
1
Quantitative Assay
of Aspirin Tablets
Back Titration

2
There are two types of analytical methods :
(1) Qualitative Analysis: -
It determines the presence or absence of a particular compound, but
not the mass or concentration. By definition , qualitative analysis do
not measure quantity.
(2) Quantitative Analysis: -
It determines how much of each component , or of specified
components is present in a given sample.
Methods of Quantitative Chemical Analysis :
1-Volumetric ( Titrimetric ) analysis.
2-Gravimetric analysis.
3-Spectrophotometric analysis.

3
Requirements For a Titrimetric Assay:
(1) The reaction can be represented by a chemical equation.
(2)The reaction should be relatively fast.
(3)The reaction should be complete & irreversible.
(4) The end point should be easily detected.
Types of Titration:
1- Forward titration (direct titration).
2- Back titration (indirect titration).
Back Titration:
It includes the addition of an excess of a std. solution to a weighted
amount of a sample and then the excess unreacted std. solution is
determined by titration with another std. solution.

4
Back Titration Is Used For:
1- Volatile substances, e.g., NH
3
.
2- Insoluble or slightly soluble substances, e.g. CaCO3
3- Substances for which the quantitative reaction proceeds rapidly
only in the presence of excess of reagent, e.g., Lactic acid & Aspirin.
4- Substances which decompose on heating, e.g. , Formaldehyde.
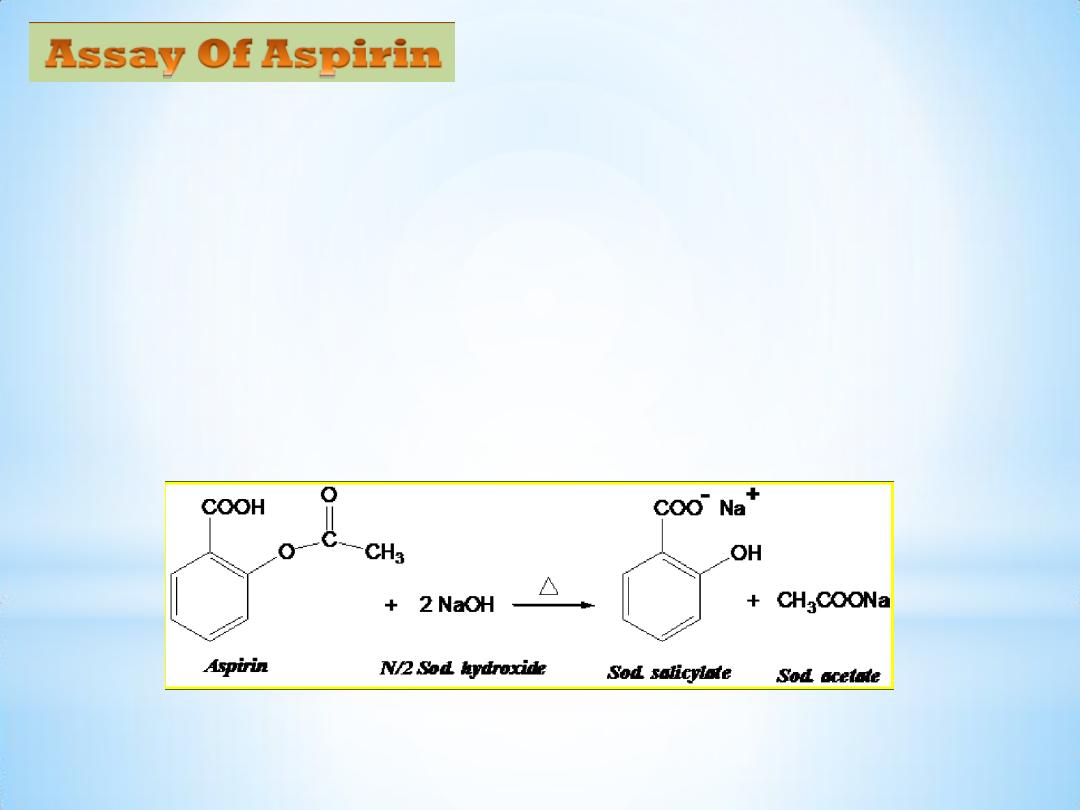
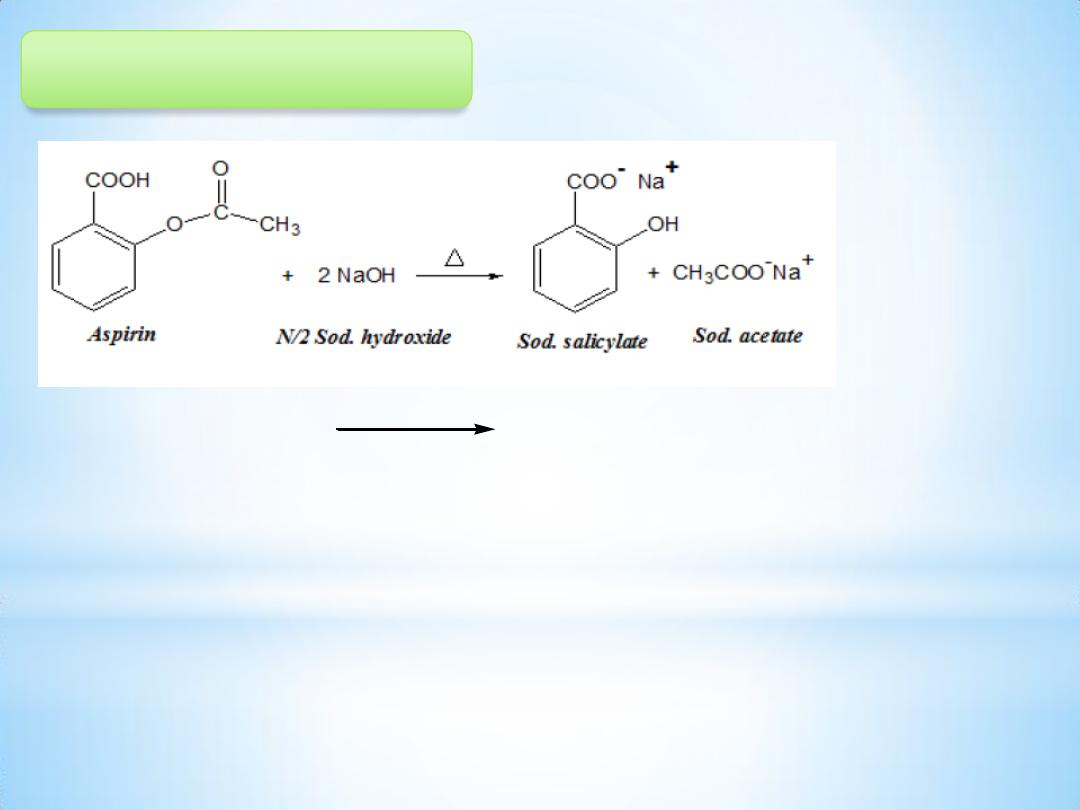
5
Principle:
The determination of the amount of aspirin present in a tablet dosage
form is done by alkaline hydrolysis of aspirin using N/2 NaOH standard
solution followed by back titrating of the excess unreacted alkali using
N/2 HCl std. solution & phenol red as indicator.

6
• Aspirin readily dissolved in dilute NaOH solution and hydrolyzed
completely by heating for 10 minutes with an excess of a base.
• Titration of the excess unreacted alkali with N/2 HCl std. solution
using phenol red indicator

7
• As in other quantitative determination involving boiling with a
standard alkali , cooling and back titrating the excess,
it’s
necessary to carry out a blank experiment without the aspirin .
• In order to:
1- Minimize any error due to small unavoidable losses.
2- Heating and cooling an alkaline liquid results in an apparent
change in strength if certain indicators are used .
• This change may be due to the interaction of the reagent with the
glass or due to , the absorption of atmospheric CO2 ,
• CO2 is rapidly absorbed by the hot alkaline solution to form
sodium carbonate .
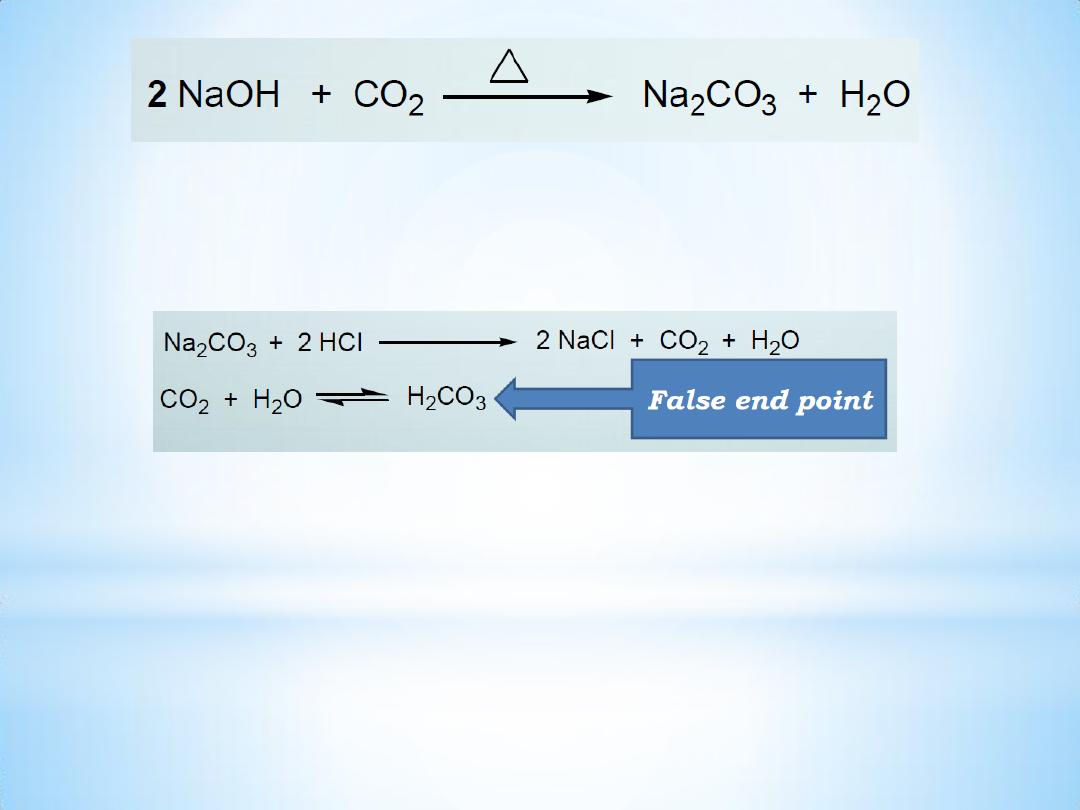
8
• In the back titration with the standard acid the liberated CO2
causes a color change of the indicator before the actual end point.
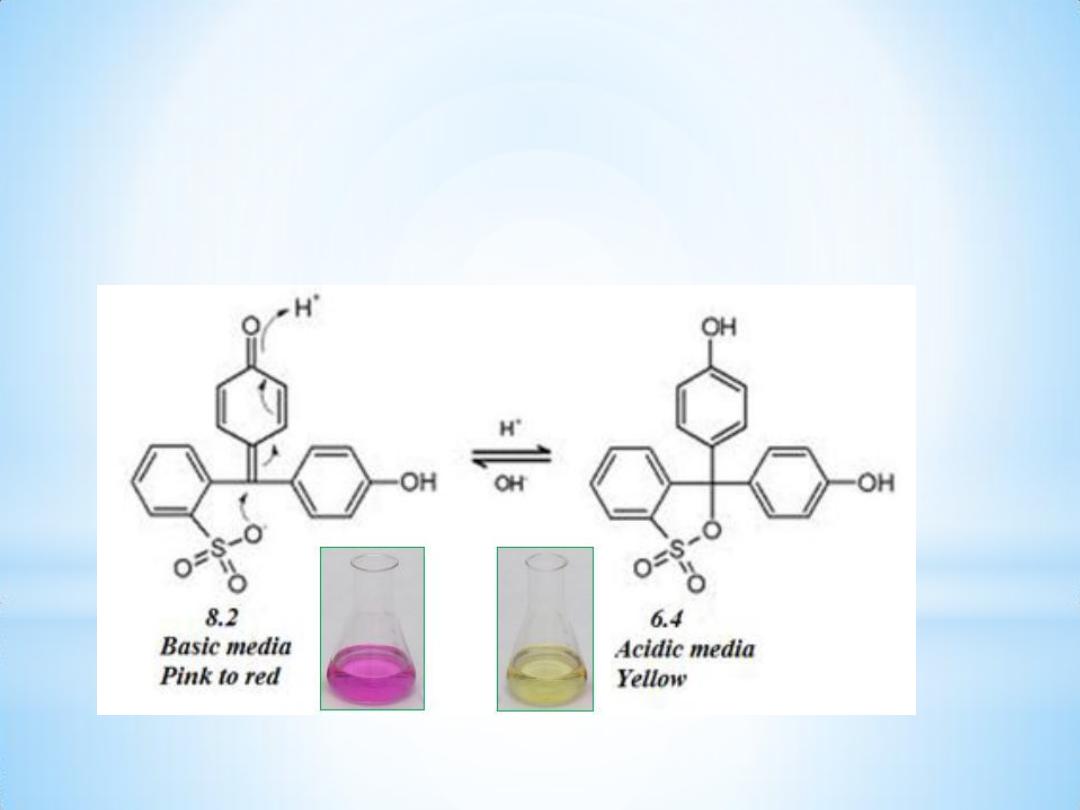
9
Phenol Red Indicator:
It’s also known as phenolsulfonphthalein . pH indicator ( PSP ) is a
pH indicator .
10
Calculations :
NaOH
+
HCl
NaCl
+
H
2
O
excess
unreacted
0.5 N
1 mole of aspirin = 2 mole of NaOH
0.045 g of aspirin = 1 ml of 0.5 N NaOH
11
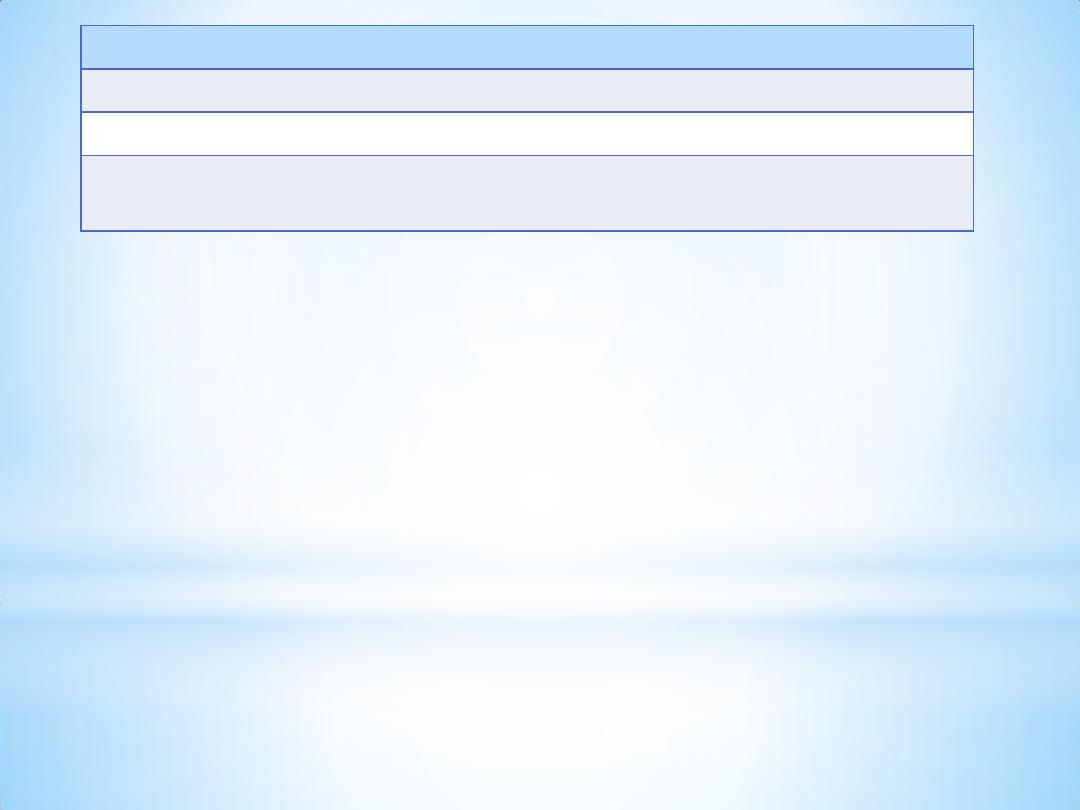
Sample exp.
Blank exp.
Weight of the powdered aspirin used
0.5 g
-
Volume of 0.5 N NaOH used
30ml
30ml
Volume of 0.5 N HCl used
19ml
28ml
Each 0.045 g of aspirin = 1 ml of 0.5 N NaOH Std. sol.
Each 0.045 g of aspirin = 1 ml of 0.5 N HCl Std. sol.
V2-V1 = 28-19 = 9 ml
9 x 0.045 =
0.405 g
of aspirin in the sample
