Water Balance in Human Body
د.عبدالحق ألنعيميBody Fluid Compartments
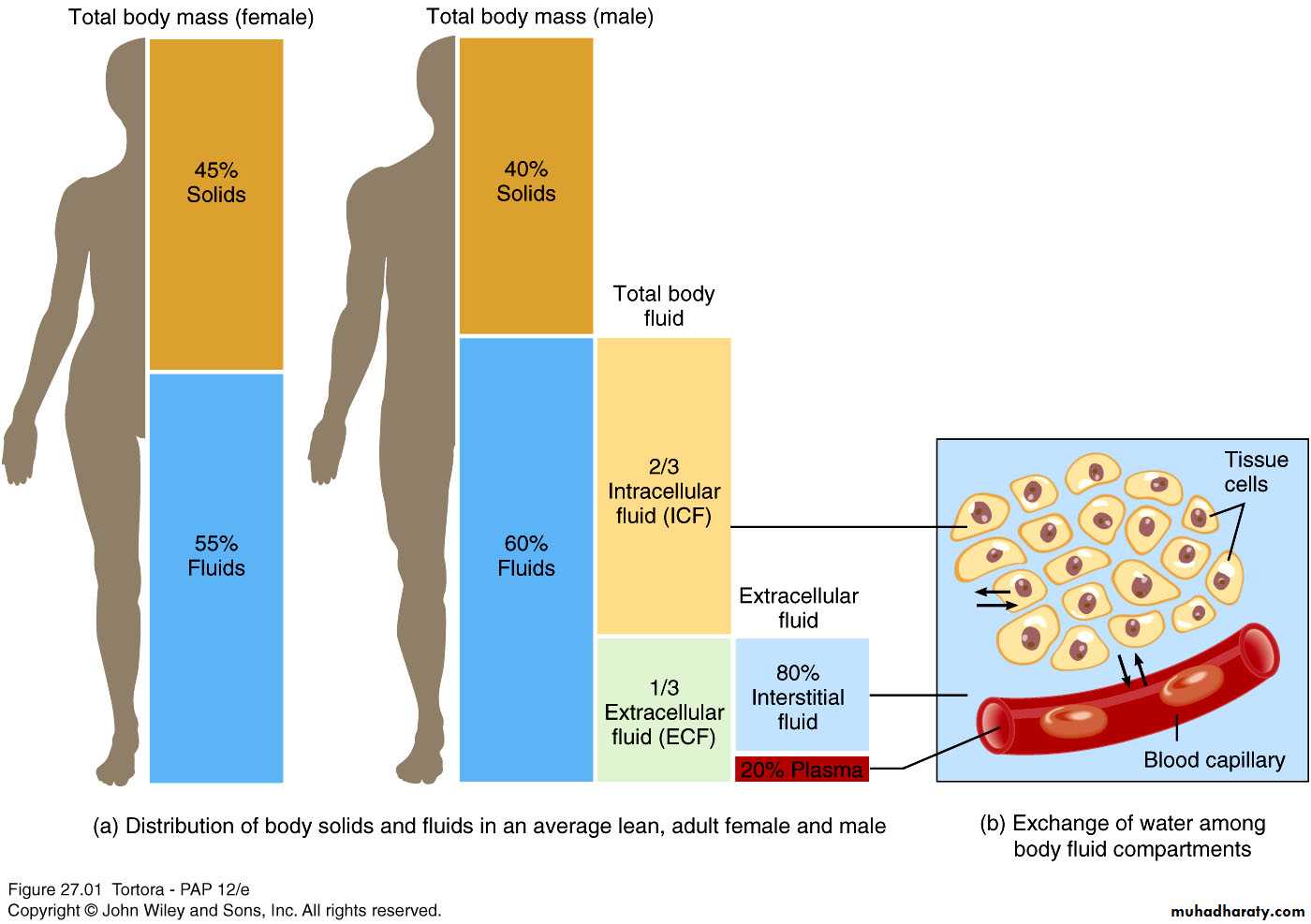
In lean adults, body fluids constitute 55% of female and 60% of male total body massIntracellular fluid (ICF) :
About 2/3 of body fluid
Extracellular fluid (ECF) :
1/3 of body fluid which include Interstitial fluid & Plasma
Interstitial fluid between cell is 80% of ECF
Plasma in blood is 20% of ECF
Also includes lymph, cerebrospinal fluid, synovial fluid, aqueous humor, vitreous body, endolymph,, pleural, pericardial, and peritoneal fluids
Sources of Body Water Gain and Loss
* Fluid balance related to electrolyte balance* Kidneys excrete excess water through dilute urine or excess electrolytes through concentrated urine
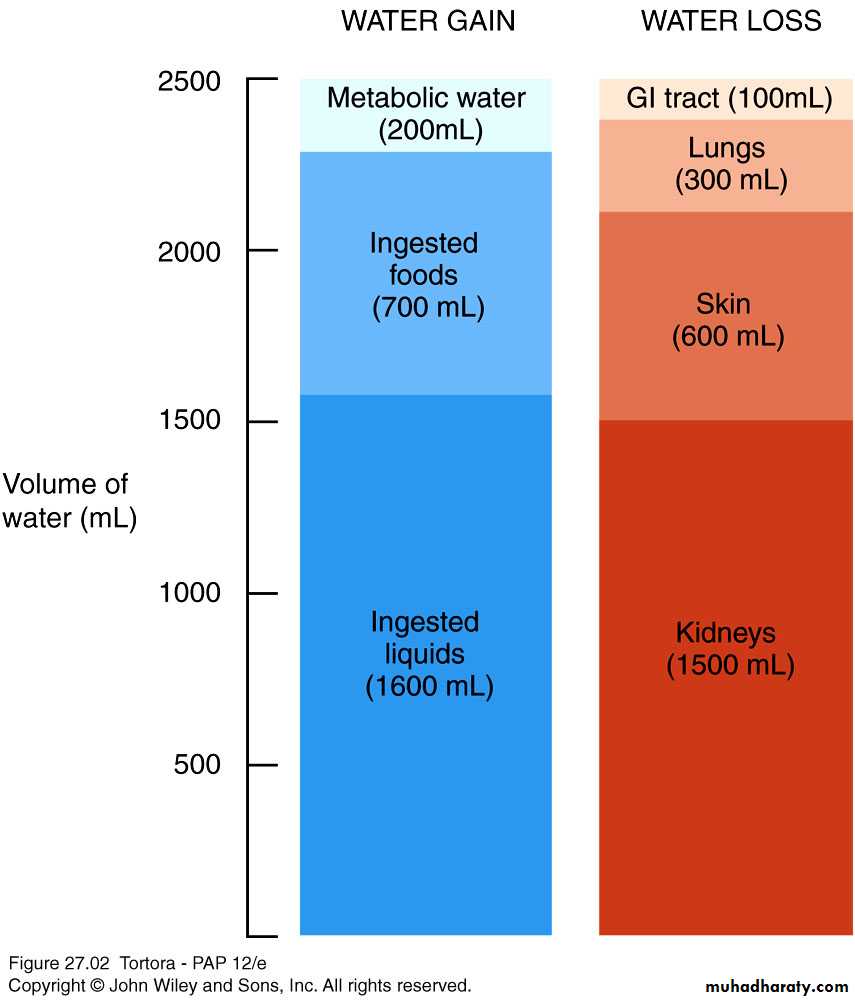
Body can gain water by
Ingestion of liquids and moist foods (2300mL/day)
Metabolic synthesis of water during cellular respiration (200mL/day)
Body loses water through
Kidneys (1500mL/day)
Evaporation from skin (600mL/day)
Exhalation from lungs (300mL/day)
Feces (100mL/day)
Body Fluid Compartments
Daily Water Gain and Loss
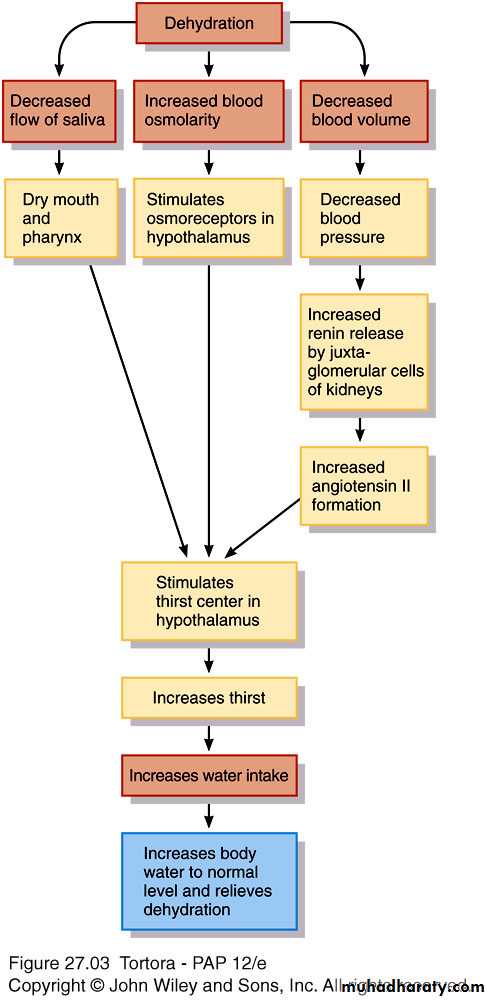
Regulation of body water gain
Mainly by volume of water intakeDehydration – when water loss is greater than gain
1.Decrease in volume,
2.increase in osmolarity of body fluids
Stimulates thirst center
in hypothalamus
Regulation of water and solute loss
Extent of urinary salt loss is the main factor that determines body fluid volume
Main factor that determines body fluid osmolarity is extent of urinary water loss
3 hormones regulate renal Na+ and Cl- reabsorption
Angiotensin II and aldosterone promote urinary Na+ and Cl- reabsorption of (and water by osmosis) when dehydrated
Atrial natriuretic peptide (ANP) promotes natriuresis, excretion of Na+ and Cl- followed by water excretion .
27_table_01
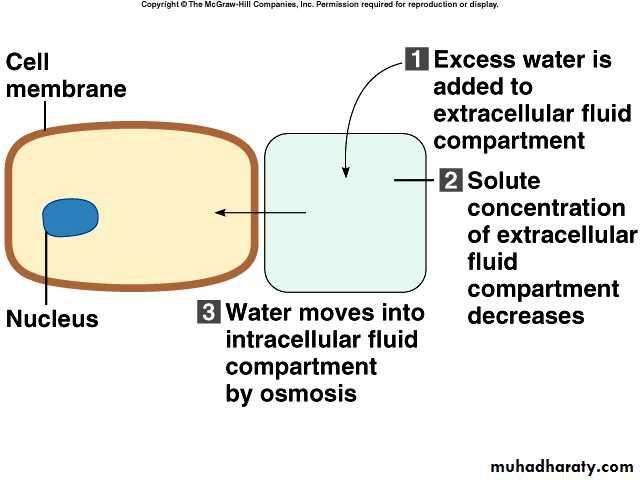
Movement of water between compartments
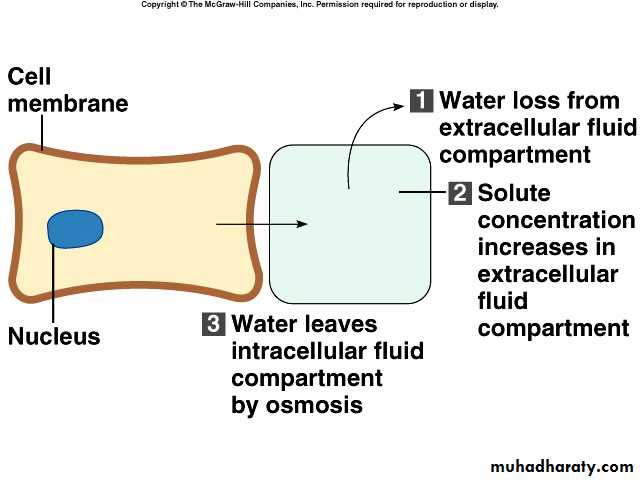
Normally, cells neither shrink or swell because intracellular and interstitial fluids have the same osmolarityIncreasing osmolarity (tonicity) of interstitial fluid draws water out of cells and cells shrink
Decreasing osmolarity of interstitial fluid causes cells to swell
Changes in osmolarity most often result from changes in Na+
concentration
Water excess & intoxication – drinking water faster than the kidneys can excrete it
Can lead to convulsions, coma or even death
Osmosis is the primary method of water movement into and out of body fluid compartments.
Osmosis is the net movement of water molecules through a selectively permeable membrane from an area of high water concentration to an area of lower water concentration.The concentration of solutes determines the direction of water movement.
Most solutes in the body are electrolytes – inorganic compounds which dissociate into ions in solution.
“Where sodium goes, water follows.”
<#>
Solutes : dissolved particlesElectrolytes – charged particles
Cations – positively charged ions
Na+, K+ , Ca++, H+
Anions – negatively charged ions
Cl-, HCO3- , PO43-
Non-electrolytes - Uncharged
Proteins, urea, glucose, O2, CO2
ICF differs considerably from ECF
ECF : most abundant cations are Na+ followed by Ca++ECF anions : Cl- , HCO3-
Sodium-functions
Impulse transmission, muscle contraction, fluid and electrolyte balance
Chloride
Regulating osmotic pressure, forming HCl in gastric acid
ICF differs considerably from ECF
ICF : most abundant cation is K+ followed by Mg++
ICF anions : negatively charged proteins and phosphates (HPO42-) ICF contain more protein than plasmaPotassium - function
Resting membrane potential ,
action potentials of nerves and muscles
Maintain intracellular volume
Regulation of pH
Controlled by aldosterone
Na+ /K+ pumps play major role in keeping K+ high inside cells and Na+ high outside cell
Manifestation of disorders of water , electrolytes and acid-base status
Clinical effectsAltered physiology
Primary disturbance
Circulatory changes
ECF
Na+
Cerebral changes
ECF osmolarity
Water
Neuromuscular weakness , Cardiac effects
Action potential in excitable tissues
K+
Altered tissue function , respiratory compensation
Acid-base balance(PH)
H+
Neuromuscular ,vascular &cardiac effects
Cell membrane stability
Magnesium
Wide spread tissue effects
Cell energetics
Phosphate
How to interpret electrolyte , urea and creatinine results
Na+ (sodium)• Largely reflects reciprocal changes in body water content
K+ (potassium)
• May reflect K shifts in and out of cells
• Low levels usually mean excessive losses (gastrointestinal or renal)
• High levels usually mean renal dysfunction
Cl− (chloride)
• Generally changes in parallel with plasma Na
• Low in metabolic alkalosis • High in some forms of metabolic acidosis
HCO3− (bicarbonate)
• Abnormal in acid–base disorders
Urea
• Increased with a fall in glomerular filtration rate (GFR),reduced renal perfusion or urine flow rate, and in high protein intake or catabolic states
Creatinine
• Increased with a fall in GFR, in individuals with high musclemass, and with some drug