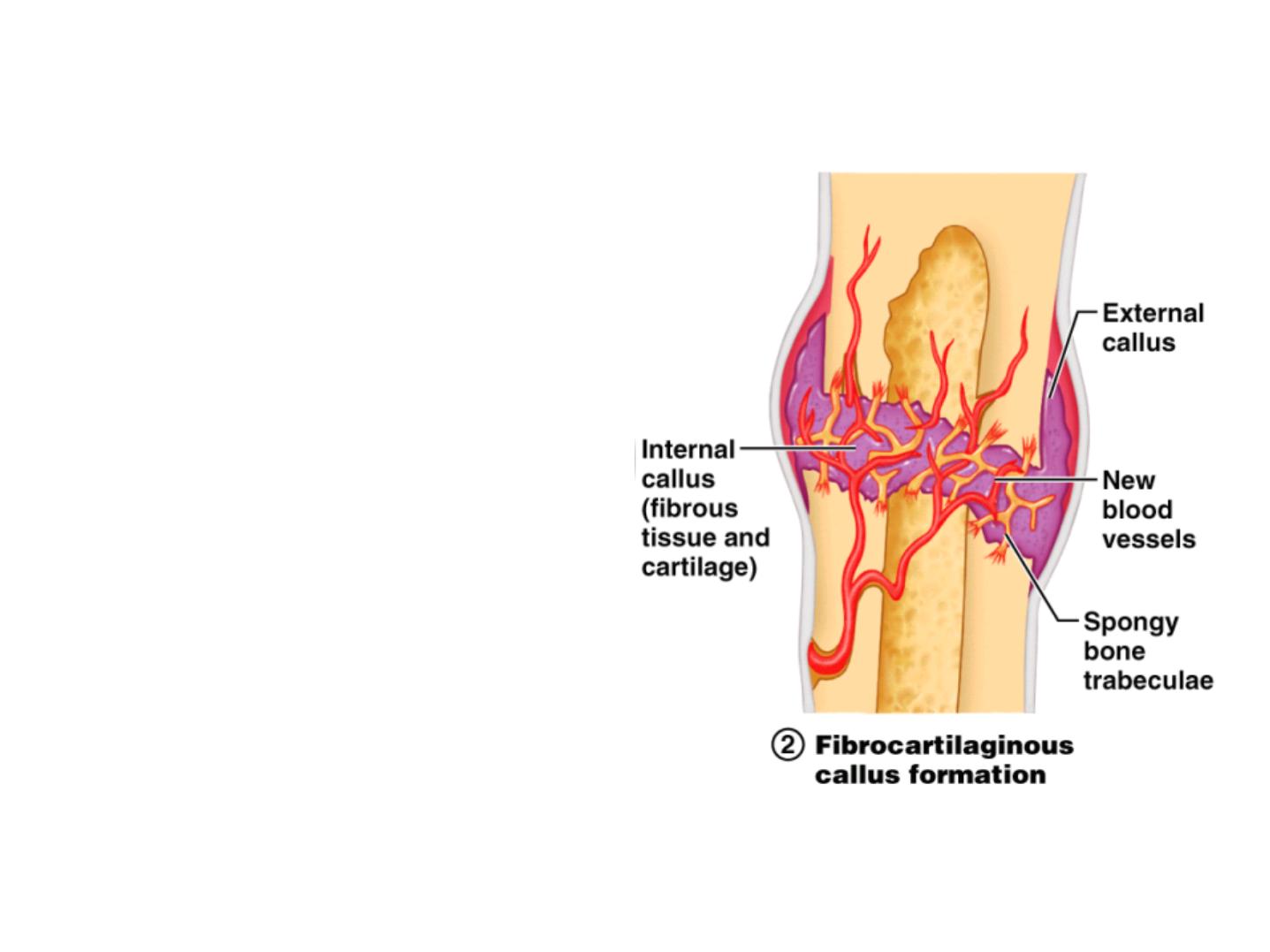
Fracture Repair Step 2: Soft Callus
• Cells of the endosteum and
periosteum divide and migrate into
fracture zone
• Granulation tissue (soft callus)
forms a few days after the fracture
from fibroblasts and endothelium
• Fibrocartilaginous callus forms to
stabilize fracture
- external callus of hyaline cartilage
surrounds break
- internal callus of cartilage and
collagen develops in marrow cavity
• Capillaries grow into the tissue and
phagocytic cells begin cleaning
debris
Figure 6.13.2
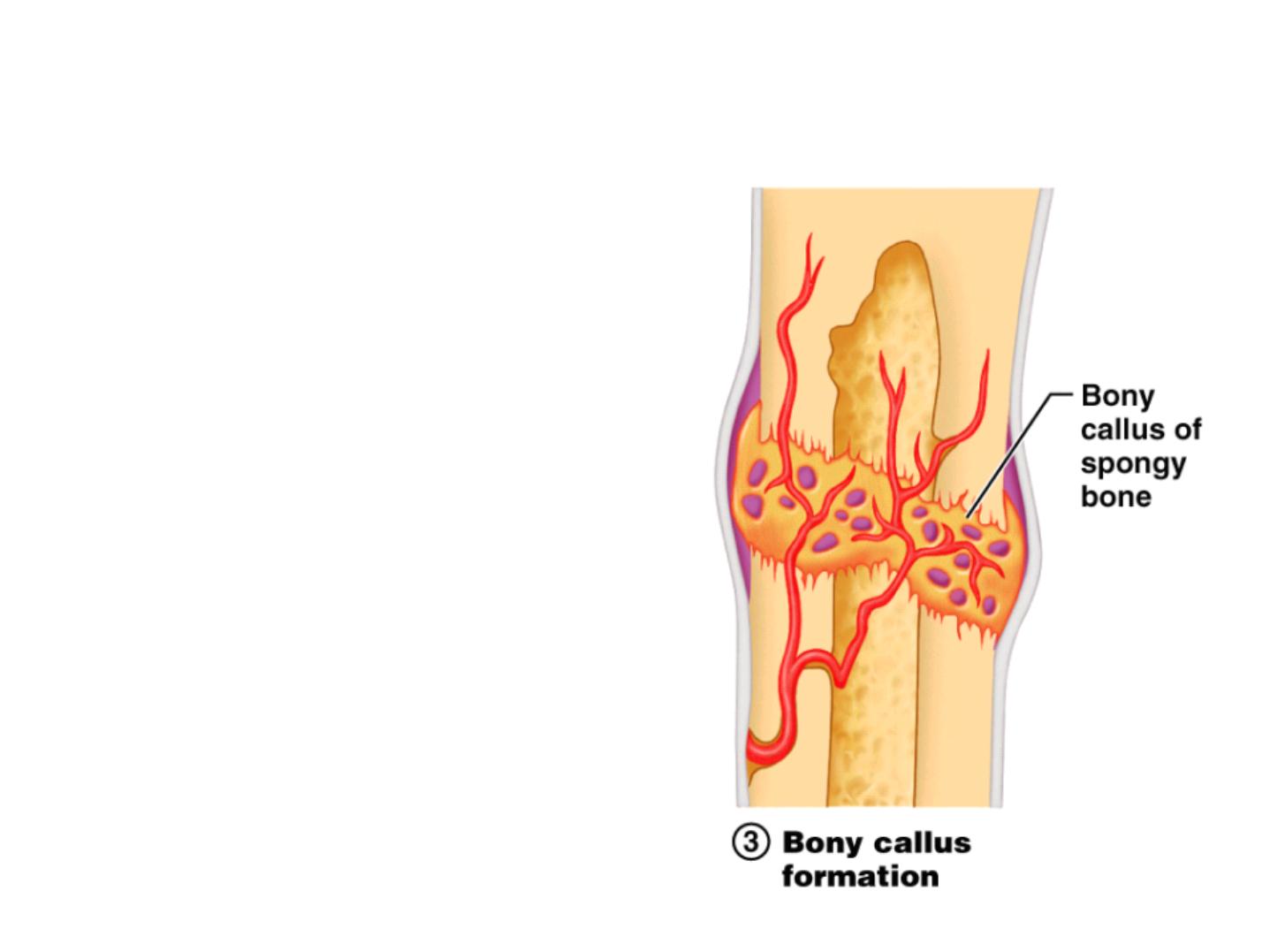
Fracture Repair Step 3: Bony Callus
• Bony callus formation
- New spongy bone
trabeculae appear in the
fibrocartilaginous callus
- Fibrocartilaginous callus
converts into a bony (hard)
callus
- Bone callus begins 3-4
weeks after injury, and
continues until firm union
is formed 2-3 months later
Figure 6.13.3
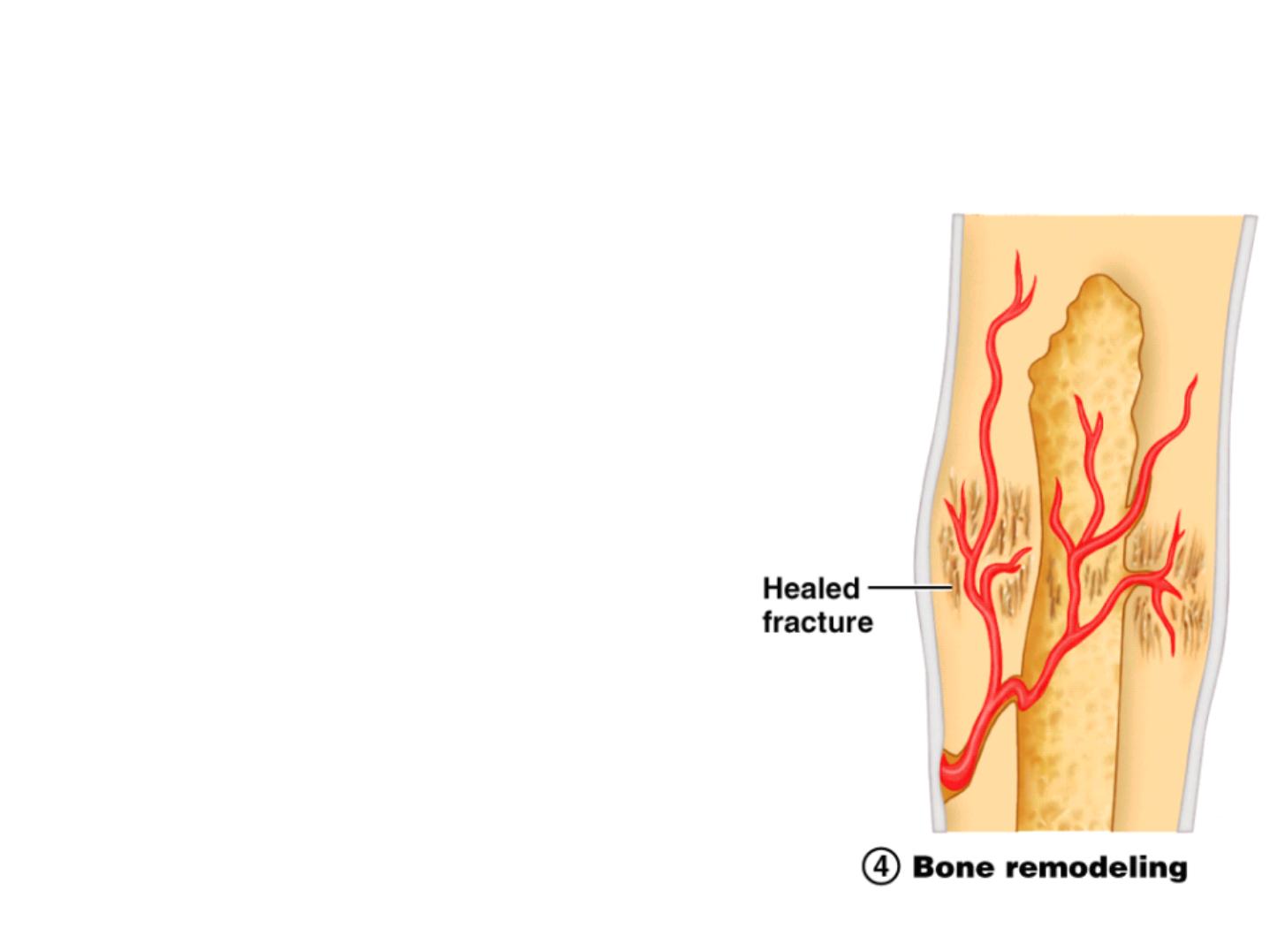
Fracture Repair Step 4: Remodeling
• Bone remodeling
- Excess material on the bone
shaft exterior and in the
medullary canal is removed
- Compact bone is laid down to
reconstruct shaft walls
- Remodeling for up to a year
• reduces bone callus
• may never go away completely
- Usually heals stronger than
surrounding bone
Figure 6.13.4

Aging and Bones
• Bones become thinner and weaker with age
• Osteopenia begins between ages 30 and 40
• Women lose 8% of bone mass per decade,
men 3%

Hormones and Bone Loss
• Estrogens and androgens help maintain bone
mass
• Bone loss in women accelerates after
menopause

Cancer and Bone Loss
• Cancerous tissues release osteoclast-
activating
factor
:
- stimulates osteoclasts
- produces
severe osteoporosis
